Back to Journals » Medical Devices: Evidence and Research » Volume 13
Multicenter Real-World Assessment of the Effectiveness of V-Go Wearable Insulin Delivery Device in Adult Patients with Type 2 Diabetes (ENABLE Study): A Retrospective Analysis
Authors Hundal R , Kowalyk S , Wakim A, Nikkel C, Sink II JH, Doyle M
Received 21 June 2020
Accepted for publication 2 September 2020
Published 22 September 2020 Volume 2020:13 Pages 283—291
DOI https://doi.org/10.2147/MDER.S265869
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Ripu Hundal,1 Stephan Kowalyk,2 Amanda Wakim,3 Carla Nikkel,4 John H Sink II,4 Melissa Doyle5
1First State Endocrinology, Newark, DE, USA; 2Endocrinology Specialists PC, Greensburg, PA, USA; 3Center of Endocrinology, Wheeling Hospital, Wheeling, WV, USA; 4Medical Affairs, Zealand Pharma, Boston, MA, USA; 5Progressive Diabetes Care, PLLC, Erwin, NC, USA
Correspondence: Carla Nikkel
Medical Affairs, Zealand Pharma, 34 Farnsworth St, Floor 4, Boston, MA 02210, USA
Tel +1 405-509-0401
Fax +1 405-330-4040
Email [email protected]
Purpose: Patch-like wearable insulin delivery devices are gaining acceptance as a treatment modality for insulin delivery in patients with diabetes. These devices aim to simplify and optimize insulin delivery while reducing barriers associated with a basal-bolus insulin regimen. As clinicians aim to learn more about this method of insulin delivery, real-world evidence can provide insight for patient identification and treatment guidance. This study was performed to evaluate the change in glycemic control (A1C) and insulin total daily dose (TDD) after switching to V-Go wearable insulin delivery device in a type 2 diabetes population with suboptimal control using conventional insulin delivery regimens.
Patients and Methods: Electronic health records were queried to identify patients meeting inclusion criteria. Study objectives evaluated change in A1C and insulin TDD compared to baseline. A total of 283 patients were enrolled across 9 diabetes specialty sites.
Results: A1C significantly decreased from baseline at 3 months (− 1.01% ± 0.09; P=0.0001) and 7 months (− 1.04% ± 0.10; P< 0.0001) after switching to V-Go. TDD of insulin significantly decreased at 3 months (− 17 ± 3 U/day; P< 0.0001) and 7 months (− 14 ± 3 U/day; P< 0.0001). Stratifying by prescribed baseline insulin regimen (basal-bolus, basal only or premix) or diabetes duration (< 5 years to > 20 years) demonstrated significant glycemic improvements from baseline with V-Go regardless of baseline regimen or duration of diabetes. After 7 months of V-Go use, the percent of patients considered high risk (A1C > 9.0%) was reduced by nearly half (46% to 24%), and 52% of patients overall achieved an A1C < 8%.
Conclusion: This study represents the largest real-world study of the effectiveness of V-Go in patients with type 2 diabetes. Significant improvements in glycemic control with a reduction in insulin utilization were achieved across varying baseline insulin regimens and regardless of diabetes duration supporting the clinical benefits of this patch-like wearable insulin delivery device.
Keywords: basal-bolus, insulin, insulin delivery device, patch, type 2 diabetes, V-Go
Introduction
Over 30 million adults in the United States have diabetes,1 and despite increasing availability and use of various non-insulin glucose-lowering medications (NIGLM), nearly 50% of patients with diabetes fail to achieve target hemoglobin A1C (A1C).2 Insulin is the most potent glucose-lowering agent and offers patients the highest potential to achieve glycemic control,3,4 however over 70% of physicians report that patients do not use insulin as prescribed.5
A basal-bolus insulin regimen is the most effective insulin regimen and offers flexibility for patients with variable mealtimes and carbohydrate content,6 but intensified insulin regimens are complicated to administer both for the patient and clinician. American Association of Clinical Endocrinologists (AACE) treatment recommendations state that the choice of therapy should factor in ease of use, and the regimen should be as simple as possible, especially for an elderly population.3 Simplifying treatment can improve patient compliance leading to improved glycemic control. Advances in insulin delivery have simplified insulin therapy,7 including completely disposable patch-like basal-bolus insulin delivery devices, which have recently been incorporated into the American Diabetes Association (ADA) standards of care as an alternative to insulin pen or syringe delivery.8 These devices aim to simplify and optimize insulin delivery while addressing many patient concerns that may have contributed to poor adherence to a basal-bolus insulin regimen.
As clinicians adopt advances in insulin delivery such as disposable patch-like basal-bolus insulin delivery devices, real-world evidence (RWE) can provide insight for patient identification and treatment guidance. Real-world data (RWD) include data from broad, diverse populations routinely collected from sources including electronic medical records (EMR), claims and billing data. Randomized controlled trials (RCTs) are necessary to assess efficacy; however, they often have specified inclusion and exclusion criteria to enroll a relatively homogeneous population and therefore may not reflect a product’s performance in a broader diverse population, especially patients with serious comorbidities,9 as is often the case in patients with diabetes. RCTs also commonly have frequent and regimented follow-up; therefore, patient enrollment and compliance tend to be higher in RCTs, which may lead to an unrealistic expectation of efficacy in actual clinical practice.9 RWE provides relevant data that can guide clinical decisions made by patients and provider, for instance when choosing an alternative insulin delivery method.
V-Go® (Zealand Pharma, Denmark) is a wearable basal-bolus insulin delivery device that can deliver a continuous basal infusion (20, 30, or 40 units/24 h) and allows for up to 36 additional units of insulin for mealtime dosing in 2-unit increments.10 It is indicated for patients 21 years of age and older requiring insulin and was launched in the USA in 2012. It is fully mechanical with no tubing or electronics, and it does not require any programming or control from an external device, differentiating it from other wearable devices. V-Go is filled with U-100 fast-acting insulin (eg, insulin lispro or insulin aspart) and is affixed to the skin like a patch using a hypoallergenic and latex-free adhesive. V-Go is fully disposable and is designed to be replaced every 24 h. To initiate the continuous basal rate of insulin, the patient applies V-Go to the skin and presses a button to insert a 4.6-mm, 30-gauge stainless steel needle into the subcutaneous space. On-demand mealtime bolus insulin doses can be administered by pressing the bolus-ready button and the bolus-delivery button through clothing, allowing for discreet insulin administration.
Previous studies showed that switching patients from traditional insulin delivery modes (insulin pens or syringes) to V-Go was associated with significantly improved glycemic control.7,11–19 This retrospective study represents the largest RWE study to date evaluating the use of V-Go across multiple practices. The objective of the study was to evaluate clinical outcomes in patients with sub-optimally controlled diabetes transitioned from other traditional modes of insulin delivery to V-Go. These findings will support and build upon the current evidence for V-Go to guide clinical decision-making.
Materials and Methods
This study was a retrospective review of patients who were using insulin and were changed to insulin delivery with V-Go across 9 sites spanning 8 states in the Northeast, Southeast and Southwest. Sites ranged from large specialized diabetes centers to independently owned practices. A query of the investigators’ EMR databases identified patients who initiated V-Go between January 1, 2014 and March 31, 2017. Adult patients ≥21 years with type 2 diabetes diagnosis, A1C value ≥7% and ≤14% within 60 days prior to initiating V-Go or up to 7 days after, prior use of insulin with documented determinable dosing immediately preceding V-Go initiation, and at least one documented follow-up visit on V-Go with an A1C measurement within 6 months after initiation were eligible for inclusion. Patients who used concentrated (U-500) insulin immediately preceding V-Go initiation and/or to fill V-Go, or used a traditional insulin pump immediately preceding V-Go initiation were excluded from the study. The study was reviewed and approved by Allendale Investigational Review Board and a waiver of patient consent was granted.
A1C measurements, insulin dosing, insulin regimens (eg basal only, premix, basal-bolus), weight, and NIGLM were obtained from the EMR records at baseline and for up to two follow-up office visits where an A1C measurement was documented. The prescribed dosages for insulin dosing were documented and recorded for study analysis. Primary outcome measures included changes in A1C and insulin dosing from baseline.
Descriptive statistics were used to report baseline variables and subject characteristics. Means and standard deviations (SD) were reported for continuous measures and relative frequencies were reported for categorical measures. Paired t-tests were used to assess statistical significance between baseline and follow-up values for continuous measures, and McNemar’s tests were used for categorical measures. To test for differences in change in A1C and TDD from baseline to study end based on changes to NIGLM, a one-factor repeated-measures ANOVA was performed for each variable with change in concomitant medication as the factor. P values <0.05 were considered to be significant. Missing data for continuous variables were imputed using the multiple imputation feature in SPSS and pooled data were used to express changes from baseline in means and standard error (SE). Outcomes were assessed for the overall population, as well as stratified by baseline insulin regimen, duration of diabetes for patients with known duration of diabetes and utilization of NIGLM. All statistical analyses were performed using SPSS (v. 25.0 (Armonk, NY)).
Results
In total, 580 patients were identified by the database query, and 283 patients across 9 sites met study inclusion and exclusion criteria and were enrolled. Primary reasons for ineligibility included no documented follow-up visit with an A1C measurement within 6 months of V-Go start, and no documented A1C within the protocol-specified range 60 days prior to V-Go start or within 7 days of initiating V-Go. Of the 283 patients enrolled, 227 patients had an A1C measurement from a second visit as well. The mean time from the start of V-Go to the first visit was 2.9 ± 1.3 months, and the mean time from the start of V-Go to the second visit was 7.1 ± 3.0 months, henceforth the visits will be referred to as the 3-month visit and the 7-month visit.
Patient demographics and baseline diabetes regimen are shown in Tables 1 and 2. Mean baseline A1C across the patient population was >9%, and the majority of patients were using a basal-bolus insulin regimen prior to V-Go (66%).
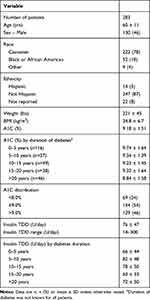 |
Table 1 Baseline Patient Demographics and Characteristics |
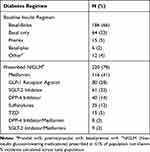 |
Table 2 Baseline Diabetes Regimen |
Clinical Outcomes
Effectiveness of insulin delivery with V-Go for the study population was assessed based on glycemic control (A1C) and total daily dose (TDD) of insulin prescribed over time (Figure 1), and the change in the distribution of the proportion of patients meeting A1C targets (Figure 2). In the overall cohort, there were significant mean reductions in A1C at both 3 months (−1.01% ± 0.09; P=0.0001) and 7 months (−1.04% ± 0.10; P<0.0001) after switching to V-Go, which were accomplished with a mean decrease in TDD of insulin (3 months: −17 ± 3 U/day; P<0.0001 and 7 months: −14 ± 3 U/day; P<0.0001). At baseline, the ratio of prescribed basal to prandial insulin was 64% to 36%, which shifted to 57% to 43% at the study end on V-Go. After 7 months of V-Go use, the percent of patients at high risk (A1C >9.0%) was reduced by nearly half; over 50% of patients overall achieved an A1C <8%; and 23% of patients achieved A1C <7%.
A1C and insulin TDD were analyzed stratified by the baseline regimen (basal-bolus, basal only, or premixed insulin) prior to V-Go use (Table 3). Basal-plus (n=6), prandial (n=5), premix/prandial (n=3), and basal/premix (n=4) strata were not individually analyzed due to the small sample sizes. Significant reductions in mean A1C were observed at both the 3-month and 7-month visits in all subsets except the 3-month premix subset. TDD of insulin was significantly decreased at both the 3-month and 7-month visits in the basal-bolus and premix subsets. There was a significant increase in the insulin TDD in patients prescribed basal insulin only, prior to V-Go; however, the basal dose of insulin in this group decreased by 28% at both the 3-month visit (47 ± 3 at baseline to 34 ± 1 at 3 months; n=64; P<0.0001) and the 7-month visit (48 ± 4 at baseline to 34 ± 1 at 7 months; n=55; P<0.0001), confirming that the increase in TDD of insulin in the basal only subset reflects the addition of insulin to cover meals.
 |
Table 3 Changes in A1C and TDD in Population by Baseline Insulin Regimen Prior to V-Go |
Patient using a basal-bolus regimen prior to V-Go were divided into tertiles based on insulin prescribed at baseline (<60 U; 60–90 U; >90 U), and A1C and insulin TDD were analyzed stratified by tertile (Table 4). Significant reductions in mean A1C were observed across all tertiles, and TDD of insulin was significantly reduced in tertiles 2 and 3, with a mean reduction of 63 units in patients prescribed >90 U/month prior to V-Go initiation.
 |
Table 4 Changes in A1C and TDD in Basal-Bolus Population Based on Baseline Insulin TDD Tertiles |
A1C and insulin TDD were also analyzed stratified by duration of diabetes for patients with known duration (n=186) (Figure 3). A1C was significantly decreased at both the 3-month and 7-month visits across all strata, and TDD was significantly decreased in the 5 to10 and 10 to 15-year strata, which had the highest baseline TDD.
To assess whether changes to NIGLM may have impacted study results, changes to NIGLM were reviewed and data analyzed in patients with two follow-up visits. Sixty percent of patients had no change to NIGLM, 19% had NIGLM added and 16% had NIGLM discontinued by study end. Multiple changes to NIGLM were reported in 5% of patients. No significant between-group differences at baseline for A1C or TDD were observed. All groups benefited from significant reductions (−0.92% to −1.35%, P<0.01) in A1C after switching to V-Go, with no significant difference in change in A1C between groups. Reductions in TDD from baseline were observed across all groups with significant reductions in both the group with no change to NIGLM (−15 U/day; P<0.0001) and the group where NIGLM was added (−23 U/day; P<0.001). Change in TDD was not significantly different between groups for those with no change in NIGLM and those with NIGLM added or discontinued.
At 7 months, 80% of all patients in the study had documented continued V-Go use, 14% of patients had documented discontinuation and 5% were lost to follow-up. Reasons for V-Go discontinuation include cost/insurance coverage (n=12), patient preference (n=10), skin irritation (n=6), V-Go would not stay adhered to the skin (n=4), difficulty using (n=3), or started insulin pump (n=3). Reason for discontinuation was not reported in 2 patients.
After 3 months, there was a significant increase in weight (2.3 lbs; P<0.0001); however, the change was not significant at 7 months (2.1 lbs; P=0.106).
Discussion
This multicenter study conducted at 9 sites in a real-world setting included a population not achieving glycemic targets, with 45% of patients diagnosed with diabetes over 15 years ago, and 46% of the population having an A1C >9%. Even in this sub-optimally controlled patient population with a longer duration of diabetes, patients benefitted from improved glycemic control while using a lower TDD of insulin after switching to V-Go. Improvements were consistently seen across varying baseline insulin regimens and significant improvements in glycemic control were achieved by patients newly diagnosed with diabetes as well as those diagnosed over 20 years ago. Significant reductions in A1C using a lower TDD of insulin after switching to V-Go were also observed regardless of changes in NIGLM, suggesting the clinical benefits observed were related to the use of V-Go. Important glycemic control goals were achieved after switching to V-Go; after 7 months of V-Go use the proportion of patients considered high risk (A1C>9%) was reduced by nearly half (46% to 24%) and 23% of patients met the glycemic target set by ADA of A1C <7%.20 This is the largest body of data generated in a multicenter real-world setting to date supporting V-Go effectiveness, and together with the existing body of evidence on V-Go use, it helps provide realistic guidance to clinicians to consider this strategy to improve glycemic control and potentially reduce the risk of complications.
The current body of evidence using V-Go includes a variety of study designs and approaches, with consistent improvements in glycemic control, achieved using less insulin. In 2015, Lajara and colleagues conducted a retrospective real-world study in 204 patients with A1C >7% prior to starting V-Go and showed that after initiation of V-Go, patients experienced significant decreases in A1C at both 14 weeks (−1.53%; P<0.001) and 27 weeks (−1.79%; P<0.001).11 These improvements in glycemic control were achieved with significantly less insulin (86–99 U/day at baseline to 58 U/day at 27 weeks; P<0.001). A recent retrospective study by Everitt and colleagues analyzed patients with baseline A1C ≥7% who were switched to V-Go.17 In this study, patients who discontinued V-Go and resumed conventional insulin delivery (CID) served as a natural comparator to assess V-Go versus CID following persistent (>5 months) use of both therapies. Following similar durations of therapy (8-month V-Go vs 7.5-month CID), patients using V-Go benefitted from significantly greater reductions in A1C (−1.42% V-Go vs −0.20% CID; P=0.003) and required significantly less insulin (change from baseline −4 U/day V-Go vs 13 U/day CID; P=0.003). A prospective study conducted by Cziraky and colleagues randomized 52 study sites via cluster randomization to either V-Go or standard treatment optimization (STO).19 Patients with baseline A1C between 8% and 14% were eligible for study enrollment. Significant decreases across the overall population in A1C were seen after 4 months in both groups (V-Go −1.0%; P<0.001 vs STO −0.5%; P<0.001); however, the A1C decrease in V-Go patients was significantly larger (P=0.002). There was also a significant decrease in TDD of insulin with V-Go (0.76 U/kg to 0.57 U/kg; P<0.001), but not with patients receiving STO (0.76 U/kg at baseline and at study end). Within the overall study group, 50% of patients were prescribed basal-bolus therapy requiring multiple daily injections (MDI) at baseline. A subset analysis of this MDI group demonstrated similar results in the overall population for improvement in glycemic control with the V-Go group experiencing a significantly larger decrease in A1C from baseline compared to the STO group (−1.0% vs −0.4%; P=0.006, respectively), with V-Go patients requiring significantly less insulin.
The body of RWE across single and multicenter studies, and evidence from a prospective randomized trial consistently support the effectiveness of V-Go, and in our opinion, reinforce that the mode of insulin delivery does matter. In the current study, the subset of patients prescribed MDI insulin therapy prior to initiating V-Go further validates the effectiveness of V-Go versus MDI for basal-bolus therapy. After 3 months of V-Go use, there was a significant reduction in A1C of 1.0% achieved using 32% less insulin in this subset of patients. This improvement associated with V-Go may be due to continuous subcutaneous insulin delivery and the ability to discreetly administer mealtime bolus doses at any time, which can lead to increased compliance and improved insulin administration before meals. These differences are important to consider when making therapeutic choices for insulin delivery and support why patch-like devices such as V-Go have gained acceptance as an insulin delivery option in the 2019 Standards of Medical Care in Diabetes issued by the ADA, and continue to gain recognition as an alternative to insulin syringes and pens.8,21
The population of patients with diabetes is heterogeneous and complex, with the majority of patients having multiple comorbidities.22 Diabetes duration is associated with an increased risk of macrovascular and microvascular events and death.23 It may be expected that achieving adequate glycemic control will become more difficult in patients with a longer duration of diabetes. In this study, there were significant improvements in glycemic control after initiation of V-Go across all durations of diabetes, even in patients with greater than 20 years of living with diabetes.
This multicenter study provides real-world experience with the use of V-Go across different patient characteristics, with consistently lower A1C achieved using less insulin after switching to V-Go. It is also the first study to evaluate the impact of the duration of diabetes on the effectiveness of V-Go. Limitations of this study are that it was a retrospective study and therefore associations rather than causations can be made. Also, it was required that patients had at least one follow-up visit after initiation of V-Go to be included in the analysis; therefore, it may have been more likely that compliant patients were included. Hypoglycemia was not collected in the study data because of differences in standard practice for defining and recording hypoglycemic events across the different sites, which would not have provided standardized data. Prescribed TDD of insulin was available in the EMR and was recorded for analysis; actual doses administered may have differed.
Conclusions
This study provides further real-world evidence that patients with sub-optimally controlled type 2 diabetes prescribed a wide range of traditional insulin regimens may achieve significant A1C improvements using less insulin when transitioned to V-Go. The findings from this study also support that patch-like insulin delivery devices like V-Go can improve glycemic control regardless of the duration of diabetes.
Abbreviations
AACE, American Association of Clinical Endocrinologists; ADA, American Diabetes Association; CID, conventional insulin delivery; EMR, electronic medical record; h, hour; A1C, hemoglobin A1c; MDI, multiple daily injections; NIGLM, non-insulin glucose-lowering medications; RCT, randomized controlled trial; RWD, real-world data; RWE, real-world evidence; SD, standard deviation; SE, standard error; STO, standard treatment optimization; TDD, total daily dose; U, units.
Data Sharing Statement
The data sets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study protocol was reviewed and approved by the Allendale Investigational Review Board and a waiver of informed consent and HIPAA granted due to the retrospective nature of this research meeting the general requirements of 45 CFR 46.116(d). This retrospective analysis involved minimal risk to the subjects while not adversely affecting the rights and welfare of the subjects, the integrity of the data is to be assured and maintained over its entire life-cycle and the retrospective analysis did not limit the authority of a physician to provide emergency medical care, to the extent the physician is permitted to do so under applicable federal, state, or local law. Data were extracted from electronic health records and the data then de-identified before an input in the database for further analysis. All computer entry was on a password-protected computer and conducted using patient identification numbers only. All physical records were maintained in a locked file cabinet.
Acknowledgments
We would like to acknowledge Jane A. Cases, MD; Amer Al-Karadsheh, MD; Adrienne N. Spence, DNP, FNP-C, CDE and Jamie Brewer, RN, MSN, FNP-C for their participation as investigators for the ENABLE study without whom this study and these analyses would not be possible. Editorial assistance in the preparation of this article was provided by Maria Paluselli, founder of Panaceum Clinical, LLC. Support for this assistance was funded by Zealand Pharma.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed on the journal to which the article will be submitted; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RH has received speaker fees and research support from Valeritas, Inc. now acquired by Zealand Pharma; SK has received speaker fees and research support from Valeritas, Inc. now acquired by Zealand Pharma; AW has received speaker fees and research support from Valeritas, Inc. now acquired by Zealand Pharma; CN is a current employee and stockholder of Zealand Pharma; JS at the time of the research had received speaker fees and research support from Valeritas, Inc. now acquired by Zealand Pharma and speaker fees from Novo Nordisk and is now a current employee and stockholder of Zealand Pharma; MD received research support from Valeritas, Inc. now acquired by Zealand Pharma. The authors report no other potential conflicts of interest for this work.
References
1. Centers for Disease Control and Prevention. National diabetes statistics report, 2017: estimates of diabetes and its burden in the United States. Atlanta, GA: US Department of Health and Human Services; 2017. Available from: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf.
2. Lipska KJ, Yao X, Herrin J, et al. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006–2013. Diabetes Care. 2017;40(4):468–475. doi:10.2337/dc16-0985
3. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Association of clinical endocrinologists (AACE); American college of endocrinology (ACE). Consensus statement by the American association of clinical endocrinologists and American college of endocrinology on the comprehensive type 2 diabetes management algorithm – 2017 executive summary. Endocr Pract. 2017;23(2):207–238.
4. American Diabetes Association. Standards of medical care in diabetes – 2020. Chapter 9: pharmacologic approaches to glycemic treatment. Diabetes Care. 2020;43(Supplement 1):S98–S110. doi:10.2337/dc20-S009
5. Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger P-M. Factors associated with injection omission/non-adherence in the global attitudes of patients and physicians in insulin therapy study. Diabetes Obes Metab. 2012;14(12):1081–1087. doi:10.1111/j.1463-1326.2012.01636.x
6. Leahy JL. Insulin therapy in type 2 diabetes mellitus. Endocrinol Metab Clin North Am. 2012;41(1):119–144. doi:10.1016/j.ecl.2012.03.004
7. Lajara R, Nikkel C, Abbott S. The clinical and economic impact of the V-Go disposable insulin delivery device for insulin delivery in patients with poorly controlled diabetes at high risk. Drugs Real World Outcomes. 2016;3(2):191–199. doi:10.1007/s40801-016-0075-4
8. American Diabetes Association. Standards of medical care in diabetes – 2019. Chapter 7: diabetes technologies. Diabetes Care. 2019;41(Supplement 1):S71–80. doi:10.2337/dc19-S007
9. Klonoff DC, Gutierrez A, Fleming A, Kerr D. Real-world evidence should be used in regulatory decisions about new pharmaceutical and medical device products for diabetes. J Diabetes Sci Technol. 2019;1932296819839996. doi:10.1177/1932296819839996.
10. Valeritas. V-Go: instructions for patient use; 2018. Available from: https://44yunigub221yppu525xpeh2-wpengine.netdna-ssl.com/wp-content/uploads/2018/06/ART-037_Rev-J_V-GoUpdatedIFU_05252018_FNL_LowRes.pdf.
11. Lajara R, Fetchick DA, Morris TL, Nikkel C. Use of V-Go® insulin delivery device in patients with sub-optimally controlled diabetes mellitus: a retrospective analysis from a large specialized diabetes system. Diabetes Ther. 2015;6(4):531–545. doi:10.1007/s13300-015-0138-7
12. Lajara R, Davidson JA, Nikkel CC, Morris TL. Clinical and cost-effectiveness of insulin delivery with V-Go disposable insulin delivery device versus multiple daily injections in patients with type 2 diabetes inadequately controlled on basal insulin. Endocr Pract. 2016;22(6):726–735. doi:10.4158/EP151182.OR
13. Lajara R, Nikkel C. Practical Considerations for switching to V-Go for insulin delivery in patients with type 2 diabetes. Pract Diabetol. 2016;5:10–15.
14. Rosenfeld CR, Bohannon NJ, Bode B, et al. The V-Go insulin delivery device used in clinical practice: patient perception and retrospective analysis of glycemic control. Endocr Pract. 2012;18(5):660–667. doi:10.4158/EP11362.OR
15. Johns BR, Jones TC, Sink JH, Cooke CE. Real-world assessment of glycemic control after V-Go® initiation in an endocrine practice in the southeastern United States. J Diabetes Sci Technol. 2014;8(5):1060–1061. doi:10.1177/1932296814537041
16. Sutton D, Higdon CD, Nikkel C, Hilsinger KA. Clinical benefits over time associated with use of V-Go wearable insulin delivery device in adult patients with diabetes: a retrospective analysis. Adv Ther. 2018;35(5):631–643. doi:10.1007/s12325-018-0703-3
17. Everitt B, Harrison HC
18. Winter A, Lintner M, Knezevich E. V-Go insulin delivery system versus multiple daily insulin injections for patients with uncontrolled type 2 diabetes mellitus. J Diabetes Sci Technol. 2015;9(5):1111–1116. doi:10.1177/1932296815580361
19. Cziraky MJ, Abbott S, Nguyen M, et al. A pragmatic clinical trial to compare the real-world effectiveness of V-Go versus standard delivery of insulin in patients with advanced type 2 diabetes. JHEOR. 2019;6(2):70–83. doi:10.36469/9731
20. American Diabetes Association Standards of Medical Care in Diabetes—2020. Chapter 6: glycemic targets. Diabetes Care. 2020;43(Supplement 1):S66–S76.
21. Ginsberg BH. Patch pumps for insulin. J Diabetes Sci Technol. 2019;13(1):27–33. doi:10.1177/1932296818786513
22. Iglay K, Hannachi H, Howie PJ, et al. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr Med Res Opin. 2016;32(7):1243–1252. doi:10.1185/03007995.2016.1168291
23. Zoungas S, Woodward M, Li Q, et al., ADVANCE Collaborative group. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia. 2014;57(12):2465–2474. doi:10.1007/s00125-014-3369-7
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



