Back to Journals » International Medical Case Reports Journal » Volume 14
Multidisciplinary Team Management of Severe Hemophilia A with Non-ST Elevation Myocardial Infarction
Authors Peng J, Yang H, Li J, Dai F, Wu J, Zhao X, Zheng C
Received 29 October 2020
Accepted for publication 12 January 2021
Published 27 January 2021 Volume 2021:14 Pages 15—20
DOI https://doi.org/10.2147/IMCRJ.S289483
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Jie Peng,1 Hongbin Yang,1 Jie Li,1 Feng Dai,1 Jingsheng Wu,2 Xielan Zhao,1 Changcheng Zheng2
1Department of Hematology, Xiangya Hemophilia Diagnosis and Treatment Center, Xiangya Hospital, Central South University, Changsha, People’s Republic of China; 2Department of Hematology, Anhui Provincial Hospital, Anhui Medical University, Hefei, People’s Republic of China
Correspondence: Xielan Zhao
Department of Hematology, Xiangya Hemophilia Diagnosis and Treatment Center, Xiangya Hospital, Central South University, 87 Xiangya Road, Changsha 410008, People’s Republic of China
Tel +86-731-84327564
Fax +86-731-84327497
Email [email protected]
Changcheng Zheng
Department of Hematology, Anhui Provincial Hospital, Anhui Medical University, 17 Lujiang Road, Hefei 230001, People’s Republic of China
Tel/Fax +86-551-62283114
Email [email protected]
Abstract: Elderly patients with hemophilia A have an increased risk of age-related thrombotic diseases, such as myocardial infarction. The relevant risk factors are comparable to those in the normal elderly population. However, their diagnosis and treatment are difficult. We report a case of a 53-year-old man with severe hemophilia A who presented with non-ST elevation myocardial infarction (NSTEMI), and this is the first report of successful treatment of such a patient in China. The patient presented with chest tightness, palpitations, and dyspnea after excessive alcohol consumption. He developed hypotension and shock, which rapidly progressed to respiratory and cardiac arrest and loss of consciousness. Immediate cardiopulmonary resuscitation was initiated, along with respiratory and cardiovascular management. Hematologic management with factor VIII (FVIII) replacement therapy and concurrent aspirin coupled with enoxaparin sodium, were also employed. As the patient’s condition was diagnosed as acute NSTEMI, a percutaneous coronary intervention was not performed. The patient showed significant improvement after 1 month; he was able to walk independently and was discharged. Based on the medication order, the patient was continuously treated with FVIII prophylaxis, clopidogrel tablets, and atorvastatin tablets after discharge to prevent the recurrence of cardiovascular events. The acute coronary syndrome incidence rate is similar in patients with hemophilia and the general population. Multidisciplinary collaborative management is required. The multidisciplinary team needs to develop its diagnosis and treatment process flow, and treatment should be individualized using or anticoagulation/antiplatelet therapy based on the patient’s medical history.
Keywords: factor replacement therapy, anticoagulation therapy, antiplatelet therapy, coagulation factors, elderly, case report
Introduction
With the advent of factor replacement therapy for hemophilia A, patients with the disease have experienced a significant reduction in the bleeding episodes and a marked improvement in their quality of life and life expectancy, both of which are now close to those of the general male population.1,2 However, replacement therapy has also introduced other issues such as thrombotic disease. Since Borchgrevink in 1959 reported the first case of myocardial infarction (MI) in hemophiliac patients, several cases have been reported worldwide.3 Therefore, elderly patients with hemophilia not only suffer from congenital bleeding disorders but are also at an increasing risk of age-related thrombotic diseases, such as MI. This implies that patients are at a dual risk of hemorrhage and thromboembolism, and the corresponding treatment should prevent hemorrhage while avoiding coagulation.4,5 There have only been a few relevant reports in China, and only 1–2 case reports have been published to date, of which all patients have died. The diagnosis of hemophilia A with ST-elevation myocardial infarction was confirmed only during an autopsy. Since MI in patients with hemophilia is rare, even globally, the practice of treating these patients with anticoagulant therapy is limited. We hereby report a case of severe hemophilia A combined with non-ST elevation myocardial infarction (NSTEMI), which was successfully treated with a multidisciplinary approach.
Case Presentation
A 53-year-old man presented to the emergency room of the Xiangya Hospital on December 19, 2017, with intermittent chest tightness, palpitation, and dyspnea. The patient had repeated bleeding in the joints since childhood. He underwent quantitative coagulation factor (FVIII) testing at a local hospital, and the diagnoses were as follows: severe hemophilia A, baseline factor VIII (FVIII) level <1%. Multiple follow-ups showed negative results for FVIII inhibitors; fresh frozen plasma and cryoprecipitate had been infused during previous bleeding episodes. Due to the improved financial situation in the past 20 years, the patient received occasional infusions of plasma-derived concentrates of FVIII/recombinant FVIII at irregular intervals. The patient had an approximately 16-year history of diabetes and had his blood sugar controlled through dietary restrictions and oral hypoglycemic agents, such as metformin and gliclazide. He had an approximately 3-year history of hypertension and kept his blood pressure (BP) in check through the intermittent use of therapeutic drugs, such as irbesartan and amlodipine. The patient had been hospitalized thrice in the past 5 years, owing to gastrointestinal bleeding (duodenal ulcer). The patient had no history of hepatitis C. He was a light smoker and a mild drinker.
The family and friends notified that the patient experienced chest tightness, palpitation, and dyspnea after consuming a large amount of alcohol on December 17, 2017; the symptoms were slightly relieved after administering “nitroglycerin.” December 18, his chest tightness and dyspnea significantly exacerbated. He was admitted to the emergency room of the People’s Hospital of Ningxiang, and his electrocardiogram showed ST-segment depression. The patient had hypotension and shock during the examination, which rapidly progressed to respiratory and cardiac arrest and loss of consciousness. He was administered immediate cardiopulmonary resuscitation and was managed using anti-shock therapy, tracheal intubation, assisted ventilation, and symptomatic and supportive treatment. During this period, the patient received FVIII 2000 IU once. Other specific treatments were not available. Following the stabilization of his condition, the patient was transferred to the emergency ICU on December 19. His physical examination findings were as follows: height, 170 cm; weight, 60.0 kg; BMI, 20.8 kg/m2; body temperature, 37.1°C; pulse, 112 beats/min; respiratory rate, 23 breaths/min; BP, 147/87 mmHg. The patient was managed by tracheal intubation, continuous oxygen supply, and assisted ventilation. He appeared to have moderate anemia, a heart rate of 112 beats/min, and metronomic heartbeats. Widespread moist rales were heard in both the lungs, the abdomen was soft and non-tender, and the liver and spleen were not enlarged. Joints of the right lower limb were deformed, and movement was impaired. Joints of the remaining limbs were normal. There was mild edema in the lower limbs. Pathological signs were not observed. Laboratory examination results were as follows: 1) complete blood count: white blood cells (WBC): 10.5×109/L, hemoglobin (Hb): 81 g/L↓, red blood cell (RBC): 2.69×1012/L↓, platelets: 214×109/L; 2) coagulation function: APTT, 34.4 s; FVIII: C, 160% (after FVIII infusion); FVIII inhibitor (-); 3) myocardial enzymology: lactate dehydrogenase (LDH), 393 U/L↑; creatine kinase, 210.2 U/L; creatine kinase-MB, 25.2 U/L↑; Troponin I quantification, 9.49 ng/mL↑; myoglobin, 180.00μg/L↑; brain natriuretic peptide (BNP), 15,712 pg/mL↑; C-reactive protein (CRP), 182 mg/L↑; procalcitonin (PCT), 2.22 ng/mL↑; 24 h blood glucose, 7.5–13.5 mmol/L; 4) electrocardiogram: II, III, aVF, V4–V6, significantly depressed ST segment (Figure 1); 5) bedside chest radiography: diffuse exudative changes in both the lungs (cardiogenic pulmonary edema).
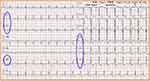 |
Figure 1 Electrocardiogram of the patient (the blue circles: II, III, aVF, V4~V6, ST segment was significantly depressed). |
Following discussions among a multidisciplinary team (MDT) including the physicians from the hemophilia center, a cardiologist, and an ICU physician, the diagnoses were as follows: 1) hemophilia A (severe); 2) coronary heart disease (CHD), acute NSTEMI, acute pulmonary edema (cardiogenic), class IV cardiac condition; 3) Type I respiratory failure; 4) hypertension (stage 2, very-high-risk group); and 5) Type 2 diabetes. The MDT developed a management plan after considering that the patient had hemorrhagic disease with the concomitant thrombotic disease. The physicians at the hemophilia center formulated the factor VIII replacement therapy regimen: 1600–1800 IU of FVIII, Q12h (target activity: 50–60%);6 the cardiologists formulated the treatment regimen for acute NSTEMI: aspirin tablets (100 mg, Qd) + enoxaparin sodium injection (4000 IU, Qd×3 days), isosorbide dinitrate (10 mg, continuous injection), metoprolol tablets (6.25 mg, Q12h), and atorvastatin tablets (20 mg, Qd). As the patient had acute NSTEMI, intervention treatment (PCI) was not necessary at that time; the ICU physicians formulated the following treatment plan: insulin for blood sugar control, assisted ventilation, antibiotics, organ function preservation, and symptomatic and supportive treatment.
Following the above treatment, the patient’s condition improved significantly after 1 month, by which time he was able to walk independently and was discharged from the hospital. Based on the medication order, the patient was continuously treated with FVIII 600 IU prophylaxis twice a week and clopidogrel and atorvastatin tablets after discharge to prevent the recurrence of cardiovascular events.
Discussion
Cardiovascular diseases, including ischemic heart disease, have drawn increasing attention among aging persons with hemophilia (PWH). However, the frequency of coronary atherosclerosis may be comparable between the PWH and the general population.5,6 Acute coronary syndrome (ACS) includes ST-elevation myocardial infarction (STEMI), NSTEMI, and unstable angina (UA).7,8 The most common cause of ACS is coronary thrombosis or thromboembolism arising from a ruptured atherosclerotic plaque.7,8 One of the important questions was whether patients with bleeding disorders could develop thrombosis. In other words, is the hypocoagulable state of patients with hemophilia a protective factor for thrombosis? A 2003 follow-up study on hemophilia carriers concluded that the disease had a direct protective effect on ischemic heart disease.9 The article analyzed the reasons underlying the low incidence of ischemic heart disease in carriers of hemophilia and found the following: 1) hemophilia was extremely rare, and many patients had a very short lifespan. 2) the hypocoagulable state in hemophilia might reduce thrombus formation. 3) the development of atherosclerosis could be uncommon. Meanwhile, it was also found that the mortality due to coronary artery disease was lower in patients with hemophilia than in the general population. However, growing evidence from extensive research suggests that hemophilia/von Willebrand disease (VWD) exerts no protective effect on atherosclerosis.5 The results of a comparison study involving 655 elderly patients with hemophilia and non-hemophilic elderly men (aged between 59 and 77 years) showed no differences in the 10-year CHD risk category distributions between men with and without hemophilia (P=0.554). At the same time, the investigators also calculated the Agatston scores on coronary artery calcification in the two groups at the University Medical Center Utrecht and found no differences in the scores between the two groups (P=0.792).5 Therefore, following a literature review, our MDT physicians were more inclined to believe that the risk of ischemic heart disease in elderly patients with hemophilia was the same as that in the non-hemophilic elderly population and that the risk would not be reduced because of the hypocoagulable state. We have found from related studies that the prevalence of cardiovascular disease in patients with hemophilia is approximately 19.5% and that of ischemic heart disease in patients with hemophilia aged over 60 years is approximately 15%.10,11 Factors such as obesity, hypertension, smoking, hypercholesterolemia, and diabetes play a leading role in the pathogenesis of MI in non-hemophilic elderly men. These risk factors for arterial occlusion are equally applicable to elderly patients with hemophilia. In particular, obesity is common among patients with hemophilia.12 Based on the patient’s medical history and laboratory examination results, our MDT physicians diagnosed hemophilia A (severe) combined with acute NSTEMI.
While arriving at the diagnosis was an important step, our next challenge was devising a patient’s treatment strategy. As physicians, we would need to determine if patients with hemophilia in a hypocoagulable state would require anticoagulation therapy; thus, determining the anticoagulation intensity and duration was important. An international, retrospective, 10-year survey was published in 2015 that evaluated the characteristics of ACS in 2380 adult patients with hemophilia and their management at each treatment center. The study found that merely 25% of physicians in the treatment centers would alter their ACS treatment strategy for patients with hemophilia and concomitant ACS, which implied that the presence of hemorrhagic disease in patients did not affect the anticoagulant therapy given by 75% of physicians. All patients with hemophilia and concomitant ACS received anticoagulant/antiplatelet drugs during the treatment period. All the patients were administered with coagulation factor concentrates before receiving anticoagulant/antiplatelet drugs. However, there was no universal standard for the predefined levels of FVIII/FIX activity. The FVIII/FIX troughs could be set at 30–50%, whereas the FVIII/FIX peaks could be set at 70–100%.6 After reviewing the relevant literature, our MDT physicians developed the process flow for treating hemophilia combined with ACS in our center (Figure 2).13 As our patient had concomitant ACS, we needed to supplement coagulation factors (FVIII), administer anticoagulation/antiplatelet therapy, and provide active symptomatic and supportive care as well (Figure 3). Following the above treatment, the patient’s condition had become stable, and he was successfully discharged. In addition, the patient was continuously treated with FVIII prophylaxis, clopidogrel tablets, and atorvastatin tablets after discharge to prevent the recurrence of cardiovascular events.
 |
Figure 2 Process flow for treating hemophilia combined with ACS. |
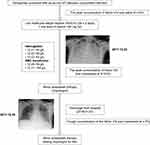 |
Figure 3 Process flow for treating our patient. |
The hemophilia treatment center should assist other departments to relieve the fear of treating hemophilia (particularly for cases involving invasive examinations and procedures). The MDT needs to develop its diagnosis and treatment process flow, and treatment should be individualized using coagulation factors or anticoagulation/antiplatelet therapy based on the patient’s medical history.
Data Sharing Statement
The data that support the findings of this study are available from Xiangya Hospital, Central South University and Anhui Provincial Hospital, Anhui Medical University but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. However, data are available from the Prof. Xielan Zhao, Prof. Changcheng Zheng upon reasonable request and with permission of Xiangya Hospital, Central South University, and Anhui Provincial Hospital, Anhui Medical University.
Statement of Ethics
Informed Consent: The patient provided written consent to publish this case details. Approval was granted by the Institutional Review Board of the Xiangya Hemophilia Diagnosis and Treatment Center, Xiangya Hospital, Central South University to publish this case report.
Acknowledgments
The authors would like to thank Editage for English language editing.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was funded by the Xiangya Hemophilia Diagnosis and Treatment Center, Xiangya Hospital, Central South University [grant number xyxyb20180201], and Anhui Provincial Hospital, Anhui Medical University [grant no. 201801070223].
Disclosure
The authors have no conflicts of interest to declare.
References
1. Darby SC, Kan SW, Spooner RJ, et al. Mortality rates, life expectancy, and causes of death in people with hemophilia A or B in the United Kingdom who were not infected with HIV. Blood. 2007;110(3):815–825. PubMed: 17446349. doi:10.1182/blood-2006-10-050435.
2. Plug I, van der Bom JG, Peters M, et al. Mortality and causes of death in patients with hemophilia, 1992–2001: a prospective cohort study. J Thromb Haemost. 2006;4(3):510–516. PubMed: 16460432. doi:10.1111/j.1538-7836.2006.01808.x.
3. Borchgrevink CF. Myocardial infarction in a haemophiliac. Lancet. 1959;1(7085):1229–1230. PubMed: 13666024. doi:10.1016/s0140-6736(59)90901-8.
4. Mauser-Bunschoten EP, Fransen van de Putte DE, Schutgens REG. Co-morbidity in the ageing haemophilia patient: the down side of increased life expectancy. Haemophilia. 2009;15(4):853–863. PubMed: 19228203. doi:10.1111/j.1365-2516.2009.01987.x.
5. Tuinenburg A, Rutten A, Kavousi M, et al. Coronary artery calcification in hemophilia A: no evidence for a protective effect of factor VIII deficiency on atherosclerosis. Arterioscler Thromb Vasc. 2012;32(3):799–804. PubMed: 22173226. doi:10.1161/ATVBAHA.111.238162.
6. Fogarty PF, Mancuso ME, Kasthuri R, et al. Presentation and management of acute coronary syndromes among adult persons with haemophilia: results of an international, retrospective, 10-year survey. Haemophilia. 2015;21(5):589–597. PubMed: 25689278. doi:10.1111/hae.12652.
7. Anderson JL, Adams CD, Antman EM, et al. 2012 ACCF/AHA Focused Update Incorporated into the ACCF/AHA 2007 Guidelines for the Management of Patients with Unstable Angina/non-ST-Elevation Myocardial Infarction: a Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(23):e663–e828. doi:10.1161/CIR.0b013e31828478ac
8. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: executive Summary: a Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(4):485–510. PubMed: 23256913. doi:10.1016/j.jacc.2012.11.018.
9. Srámek A, Kriek M, Rosendaal FR. Decreased mortality of ischaemic heart disease among carriers of haemophilia. Lancet. 2003;362(9381):351–354. PubMed: 12907007. doi:10.1016/s0140-6736(03)14021-4.
10. Mannucci PM, Mauser-Bunschoten EP. Cardiovascular disease in haemophilia patients: a contemporary issue. Haemophilia. 2010;16(Suppl.3):58–66. PubMed: 20586804. doi:10.1111/j.1365-2516.2010.02262.x.
11. Kulkarni R, Soucie JM, Evatt BL; Hemophilia Surveillance System Project Investigators. Prevalence and risk factors for heart disease among males with hemophilia. Am J Hematol. 2005;79(1):36–42. PubMed: 15849761. doi:10.1002/ajh.20339.
12. Zupančić-Šalek S, Vodanović M, Pulanić D, Skorić B, Matytsina I, Klovaite J. A case report of acute inferior myocardial infarction in a patient with severe hemophilia A after recombinant factor VIII infusion. Medicine. 2017;96(52):e9075. PubMed: 29384900. doi:10.1097/MD.0000000000009075.
13. Martin K, Key NS. How I treat patients with inherited bleeding disorders who need anticoagulant therapy. Blood. 2016;128(2):178–184. PubMed: 27106121. doi:10.1182/blood-2015-12-635094.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
