Back to Journals » Infection and Drug Resistance » Volume 15
Multicenter Italian Study on “In Vitro Activities” of Isavuconazole, Voriconazole, Amphotericin B, and Caspofungin for Aspergillus Species: Comparison between SensititreTM YeastOneTM and MIC Test Strip
Authors Lo Cascio G, Bazaj A , Trovato L , Sanna S, Andreoni S, Blasi E, Conte M, Fazii P, Oliva E, Lepera V , Lombardi G, Farina C
Received 19 March 2022
Accepted for publication 16 September 2022
Published 4 October 2022 Volume 2022:15 Pages 5839—5848
DOI https://doi.org/10.2147/IDR.S367082
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Giuliana Lo Cascio,1– 3 Alda Bazaj,1 Laura Trovato,2,4 Silvana Sanna,2,5 Stefano Andreoni,2,6 Elisabetta Blasi,2,7 Marco Conte,2,8 Paolo Fazii,2,9 Ester Oliva,10 Valentina Lepera,11 Gianluigi Lombardi,2,11 Claudio Farina2,10
1Clinical Microbiology and Virology Unit, Azienda Ospedaliera Universitaria Integrata, Verona, Italy; 2Medical Mycology Committee, Italian Society of Clinical Microbiologist, Milan, Italy; 3Clinical Microbiology and Virology Unit, Azienda USL, Piacenza, Italy; 4Clinical Microbiology, Azienda Ospedaliera Universitaria- Policlinico Vittorio Emanuele, Catania, Italy; 5Microbiology and Virology Unit, Azienda Ospedaliera Universitaria, Sassari, Italy; 6Microbiology and Virology Unit, Azienda Ospedaliero Universitaria Maggiore della Carità, Novara, Italy; 7Clinical Microbiology, Azienda Ospedaliero-Universitaria, Policlinico di Modena, Modena, Italy; 8Microbiology and Virology Unit, Grande Ospedale Metropolitano Bianchi- Melacrino- Morelli, Reggio, Calabria, Italy; 9Clinical Microbiology and Virology P.O. Spirito Santo, Pescara, Italy; 10Clinical Microbiology and Virology Unit, ASST Papa Giovanni XXIII, Bergamo, Italy; 11Clinical Microbiology, ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy
Correspondence: Giuliana Lo Cascio, Email [email protected]
Abstract: In this study the activity of Isavuconazole, Voriconazole, Amphotericin B, and Caspofungin against 224 clinical isolates of Aspergillus spp. originating from seven Italian hospitals, was comparatively evaluated with two commercial antifungal susceptibility tests (AST): SensititreTM YeastOneTM (SYO) and MIC Test Strip. More attention was focused on Isavuconazole activity, given the new introduction of the drug in widely distributed antifungal susceptibilities methods in the clinical microbiology lab. The minimum inhibitory concentrations of antifungal drug that can inhibit the growth of pathogen by 90% (MIC90) for Isavuconazole detected by SYO were 0.5, 1, 0.25, and 2 μg/mL for Aspergillus fumigatus, Aspergillus flavus, Aspergillus terreus, and Aspergillus niger, respectively, whilst they were 0.25, 0.25, 0.5, and 0.75 μg/mL by MIC Test Strip. Essential agreement between the two tested methods for Isavuconazole is 70% for all the species tested, 75.7% for A. fumigatus, 45.2% for A. flavus, 90.6% for A. terreus, and 40% for A. niger. Although the tested strains do not express any phenotypic resistance, MIC results were quite different if tested with microdilution broth or gradient agar method. This is the first Italian multicenter report on Isavuconazole MIC obtained employing the widely used SensititreTM Yeast OneTM (SYO) and MIC Test Strip on clinical isolates of Aspergillus.
Keywords: antifungal susceptibilities, Aspergillus Isavuconazole susceptibilities, Sensititre and MIC Test Strip
Introduction
The number of infections caused by Aspergillus species is constantly growing, involving either known immunocompromised patients or immunocompetent subjects, for example, those with the latter Influenza- or COVID-19-associated pulmonary aspergillosis.1,2
Currently, available antifungal drugs for the treatment of invasive fungal infections (IFI) include polyenes, azoles, and echinocandins. Voriconazole and the new mold-active Isavuconazole are the first line drugs for the treatment of aspergillosis.3–5 The emergence of azole-resistant strains of Aspergillus spp. has been increasingly reported in patients undergoing long-term antifungal treatment.6 Previous studies have shown the usefulness of commercial antifungal susceptibility testing in comparison with reference methods, such as the Clinical and Laboratory Standard Institute (CLSI) or the European Committee on Antimicrobial Susceptibility Testing (EUCAST) ones for mold azole-resistance surveillance.7,8
SensititreTM YeastOneTM (TREK Diagnostic Systems, Cleveland OH) (SYO) is a widely used broth microdilution susceptibility testing system, based on the CLSI M27-A3 microdilution reference method. Based on the alamarBlue colorimetric indicator, SYO has been approved by the FDA as a method for susceptibility testing of yeasts; nevertheless, it has also been extensively used for molds.9,10
MIC Test Strip (Liofilchem, Italy) is a gradient diffusion method extensively used in the clinical microbiology laboratory for the evaluation of susceptibility to azoles and echinocandins of both yeasts and filamentous fungi.11
The aim of this study was to evaluate the Minimum Inhibitory Concentration (MIC) distributions of Isavuconazole, Voriconazole, and Amphotericin B, and the Minimal Effective Concentration (MEC) distribution of Caspofungin obtained simultaneously by two commercial methods, SYO and MIC Test Strip, on the most frequently isolated species of Aspergillus spp. Isavuconazole activity was deeply evaluated in order to study any difference between the methods, given the new introduction of the drug in widely commercial distributed antifungal susceptibilities methods in the clinical microbiology lab. Furthermore, the study was conducted in order to highlight any differences in the evaluation of Caspofungin activity on the basis of MIC or MEC distribution.
Materials and Methods
Two hundred and twenty-four Aspergillus strains isolated from clinical respiratory samples were collected anonymously between November 2018 and January 2019 in seven Italian Clinical Microbiology laboratories. In detail, 51 strains were collected in Azienda Ospedaliera Universitaria Integrata (AOUI) of Verona, 48 in Azienda Ospedaliera Universitaria (AOU) Policlinico Vittorio Emanuele–Catania, 35 in Bergamo, 29 in Milan-Niguarda hospital, 24 in Novara, 31 in Sassari, and six strains in Modena hospital. Species-level morphological identification was confirmed, if necessary, by MALDI-TOF mass spectrometry analysis (VITEK MS system equipped with the v3.2 IVD database or Bruker equipped with Compass 1.4 database). Few cases needed DNA-based identification by sequencing of the internal transcribed spacer (ITS). The collected Aspergillus strains included the sections Fumigati (A. fumigatus complex, n=127, Aspergillus thermomutatus, n=1), Flavi (A. flavus, n= 30, A. oryzae, n=1), Nigri (n=25), Terrei (n=32), Nidulantes (n=2), one A. ochraceus, one A. versicolor, one A. ustus, and three Aspergillus spp. Approval of the ethics committee was not needed, as the strains were collected retrospectively and anonymously.
SYO and MIC Test Strip were performed according to manufacturer’s instructions.12,13 Briefly, SYO color endpoints were determined for triazole and Amphotericin B. In detail, the lowest drug concentration corresponding to the first blue or purple well after 48 hours incubation was considered the MIC result. In cases of slow or insufficient growth, the microplates were incubated for an additional 24 hours. Due to a lack of manufacturer indications, the activity of Caspofungin was read looking at the MIC, as described above, in 50% of the laboratory involved in the study. Residual lab read the MEC as the lowest concentration at which short, stubby, and highly branched hyphal clusters were microscopically observed, compared with the growth control well after 48 hours of incubation.
The SYO panel used was Y-010 and the antifungal concentration of this panel ranged from 0.008–8 µg/mL for Isavuconazole and Voriconazole, from 0.015–8 µg/mL for Caspofungin, and from 0.12–8 µg/mL for Amphotericin B. The MIC Test Strip was performed by plating a 0.5 McFarland conidial suspension into RPMI 1640 medium 2% glucose agar plates (Liofilchem, Italy). Strip MICs were read after 48 hours incubation by three independent technicians. The MIC corresponded to the lowest drug concentration at which the pointed end of the inhibition ellipse intercepted the scale on the antifungal strip; small colonies inside the ellipse were not ignored for amphotericin B and triazoles. MEC for Caspofungin was read ignoring small colonies inside the ellipse, as the fungistatic activities of the molecule. Minimum Inhibitory Concentration required to inhibit the growth of 50% or 90% of organisms, MIC/MEC 50 and MIC/MEC 90, respectively, was calculated for the species with more than 10 strains represented. Candida krusei ATCC 6258 and C. parapsilosis ATCC 22019 were used as Quality Control strains. MIC ranges and MIC50/MIC90 values were calculated for each species and method. The high off-scale MICs were converted to the next highest concentration and the low off-scale MICs were recorded as the lowest tested concentrations. For the comparison with SYO microdilution data, MIC results for MIC Test Strip were rounded up to the next double dilution step value. Essential agreement (EA) was calculated for the two methods as the percentage of isolates with MIC within one 2-fold dilution for each species and each drug.
Results
SensititreTM YeastOneTM microdilution broth method results are shown in Table 1, which depicts the MIC distribution for Isavuconazole, Voriconazole, Amphotericin B, and MIC/MEC for Caspofungin. Most of the isolates (90%) showed a MIC ≤1 µg/mL for Isavuconazole, below the CLSI ECV for all the Aspergillus species. Most of the species showed Isavuconazole MICs similar to Voriconazole MICs, the only exception was A. niger, which showed the highest Isavuconazole MIC values (MIC90 2 µg/mL), conversely A. terreus isolates were the most susceptible (MIC90 0.25 µg/mL). The susceptibility to Voriconazole matches with that recently reported by Espinel-Ingroff in Aspergillus not harboring a cyp51A mutation (range=0.25–0.5 µg/mL).14 MIC values for Amphotericin B ranged from 1–2 µg/mL for more than 90% of A. fumigatus and A. niger and 80% of A. flavus. Few A. fumigatus (seven strains, 5.4%) showed MIC over 2 µg/mL. As expected, A. terreus shows the highest MIC, as previously reported.10 Caspofungin MIC values obtained by half of the center with SYO, conforming to manufacturer instructions, were always >8 µg/mL. The MEC results, after 48 hours, were <0.5 µg/mL for 85% of A. fumigatus complex, <0.12 µg/mL for 100% A. flavus, <0.5 µg/mL for 92% A. terreus, and <0.25 µg/mL for 100% A. niger. The results obtained from uncommon clinically relevant Aspergillus spp. were reported on a single line.
 |
Table 1 MIC and MEC (µg/mL) Distributions for Aspergillus Species by SYO |
Table 2 shows the MIC Test Strip distribution for all the antifungal drugs tested on clinical isolates. Isavuconazole strip MIC values were lower than SYO MIC for all the Aspergillus species, with the lowest result being obtained with A.fumigatus (mode 0.19 µg/mL). Unexpectedly few A. fumigatus strains (9, 7%) showed high Amphotericin MIC (>4 µg/mL), which is uncommon, and was not confirmed by SYO results. Amphotericin data were higher than SYO for A. terreus, in line with those previously reported by other authors.10 Many strains of A. flavus (19, 63%) were not readable (Figure 1) because it is undeniably difficult to establish a real MIC on the intercept ellipse of the strip. MEC for Caspofungin were considerably lower than SYO results, even if it was difficult to establish for a few strains of A. fumigatus and A. terreus, as is shown in Figure 2.
 |
Table 2 MIC and MEC (µg/mL) Distribution for Aspergillus Species by MIC Test Strip |
 |
Figure 1 Amphotericin MIC test strip with Aspergillus flavus. |
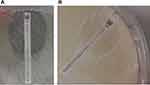 |
Figure 2 Caspofungin MIC test strip with Aspergillus fumigatus (A) and Aspergillus terreus (B). |
Voriconazole, Amphotericin B, and Caspofungin were not determinable for five strains of A. niger as the high level of plate growth after 48 hours incubation.
The MICs for the quality control and reference strains for both methods were within the expected ranges.
Table 3 illustrates the Essential Agreement (EA) and the MIC50/MIC90 with more than 10 strains represented for each species, compared with both CLSI and EUCAST epidemiological cut-off (ECV).11,14,15 No break point criteria are available for triazole susceptibility testing with the widely used SensititreTM YeastOneTM and MIC Test Strip, but for Voriconazole and Amphotericin B a proposed method dependent ECV was reported.
 |
Table 3 Essential Agreement (EA) and MIC50/MIC90 (µg/mL) by Sensititre (SYO) and MIC Test Strip for the Most Clinically Relevant Aspergillus spp |
Essential agreement, considered as ± one 2-fold dilution between SYO results and MIC test strip, turned out to be disappointing (Table 3). Isavuconazole and Voriconazole reached the best performance with an EA of 69.7% and 70%, respectively, instead of 52% for Amphotericin and 63% for Caspofungin MEC. The analysis by species reveals an excellent agreement for Isavuconazole MIC with 90% of EA for A. terreus, followed by A. fumigatus (EA 75%). Voriconazole showed for A. fumigatus and A. flavus acceptable results, with an EA of 78.9 and 77.4% MIC agreement.
Discussion
Aspergillus species are the most common causes of invasive fungal infections in immunocompromised patients. Nowadays the COVID-19 pandemic underlines the strict relationship between viral interstitial pneumonia and aspergillus opportunistic infections, especially in Intensive Care Units patients.16 The introduction of new antifungal agents and recent reports of resistance emerging during treatment of Aspergillus infections have highlighted the need for in vitro susceptibility testing.16 Isavuconazole or Voriconazole are considered first line therapy, alternatively liposomal amphotericin B could be used. Echinocandins could be used in combination in suspected azole resistance.17 Broth microdilution Antifungal susceptibility methods (BDM) have been standardized by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and the Clinical and Laboratory Standards Institute (CLSI) but are labor-intensive for the clinical microbiology lab. Few commercial methods have been proposed to evaluate the susceptibility to azoles, amphotericin, or echinocandins for molds, among them SensititreTMYeastOneTM (Thermofisher) and gradient agar test (Etest or MIC test strip) are the most widely distributed. To explore the correlation of the MIC/MEC results obtained with SensititreTMYeastOneTM and the MIC test strip on Aspergillus clinical strains, an Italian multicenter study was done. Moreover, Isavuconazole MIC distribution on Sensititre BMD was never reported. Our study provides range data on the susceptibility of main clinical Aspergillus strains derived from many geographically distributed Italian mycology laboratories. Both the assays employed in this study revealed Isavuconazole MIC90 values within the ECV established by CLSI for all the species tested, included A. niger which evidenced the highest MIC for Isavuconazole, but in accordance with other authors.18,19 SYO has been demonstrated as a suitable method for assessing the susceptibilities of Aspergillus common species to Voriconazole and Amphotericin B in comparison with CLSI method (10). MIC90 detected in our study by SYO for Voriconazole and Amphotericin B are in line with results obtained by Wang et al10 for Wild type isolates, below the CLSI ECV. As Wang et al previously reported, the SYO method showed an increase of Amphotericin B MIC of 1.6-fold higher geometric mean MIC than the CLSI M38-A method.
Amphotericin B MIC90 by the MIC Test Strip was >32 µg/mL for A. flavus, above the ECV value which should be ≤2. This is in line with the knowledge that A. flavus exhibits variable MIC to Amphotericin B with evidence of intrinsic resistance to polyenes.10,14 Caspofungin high MIC obtained by using SYO can be explained by 48 hours reading according to manufacturer’s instructions, not specifying how to read the Minimal Effective Concentration (MEC). As reported by Siopi et al,9 concerning echinocandins the best agreement between CLSI and SYO was found with an incubation of 20 hours for A. flavus and after 30 hours for A.fumigatus and A.terreus. MEC shown in Table 1 were lower and in line with other recent reports.20,21
MIC test strip showed Isavuconazole MIC lower than Voriconazole MIC, even if only slightly one 2-fold dilution. Conversely, MIC test strip showed high MIC for other drugs, especially amphotericin B in A. flavus and A. niger group. Few A. fumigatus evidenced Amphotericin B resistance, not seen in SYO BMD. MIC test strip overestimation was previously described by other authors.22 These phenotypes could be correlated to cryptic species as A. lentulus, morphologically indistinguishable from A. fumigatus sensu stricto, or other cryptic species in flavi and nigri sections, revealing the emerging importance of molecular species identification, as reported by Nargesi et al 23,24
A limitation of our study is that the SYO and MIC Test Strip results were not compared to CLSI or EUCAST reference methods, although other authors showed good correlation of Isavuconazole MIC Test Strip and EUCAST method for Aspergillus and Scedosporium clinical isolates (18). In addition, no molecular study has been done to evaluate azole resistance and few clinical strains of A. flavus complex, A. terreus complex, and A. niger complex have been tested. Therefore, further studies are necessary to increase the susceptibility data of clinical isolates.
In conclusion, our results confirm that Isavuconazole has an excellent activity against Aspergillus, as previously reported by using other standard methods.11,25 We showed that commercial SYO and MIC Test Strip methods could be used to determine Isavuconazole and Voriconazole susceptibility in molds, even though a comparative evaluation with the standardized EUCAST or CLSI method is recommended, especially if high azole MIC are found. In contrast, more attention should be paid to test Amphotericin B and Caspofungin, in line with previous reports.8,9 Overestimation of Amphotericin B MIC with SYO and MIC test strip could determine a false resistant phenotype. The difficulty to establish a simple and standardized method to read Caspofungin MEC is a concern and allows laboratories to fall in false resistance pattern if commercial company do not upgrade their technical instructions on correct incubation time and reading modality.
Essential Agreement between the two methods is rather good for Isavuconazole and Voriconazole, with an EA of 69.7% and 70%, respectively, but not enough for Amphotericin B and Caspofungin.
To our knowledge, this is the first Italian study investigating Isavuconazole activity against clinical Aspergillus strains by using the two commercial assays most widely used, namely MIC Test Strip and SensititreTMYeastOneTM. This study showed the susceptibility patterns of the main frequent Aspergillus clinical isolates in Italy and may help to understand the operational relevance of commercially available methods in clinical setting where Isavuconazole susceptibility tests are urgently demanded.
Acknowledgments
Isavuconazole MIC Strip and SensititreTM YeastOneTM plates were kindly provided at no cost by Basilea. We thank Dr. Tatiana Wictorowicz and Dr. Antonio Cassin for logistic and technical support. We thank Sofia Piffer, Ottolini Paola, and many other technicians for excellent technical assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Koehler P, Bassetti M, Chakrabarti A, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2020. PMID: 33333012. doi:10.1016/S1473-3099(20)30847-1
2. Dewi IM, Janssen NA, Rosati D, et al. Invasive pulmonary aspergillosis associated with viral pneumonitis. Curr Opin Microbiol. 2021;62:21–27. doi:10.1016/j.mib.2021.04.006
3. Douglas AP, Smibert OC, Bajel A, et al.; Australasian Antifungal Guidelines Steering Committee. Consensus guidelines for the diagnosis and management of invasive aspergillosis, 2021. Intern Med J. 2021;51(Suppl 7):143–176. doi:10.1111/imj.15591
4. Lass-Florl C. Susceptibility testing in Aspergillus species complex. Clin Microbiol Infect. 2014;20:49–53. doi:10.1111/1469-0691.12514
5. Mortensen KI. A prospective survey of Aspergillus spp. in respiratory tract samples: prevalence, clinical impact and antifungal susceptibility. Eur J Clin Microbiol Infect Dis. 2011;30(11):1355–1363. doi:10.1007/s10096-011-1229-7
6. Van der Linden JW, Camps SM, Kampinga GA, et al. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin Infect Dis. 2013;57(4):513–520. doi:10.1093/cid/cit320
7. Mello E, Posteraro B, Vella A, et al. Susceptibility testing of common and uncommon Aspergillus species against Posaconazole and other mold-active antifungal azoles using the Sensititre method. Antimicrob Agents Chemother. 2017;61(6):e00168–17. doi:10.1128/AAC.00168-17
8. Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID- ECMM_ERS guideline. Clin Microbiol Infect. 2018;24 Suppl 1(Suppl 1):e1–38. doi:10.1016/j.cmi.2018.01.002
9. Siopi M, Pournaras S, Meletiadis J. Comparative evaluation of Sensititre YeastOne and CLSI M38-A2 reference method for antifungal susceptibility testing of Aspergillus spp. against echinocandins. J Clin Microbiol. 2017;55(6):1714–1719. doi:10.1128/JCM.00044-17
10. Wang H-C, Hsieeh M-I, Choi P-C, Wu C-J. Comparison of the Sensititre YeastOne and CLSI M38-A2 microdilution methods in determining the activity of amphotericin B, itraconazole, voriconazole, and posaconazole against Aspergillus species. J Clin Microbiol. 2018;56(10):e00780–18. doi:10.1128/JCM.00780-18
11. Lamoth F, Alexander BD. Comparing Etest and broth microdilution for antifungal susceptibility testing of the most-relevant pathogenic molds. J Clin Microbiol. 2015;53(10):3176–3181. doi:10.1128/JCM.00925-15
12. ThermoFisher Scientific. Thermo Scientific Sensititre Plate Guide for Antimicrobial Susceptibility Testing. Available from: https://assets.thermofisher.com/TFS-Assets/MBD/brochures/Sensititre-Plate-Guide-Booklet-EN.pdf
13. © Liofilchem®. MIC test strip technical sheet Isavuconazole - MTS40 - Rev.0; 2015.
14. Espinel-Ingroff A, Turnidge J, Alastruey-Izquierdo A, et al. Method-dependent epidemiological cutoff values for detection of triazole resistance in Candida and Aspergillus species for the sensititre YeastOne colorimetric broth and etest agar diffusion methods. Antimicrob Agents Chemother. 2018;63(1):e01651. doi:10.1128/AAC.01651-18
15. Espinel-Ingroff A, Arendrup M, Cantón E, et al. Multicenter study of method-dependent epidemiological cutoff values for detection of resistance in Candida spp. and Aspergillus spp. to Amphotericin B and echinocandins for the Etest agar diffusion method. Antimicrob Agents Chemother. 2016;61(1):e01792–16. doi:10.1128/AAC.01792-16
16. Egger M, Bussini L, Hoenigl M, Bartoletti M. Prevalence of COVID-19-Associated pulmonary Aspergillosis: critical review and conclusions. J Fungi. 2022;8(4):390. PMID: 35448621; PMCID: PMC9027069. doi:10.3390/jof8040390
17. Risum M, Hare RK, Gertsen JB, et al. Azole resistance in Aspergillus fumigatus. The first 2-year’s data from the Danish National surveillance study, 2018–2020. Mycoses. 2022;65(4):419–428. PMID: 35104010; PMCID: PMC9302650. doi:10.1111/myc.13426
18. Guinea J. Updated EUCAST clinical breakpoints against Aspergillus, implications for the clinical microbiology laboratory. J Fungi. 2020;6(4):343. doi:10.3390/jof6040343
19. Trovato L, Scalia G, Palermo CI, Costanzo CM, Oliveri S. Evaluation of isavuconazole MIC strips for susceptibility testing of Aspergillus and Scedosporium species. Med Mycol. 2019;57(4):429–433. doi:10.1093/mmy/myy071
20. Lackner N, Vahedi-Shahandashti R, Jähnig S, Schönherr L, Lass-Flörl C. Echinocandins and their activity against aspergillus terreus species complex: a novel agar screening method. Antimicrob Agents Chemother. 2022;66(2):e0190921. PMID: 34902268; PMCID: PMC8846437. doi:10.1128/AAC.01909-21
21. Meletiadis J, Siopi M, Kanioura L, et al. Development and multicentre validation of an agar-based screening method for echinocandin susceptibility testing of Aspergillus species. J Antimicrob Chemother. 2019;74(8):2247–2254. PMID: 31106352; PMCID: PMC6640300. doi:10.1093/jac/dkz154
22. Idelevich EA, Groß U, Becker K, Bader O. Comparative evaluation of different gradient diffusion tests for detection of azole resistance in Aspergillus fumigatus. Diagn Microbiol Infect Dis. 2018;91(1):52–54. PMID: 29422272. doi:10.1016/j.diagmicrobio.2018.01.003
23. Nargesi S, Jafarzadeh J, Najafzadeh MJ, et al. Molecular identification and antifungal susceptibility of clinically relevant and cryptic species of Aspergillus sections Flavi and Nigri. J Med Microbiol. 2022;71(4). PMID: 35451946. doi:10.1099/jmm.0.001480
24. Nematollahi S, Permpalung N, Zhang SX, Morales M, Marr KA. Aspergillus lentulus: an under-recognized cause of antifungal drug-resistant Aspergillosis. Open Forum Infect Dis. 2021;8(8):ofab392. PMID: 34466628; PMCID: PMC8403229. doi:10.1093/ofid/ofab392
25. Astvad KMT, Hare RK, Arendrup MC. Evaluation of in vitro activity of Isavuconazole and comparator Voriconazole against 2635 contemporary clinical Candida and Aspergillus isolates. Clin Microbiol and Infect. 2017;23(11):882–887. doi:10.1016/j.cmi.2017.03.023
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
