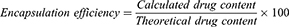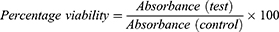Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 13
Mucoadhesive Microspheres of Maraviroc and Tenofovir Designed for Pre-Exposure Prophylaxis of HIV-1: An in vitro Assessment of the Effect on Vaginal Lactic Acid Bacteria Microflora
Authors Ekama SO , Ilomuanya MO , Azubuike CP, Bamidele TA, Fowora MA , Aina OO, Ezechi OC , Igwilo CI
Received 10 November 2020
Accepted for publication 4 March 2021
Published 8 April 2021 Volume 2021:13 Pages 399—413
DOI https://doi.org/10.2147/HIV.S291065
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bassel Sawaya
Video abstract presented by Sabdat Ekama.
Views: 358
Sabdat O Ekama,1,2 Margaret O Ilomuanya,1 Chukwuemeka P Azubuike,1 Tajudeen A Bamidele,2 Muinah A Fowora,2 Oluwagbemiga O Aina,2 Oliver C Ezechi,2 Cecilia I Igwilo1
1Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, University of Lagos, Lagos, Nigeria; 2Clinical Sciences Department, Nigerian Institute of Medical Research, Lagos, Nigeria
Correspondence: Sabdat O Ekama
Clinical Sciences Department, Nigerian Institute of Medical Research, 6 Edmund Crescent, P.M.B 2013, Yaba, Lagos, Nigeria
Tel +234 8134761356
Email [email protected]
Purpose: To formulate and evaluate microspheres of the antiretroviral drugs maraviroc and tenofovir intended for a candidate vaginal microbicide and assess its effect on the vaginal lactic acid bacteria microflora.
Methods: Ionic gelation technique was used to formulate maraviroc and tenofovir microspheres with subsequent characterization. The effect of varying concentrations of the polymer, crosslinking agent and the curing time on the outcome variables viz: particle size, mucoadhesion and encapsulation efficiency were investigated. Lactic acid bacteria were isolated from the vagina of healthy women using standard microbiologic methods. The analysis of their 16S rRNA sequence data identified Lactobacillus fermentum and Enterococcus faecalis strains which were assigned GenBank accession numbers. The efficacy of the microspheres on HIV-1BaL strain was evaluated using TZM-bl indicator cells.
Results: The optimal maraviroc and tenofovir microspheres had particle sizes of (434.82 μm and 456.18 μm), mucoadhesion of (93.3% and 90%) and encapsulation efficiency (92.80% and 78.9%) respectively. Maraviroc release kinetics followed a zero-order model and tenofovir was released via Higuchi model. The assay of a 1 mg/mL suspension of the microspheres on the strains of Lactobacillus fermentum and Enterococcus faecalis showed a viability of 93.9% and 89.7%, respectively. There was a statistically significant difference between the mean absorbance readings of the test agent and that of the positive control (P = 0.001). The microspheres elicited a progressive decline in HIV infectivity until at a concentration of 1 μg/mL.
Conclusion: The antiretroviral drugs loaded in the microspheres, had good mucoadhesion which is a potential for prolonged residence time in the vagina. The antiretroviral drugs were adequately released from the microspheres and showed efficacy against the HIV-1 BaL virus strain. There was no significant disruption in the growth of the lactic acid bacteria which constitute valuable bacteria microflora of the vagina.
Keywords: vaginal microbicides, HIV prevention, Lactobacillus fermentum, Enterococcus faecalis
Introduction
Heterosexual intercourse is a major mode of male-to-female transmission of HIV-1 infection and women account for a greater percentage of HIV prevalence globally. Pre-exposure prophylaxis measures using oral antiretroviral drugs and microbicides are advocated for HIV prevention. Microbicides are agents containing drugs that are inserted into the vagina or rectum to prevent the transmission of HIV or other sexually transmitted diseases.1,2
Microbicide dosage forms have evolved from the use of nonspecific agents to specific antiretroviral drugs. Furthermore, efforts are targeted towards improving on the lacunas observed with the early specific antiretroviral microbicides such as coital dependent use and short residence time in the vagina.
The antiretroviral drugs tenofovir and dapivirine have shown some level of success in clinical trials when administered as 1% tenofovir gel and dapivirine ring microbicides; in the CAPRISA 004 and the MTN-020-ASPIRE trials, respectively. However, subsequent clinical trials to validate the claims of the former in the VOICE and FACTS trials proved abortive.1
The disappointing outcome of these trials has directed researchers in the exploration of combination antiretroviral drugs for microbicide formulation.
In this quest, Date et al investigated the formulation of raltegravir and efavirenz nanoparticle combination as a microbicide for pre-exposure prophylaxis.
The use of combination antiretroviral agents acting at different points of the HIV lifecycle has been proven to be more effective in oral highly active antiretroviral therapy compared to single agents; this has been extended to microbicide formulations.3,4
Dobard et al formulated a rectal specific gel of 1% maraviroc and 1% tenofovir for the prevention of HIV transmission.5 However, the challenges of conventional gel formulations have necessitated the development of targeted drug delivery systems.
Employing novel drug delivery systems in microbicide formulations such as mucoadhesive microspheres (microparticles) is an effort to target the drug to the site of action, increase residence time in the vaginal environment and ultimately design formulations whose administration are coital independent.6,7
The natural ecosystem balance of the vaginal environment is maintained by resident beneficial organisms. Lactic acid bacteria (LAB) are a group of gram-positive bacteria found in different niches including the vaginal environment where they confer beneficial and protective properties.8,9 These groups of organisms maintain vaginal health and exert these valuable properties by producing lactic acid which helps to maintain the acidic vaginal pH (3.5–4.5). Common lactic acid bacteria groups are affiliated to the genus Lactobacillus, Enterococcus and Lactococcus among others.10,11
In this study, the antiretroviral drugs maraviroc and tenofovir which are entry inhibitors and nucleotide reverse transcriptase inhibitors, respectively; are formulated as microspheres to be ultimately incorporated into a vaginal microbicide gel. A mucoadhesive polymer chitosan has been selected to formulate the microspheres and confer mucoadhesive properties in a bid to prolong the residence time of the microspheres upon administration in the vagina. This antiretroviral drug combination is not available in oral form and this study intends to explore its combined efficacy when used as a microbicide designed for targeted delivery. It is required that an ideal candidate microbicide preserves the viability of the resident beneficial vaginal microflora. This study, therefore, aims to evaluate the efficacy and assess the effect of this candidate microbicide containing maraviroc and tenofovir microspheres on the vaginal lactic acid bacteria microflora.
Materials and Methods
Materials
Maraviroc was purchased from Shanghai Yudiao Chemistry Technology Co Ltd. (Shanghai, China), Tenofovir and Sodium tripolyphosphate were supplied by Macklin Biochemical Co. Ltd. (Shanghai, China), Chitosan (Molecular weight of 100–180 kDa and 70–85% deacetylated) was supplied by Dideu Medichem Ltd. (China), MRS Agar was procured from HiMedia Laboratory Pvt. Ltd. (Mumbai, India) Milli Q water (Milli Q water purification system, Merck, Germany) was used for all the experiments. All other chemicals were of analytical grade and used as obtained from the suppliers.
Experimental Design for Microsphere Formulation
A full factorial experimental design at two levels of concentrations requiring 2k runs was used to determine the varying effect of independent variables (factors) such as concentration of the polymer, crosslinking agent and curing time on the dependent variables viz: particle size, mucoadhesion, and encapsulation efficiency. Where k is the number of independent variables thus requiring eight experimental runs. Table 1 summarizes the experimental runs with the corresponding independent and dependent variables (response). A full factorial experimental design gives the opportunity to look at all the interactions that exist in a system; however, it is realistic to implement when the factors are fewer to avoid having run designs that are too cumbersome and expensive to manage.
 |
Table 1 Full Factorial Experimental Design of Independent Variables and Corresponding Dependent Variables (Response Variables) |
Design expert software was used to optimize the formulation and identify dominant factors/interactions terms that govern the system and influence the outcome of the dependent variables. The formulation chart for the experimental data is shown in Table 2.
 |
Table 2 Experimental Data for Microsphere Formulation |
Methods
Preparation of Microspheres
Microspheres of maraviroc and tenofovir were prepared using ionic gelation technique in which the polymer (chitosan) containing the antiretroviral drugs was crosslinked with sodium tripolyphosphate (TPP) to form microparticle carriers for the drugs. Varying weights of chitosan (0.1 g and 0.2 g) were dissolved in 10 mL (4%v/v) acetic acid and stirred using a magnetic stirrer for 30 min with subsequent addition of 10 mg of the individual antiretroviral drugs. The resultant dispersion was collected in a syringe with 23G needle and added dropwise into a (2%w/v and 4%w/v) solution of sodium tripolyphosphate.12 The gel-like beads formed were allowed to stand for a curing time range of 15 to 30 min, then washed three times with Mili Q water. The wet microspheres were air dried overnight and further dried in an oven at 45°C for 12 h.13 The dried microspheres were stored at 25°C ± 1°C in sealed plastic tubes.
Preparation of Vaginal Fluid Simulant (VFS) and Seminal Fluid Simulant (SFS)
Vaginal fluid simulant and seminal fluid simulant were prepared in accordance with the method of Owen and Katz.14,15
Characterization of Microspheres
Particle Size Determination
The particle size of the microspheres was determined by placing 20 particles from each batch on a glass slide, which was then mounted on the stage plate of an optical microscope (Motic stereo zoom microscope, SMZ-171 series, Hong Kong). The microspheres were observed via the eyepiece under 10X magnification with corresponding capturing of the image and recording of the particle radius. The mean particle diameter and standard deviations were calculated from these readings.
Percentage Yield Determination
The percentage yield of the microspheres was calculated by dividing the weight of the dried microspheres by the total amount of all the initial dry weights of starting materials and this was expressed as a percentage.12,13
Mucoadhesion Test Using Sheep Vaginal Tissues
Sheep vaginal tissue was obtained from a freshly slaughtered sheep in an abattoir. The vagina tissue was cut into the size of a glass slide rinsed with the prepared vaginal fluid simulant and attached to a glass slide using cyanoacrylate glue. The microspheres were counted, and 30 pieces were attached by placing unto the surface of the vaginal tissue and then immersed into a beaker containing 900 mL of the vaginal fluid simulant at 37°C ± 0.5°C. A loose string was used to connect the slide to the arm of a disintegration apparatus (Copley Scientific, UK) and it was given a vertical reciprocating movement of approximately 30 cycles per minute. At the end of 1 h and 12 h, the number of attached microspheres was counted and percentage mucoadhesion was calculated by expressing this value as a percentage of the originally attached number of microspheres.13 A 1% hydroxyethyl cellulose gel containing the drugs was used as negative control.
Encapsulation Efficiency
The prepared microspheres were crushed, dispersed in a sealed test tube containing 10 mL of 0.1 N HCl and then placed in an orbital shaker (Stuart Orbital shaker, UK) for 24 h with continuous rotation to extract the active ingredients. The resultant solution was sterile filtered (0.22 μm syringe filter) and the samples were analyzed at 215 nm (for maraviroc) and 259 nm (for tenofovir) using HPLC.
The HPLC system (Agilent 1200 series) consists of a quaternary pump system, an Agilent 1100 autosampler, a 1260 dual detector and a Zobax eclipse XDB C18 column (150 mm x 4.6 mm, with particle size 5 μm). The mobile phase was potassium dihydrogen orthophosphate buffer (pH was adjusted using orthophosphoric acid; Maraviroc (3.0), Tenofovir (3.5) and acetonitrile (70:30) v/v delivered at a flow rate of 0.5 mL/min).16 The standard curve was Y=33615X – 431.75, R2=0.9988 (for maraviroc) and Y= 29453X – 349.84, R2 = 0.9993 (for Tenofovir). The percentage encapsulation efficiency was calculated using the formula:
Where calculated drug content was determined from HPLC and the theoretical drug content is calculated from the mass ratio of the drugs and mass of the microspheres containing the drugs.
In vitro Release Study
The formulated maraviroc and tenofovir microspheres were each resuspended in 3 mL of the release medium which is a mixture of vaginal fluid simulant and seminal fluid simulant (pH 7.6) prepared at a 1:4 ratio. The dispersion was transferred into a 10 mL capacity dialysis membrane (Spectra/Por, Float-A-Lyzer G2, MWCO 3.5–5KD, Spectrum Laboratories Inc., USA). The membrane was maintained in a 20 mL release medium (VFS & SFS, pH 7.1) contained in the dialysis tube and the whole system was placed in a conical flask, then put in a shaking water bath (Julabo SW-21C, Germany) which was maintained at a temperature of 37°C ± 0.5°C. At periodic time intervals of 0, 1, 3, 6, 9, 12, 24, 48 and 72 h, 1 mL sample was withdrawn from the release medium for analysis with an equivalent replacement with 1 mL of the release medium to maintain sink condition. The amount of maraviroc and tenofovir released was determined using HPLC analysis under the same conditions as described above for encapsulation efficiency.17 The control were blank microspheres with no drugs loaded into it.
Mathematical Models Employed to Interpret Drug Release
The release data were fitted into various kinetic models viz: zero order, first order, Higuchi model and Korsmeyer–peppas model to ascertain the model that best described the drug release. This is deciphered by the model with the highest correlation coefficient (R2).
A plot of the cumulative % drug released versus time (h) is used to determine the zero-order model which is defined as a process of constant drug release. The first-order model is described as a process in which the rate of drug release is directly proportional to the concentration of the drug and it is determined by a plot of log cumulative % drug remaining versus time (h). Higuchi model describes the process of drug release as diffusion based and this model is determined by a plot of cumulative % drug released versus square root of time.
The Korsmeyer–peppas model is used to determine the type of drug transport mechanism through which drugs are released. It is evaluated by a plot of cumulative % drug released versus square root of time. In the Korsmeyer–peppas model, the release exponent n (which is the coefficient of X in the straight-line equation) represents the value for the classification of the drug transport mechanism. The exponent n is classified to follow a Fickian diffusion when n = 0.5, a non-Fickian diffusion (where 0.45 < n < 0.89), a case II transport when n = 0.89 and Super case II transport when n > 0.89.18
Fourier Transform Infrared Spectroscopy (FTIR)
The compatibility between the drugs and the excipients which constitute the microspheres was assessed using Fourier Transform Infrared Spectroscopy in accordance with the method described by Velmurugan and Ali.13
Swab Sampling and Bacteria Isolation
Ethical approval was obtained from the institutional review board of the Nigerian Institute of Medical Research (Project No:1RB/18/064) to conduct this study and consent was obtained from the participants. The participants were duly informed about the purpose of this study which was carried out according to the Declaration of Helsinki.
The women were non-pregnant, apparently healthy, of reproductive age (range, 25–35 years, mean 29.6 ± 3.2 years) and in their midcycle. Vaginal samples were collected using sterile vaginal swab sticks inserted about 4–5 cm and rotated around the walls of the vagina. The LAB organisms were isolated by streaking the swab sticks on MRS agar plates and incubated at 37°C for 48 h. The single gram-positive colonies from this growth were identified with 16S rRNA gene sequencing.
DNA Extraction and PCR Amplification
DNA Extraction was carried out using the Jena Bioscience Bacteria DNA Preparation Kit (Jena Bioscience GmbH, Germany) according to the manufacturer’s instructions. Polymerase chain reaction was carried out to identify lactic acid bacteria using BSF-8 (AGAGTTTGATCCTGGCTCAG) and BSR-534 (ATTACCGCGGCTGGC) primer pair. These primers produced an amplification size of 526bp by all lactic acid bacteria.
Sequencing and Specie Identification
All amplified PCR products (526bp) were purified using the Jena Bioscience PCR Purification Kit. Sanger sequencing of the 16S rRNA gene was carried out at Epoch Life Science (USA). The corresponding sequences were identified using the online BLAST search at http://blast.ncbi.nlm.nih.gov/Blast.cgi. The sequence data were submitted to the GenBank with subsequent assigning of GenBank accession numbers.
Lactic Acid Bacteria Viability Assay
An Eppendorf BioPhotometer (Eppendorf AG, Germany) set at an absorbance of 650 nm was used to determine the optical density of the organisms to an O.D650 of 0.062 and 0.079, respectively, corresponding to 108 CFU/mL.
An MTS (tetrazolium inner salt) assay was carried out with a suspension of the two different organisms seeded into 96 well plates with addition of 100 μL of growth medium. The first part of the well was incubated with 100 µL suspension of the microsphere suspension of maraviroc (1 mg/mL)/tenofovir (1 mg/mL); the positive control was inoculated with 100 µL of penicillin-streptomycin solution (10,000 IU/mL penicillin and 10,000 µg/mL streptomycin) while the negative control was inoculated with 100 µL of sterile broth. The 96 well plates were incubated at 37°C for 48 h. A microplate reader (EZ Read 400 Biochrom Ltd, UK) was used to determine the absorbance at a wavelength of 492 nm.17 The percentage viability of each organism was determined from the equation:
Efficacy Testing
TZM-bl Assay
TZM-bl cells assay was described by Ilomuanya et al19 in which the TZM-bl cells were plated in 96 well plates and 100 μL of a 10-fold serial dilution of each of the optimal maraviroc and tenofovir microspheres in a 50:50 ratio was applied. Nonoxynol 9 gel was used as the negative control. Several microbicide assays have been conducted using nonoxynol-9 as the control agent. It is the first microbicide candidate to be evaluated which failed in clinical trials however it has served as negative control in microbicide assays.20
Statistical Analysis
The design of experiment was analyzed using the Design-expert software (Trial version 13, Stat-Ease Inc., Minneapolis MN, USA). Statistical analysis was conducted using the student t-test, assuming a significance level (α) of 0.05 and at 95% confidence interval. Results were presented as mean ± standard deviation with n = 3 determinations.
Results
Formulated Microspheres
The formulated microspheres were discrete, free flowing and almost spherical in shape as seen in Figure 1. The particle size of the F formulations containing tenofovir ranged from 443.36 ± 50.87 µm to 633.30 ± 63.90 µm with percentage mucoadhesion values of 57% to 90%. Encapsulation efficiency values ranged from 7.7% to 78.9% with observed percentage yield of 1.9% through 11.3%. Larger particle sizes were observed with the G formulations with dimensions spanning from 490.85 ± 10.11 µm to 794.59 ± 23.6 µm; however, similarly ranged mucoadhesion values of 63.3% to 93.3%, and percentage yield of 2.4% to 13.1% were recorded as shown in Table 2.
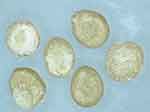 |
Figure 1 Microspheres observed under an optical microscope with 10x magnification. |
Variability of Independent Factors and Its Effect on Outcome Variables
An increase in the concentration of the polymer (chitosan) resulted in higher percentage mucoadhesion levels (F3→F4 & G3→G4) and encapsulation efficiency (F3→F4 & G3→G4) as shown in Table 1. Increasing the concentration of the crosslinking agent (tripolyphosphate), (G4→G2, F8→F6, and F4→F2) caused an increase in particle size when there is a corresponding maximum polymer concentration (2%w/v), but a decrease in particle size was observed when the polymer concentration was low (1%w/v) F7→F5 & G7→G5.
The extension of curing time from 15 min to 30 min resulted in decreased mucoadhesion with a corresponding increase in encapsulation efficiency (G2→G6 & G3→G7).
Screening, Characterization, and Optimization of Formulation Variables
The factors that were relevant to the outcome variables (responses) were identified using a half normal plot and pareto chart generated by the design expert software.
The half normal plot highlights relevant factors that influence particle size which are factors/interaction terms that do not align with the straight line (Figure 2A) with a further confirmation in the pareto chart (Figure 2B).
 |
Figure 2 Continued. |
These factors include curing time C, interaction terms AB (concentration of tripolyphosphate/chitosan) and interaction terms ABC (concentration of tripolyphosphate/chitosan/curing time).
The pareto chart demonstrates the significance of these factors by showing them to be above the t-value limit and the Bonferroni limit in the chart (Figure 2B).
Encapsulation efficiency was governed by the factor B (concentration of chitosan) and interaction factors ABC (concentration of tripolyphosphate/chitosan/curing time) as shown in Figure 2C and D. However, the outcome variable mucoadhesion was governed by only the concentration of chitosan (Figure 2E and F).
Analysis of variance (ANOVA) was used to test for the significance of the model for all the outcome variables and the level of significance, α was set at 0.05.
The ANOVA table showed the overall significance of the model for all outcome variables as well as the significance of the individual factors identified from the pareto charts. The p values obtained were less than the α value which confirmed the statistical significance of the three models (Table 3).
 |
Table 3 ANOVA for the Selected Relevant Factors and Factor Interactions for Each Response |
The fit summary (Table 4) showed a good fit with regards to the R2 values which were 0.9615, 0.9276, and 0.9040 for the outcome variable particle size, encapsulation efficiency and mucoadhesion, respectively. The proximity of these values to 1 is an indication of a close relationship between the experimental and predicted values. Furthermore, the difference between the adjusted R2 and predicted R2 is less than 0.2 for the three outcome variables which implies that the values agree and are in proximity. The adequate precision for the three outcome variables is greater than 4 which is the goal of the fit assessment as it indicates adequate signal.
 |
Table 4 Fit Statistics |
Interaction plots and pairwise comparison were used to tease out the best conditions for the optimized formulation and a prediction of the outcome variables (response) as shown in Table 5 and Figure 2G. The two sets of values presented in Table 5 showed proximity of values before optimization (F4 and F6) and after optimization.
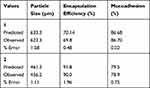 |
Table 5 Predicted and Experimental Values of the Optimal Conditions of Microspheres Obtained by Numerical Optimization |
In vitro Release Pattern of Maraviroc and Tenofovir from the Microspheres
An analysis of the in vitro drug release from the optimal maraviroc and tenofovir microspheres was carried out by fitting the release data into various kinetic models to determine the coefficient of correlation (R2) for each model (Table 6). The evaluation of the release kinetic models of maraviroc has shown that maraviroc release followed a zero-order kinetic model (highest coefficient of correlation value of R2 =0.9409) and a super case II drug transport mechanism (release exponent, n = 1.1329 obtained from the Korsmeyer–peppas model). Tenofovir release followed a Higuchi model release kinetics and a non-Fickian drug transport mechanism, demonstrated by highest values of coefficient of correlation R2 = 0.9034 and release component n = 0.5973 in the Korsmeyer–peppas model.
 |
Table 6 Mathematical Model for Maraviroc and Tenofovir Release Kinetic Data |
Compatibility Studies
Characteristic major peaks which are indicative of tenofovir and maraviroc were observed in both spectra for the drugs and polymer. The infrared spectrum of pure tenofovir compared with tenofovir microsphere as shown in Figure 3A and B, displayed a manifestation of the major peaks attributed to tenofovir, also in the vibrational spectrum of the tenofovir microsphere with no major shift in peaks which is an indication of no significant interactions.
 |
Figure 3 Continued. |
 |
Figure 3 (A) Pure Tenofovir Infrared spectrum. (B) Tenofovir and excipient Infrared spectrum. (C) Pure Maraviroc Infrared spectrum. (D) Maraviroc and excipient infrared spectrum. |
Characteristic major peaks which are indicative of tenofovir that were observed in both spectra are the C=O, carbonyl stretching (group frequency 1690–1675 cm−1), the C=C˗C aromatic ring (group frequency 1510–1450 cm−1), the N-H stretching amide group/normal polymeric OH stretch (group frequency 3400–3380 cm−1), and the vinyl C-H bend (group frequency 915–890 cm−1). In Figure 3C and D the vibration spectrum of pure maraviroc and maraviroc microspheres has shown peaks characteristic of maraviroc such as the C-F stretch of aliphatic fluoro compounds (group frequency 1150–1000 cm−1), the C=O stretch carboxylate salt (group frequency 1610–1550 cm−1), the N-H stretch aromatic nitro compounds (group frequency 1555–1485 cm−1) and the amide functional group frequency 1680–1630 cm−1.21
Lactic Acid Bacteria Species Identification
The DNA samples showed amplification for the band sharing frequency (BSF) primers producing an amplification size of 526bp by all lactic acid bacteria as shown in Figure 4. The lactobacillus bacteria identified via the online BLAST search with corresponding percentage identity and GenBank accession numbers were Lactobacillus fermentum (98.85% identity, SUB7940628 Seq_29B_BSF-8_ZTJ MT904660), Lactobacillus fermentum (98.66% identity, SUB7940628 Seq_30B_BSF-8_ZTJ MT904661) and Enterococcus Faecalis (99.4% identity, SUB7940628 Seq_28B_BSF-8_ZTJ MT904659).
 |
Figure 4 Agarose gel electropherogram for PCR detection of Lactic acid bacteria, M = 100bp DNA ladder, + = positive control (Lactobacillus fermentum). |
Viability of Lactic Acid Bacteria
L. fermentum and E. faecalis had an average viability of 93.9% and 89.7%, respectively, after a 48-h exposure to the microsphere suspension with a reduced viability of 11.2% and 11.5% observed for the positive control. The mean absorbance values for the negative control were 1.16 ± 0.17 and 1.26 ± 0.22. The test agents had absorbance values that were approximately the same (1.09 ± 0.25 and 1.13 ± 0.19) as the negative control. The positive control group had the least values of 0.13 ± 0.02 and 0.13 ± 0.01 (Table 7).
 |
Table 7 Independent Sample t-test Comparing the Means of Absorbance of the Test Agent and Controls to Test for Significance |
Assuming a significance level (α) of 0.05 and at 95% confidence interval, test for significance has shown p values of 0.706 and 0.498 for the test and negative control with values 0.002 and 0.001 obtained for the test and the positive control, respectively.
Efficacy Testing
Results from the in vitro concentration–response curve of the optimal F4 and G4 microspheres showed an initial threshold dose at a concentration of 0.001g/mL. This indicates the onset of action of a decline in HIV infectivity after which there was a progressive decline until at a concentration of 1 µg/mL. There was a contrasting difference between the response of the negative control compared to the microspheres (Figure 5).
 |
Figure 5 HIV infectivity dose–response curves for optimal maraviroc and tenofovir microspheres and nonoxynol gel incubated with HIV-1 indicator TZM-bl cells at different concentrations (n = 5 ± SD). |
Discussion
The experimental design employed in this study has aided in the screening and identification of the dominant relevant factors and interaction terms which are significant in the model. Numerical optimization was conducted with the goal of identifying the best levels of the identified dominant factors that will produce optimal responses.
The interaction factor of the combination of polymer and crosslinking agent concentration was the most important factor that influenced the particle size. This implies that high concentrations of chitosan and tripolyphosphate will lead to the formation of microspheres with larger particle size. The goal with regards to particle size is to achieve the minimal particle size possible, although the size of microspheres ranges from 1–1000 µm.
Curing time was seen to influence particle size in the pareto chart. Extending the curing time allows for extensive crosslinking and likely increase in particle size. The possibility of hydrophilic drugs like tenofovir washing off in the aqueous tripolyphosphate solution is pertinent and should be considered thus curing time is best maintained at low levels.16
The concentration of chitosan has been found to be a very important factor that influences encapsulation efficiency and mucoadhesion from the pareto chart. High concentrations of chitosan allow for maximum crosslinking with available tripolyphosphate; it increases the viscosity of the dispersion and expands the binding sites for better crosslinking with a wider surface area for improved drug encapsulation, with a resultant effect of maximum encapsulation. Interestingly, chitosan concentration is the sole factor that controlled mucoadhesion as shown in the pareto chart. Chitosan is a bioadhesive polymer and it confers the bioadhesive property to a microsphere.22 In view of this, the concentration of chitosan should be maintained at its highest. However, the concentration of the tripolyphosphate could be minimized to reduce particle size.
The predicted values for the optimal microsphere from the model showed the proximity between the optimal and predicted values of two sets of conditions after numerical optimization. The two conditions can be implemented; however, the condition with the lower particle size, higher percentage mucoadhesion values, good encapsulation efficiency which had close values to formulation F4 and G4 was selected as the optimized condition.
The choice of method employed in microsphere preparation determines to a great extent the particle size range and percentage yield of the particles. Ideally, microspheres are of size range 1–1000 µm; however, lower particle sizes are observed with spray drying method compared to ionic gelation technique.6
Zhang et al16 formulated microspheres using spray drying method and reported an optimal tenofovir microsphere particle size and percentage yield of 4.73 µm and 68.9% compared to ours which was 456.18 µm and 11.3%, respectively. The higher percentage yield observed with spray drying could be attributed to the use of less solvents coupled with an atomized drying process compared to ionic gelation technique.
The release of tenofovir from the microsphere started within 1 h and was sustained for 72 h in majority of the formulations. In comparison to a maraviroc which is moderately lipophilic, the release commenced after 12 h and was sustained for 72 h. The difference in their hydrophilicity might account for this difference in release pattern from the microsphere. Similarly, Dobard et al reported an observed slower release of maraviroc compared to tenofovir in their studies of a rectal gel containing maraviroc and tenofovir. They attributed this slower release to the ionization pH of maraviroc at 7.0 that affects tissue permeability. Irrespective of the difference in rate of release, maraviroc is reported to be several folds more potent compared to tenofovir.5
The high percentage mucoadhesion (90% and 93.3%) observed with formulations F4 and G4, respectively, portrays the potentials of a formulation that will overcome the challenges of short residence time in the vagina and coital dependent administration associated with previous conventional microbicides. The ability of the microspheres to adhere to the vaginal mucosa will allow for a longer duration of action such that the dosing and administration will not be tailored to the time of intercourse (before or after) which was a huge set back that discouraged adherence to the 1% tenofovir gel that employed the BAT24 (a dose 12 h before sex and the second dose 12 h after sex and no more than 2 doses in 24 h) dosing strategy.23
There was no significant interaction observed between the drugs and the polymers. The major peaks identified with the pure drugs were manifested in the spectrum of the drug/polymer with no major shift in peaks.
The pre-exposure prophylactic ability of the combined optimal tenofovir and maraviroc microspheres was evaluated via an in vitro concentration-response procedure using nonoxynol-9 as the control. Nonoxynol-9 is a spermicidal agent which was one of the early nonspecific antiviral agents that were tested as microbicides but failed in clinical trials.24
The HIV infectivity of the combination of F4 and G4 microspheres compared with nonoxynol-9 showed a marked difference in the HIV infectivity rate against the CCR5 tropic HIV-1 BaL strain. The continuous decline in HIV infectivity of the microspheres indicates adequate release of the drugs and anti-HIV activity.
The use of some antimicrobial medications can cause a preponderance of pathogenic bacteria that results in a condition in women termed bacterial vaginosis.25
Several studies have used the strain Lactobacillus crispatus to assess the effect of microbicides on vaginal lactic acid bacteria; however, this is not the only strain present in the vaginal environment in women. Some women have other strains of lactobacillus as the constituent of their vagina microbiota in addition to other lactic acid-producing organisms such as E. Faecalis.
Studies have reported differences in strains of Lactobacillus resident in women across races with black Americans having lesser probability to have L. crispatus compared to Caucasians and Asians.26 Studies in northeast India reported L. mucosae and E. faecalis as the dominant species in their cohort. Ethnicity and genetic variation have been found to influence vaginal microbiota composition.27
In view of this variability in vaginal microbiome composition, this study has explored other lactic acid bacteria isolated from the women in this study.
The assay of a 1 mg/mL suspension of the microsphere on the strains L. fermentum and E. faecalis showed a viability that confirmed the ability to thrive thus, asserting its safety for use as a microbicide.
Conclusion
The combination of maraviroc and tenofovir microspheres which is not available as oral dosage forms has shown efficacy against the HIV-1BaL virus strain. The microspheres intended for use in the formulation of a vaginal microbicide had good mucoadhesion which is a potential for prolonged residence time in the vagina. The antiretroviral drugs were adequately released from the carrier polymer and did not significantly disrupt the growth of the lactic acid bacteria which constitute valuable bacteria microflora of the vagina thus can be utilized for further in vivo studies.
Ethical Approval
Ethical approval for the study was obtained from the Institutional Review Board of the Nigerian Institute of Medical Research with Project No:1RB/18/064.
Acknowledgments
This project was supported by the Fogarty International Centre of the National Institutes of Health under Award Number D43TW010934. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Baeten JM, Hendrix CW, Hillier SL. Topical microbicides in HIV prevention: state of the promise. Annu Rev Med. 2020;71(1):361–377. doi:10.1146/annurev-med-090518-093731
2. Naswa S, Marfatia Y, Prasad TL. Microbicides and HIV: a review and an update. Indian J Sex Transm Dis. 2012;33(2):81–90. doi:10.4103/0253-7184.102098
3. Alexandre KB, Mufhandu HT, London GM, Chakauya E, Khati M. Progress and perspectives on HIV-1 microbicide development. Virology. 2016;497:69–80. doi:10.1016/j.virol.2016.07.004
4. Date AA, Shibata A, Goede M, et al. Development and evaluation of a thermosensitive vaginal gel containing raltegravir+efavirenz loaded nanoparticles for HIV prophylaxis. Antivir Res. 2012;96(3):430–436. doi:10.1016/j.antiviral.2012.09.015
5. Dobard CW, Taylor S, Sharma PL, et al. Protection against rectal chimeric simian/human immunodeficiency virus transmission in macaques by rectal-specific gel formulations of maraviroc and tenofovir. J Infect Dis. 2015;212(12):1988–1995. doi:10.1093/infdis/jiv334
6. Haneesha SK, Venkata RM, Rao NR. Microspheres for controlled release drug delivery: an overview on preparation, methods and characterizations. Int J Biol Pharm Allied Sci. 2020;9(3):627–637. doi:10.31032/ijbpas/2020/9.4.4995
7. Virmani T, Gupta J. Pharmaceutical application of microspheres: an approach for the treatment of various diseases. Int J Pharm Sci Res. 2017;8(8):3253–3260. doi:10.13040/ijpsr.0975-8232.8(8).3252-60
8. Alioua S, Abdi A, Fhoula I, Bringel F, Boudabous A, Ouzari IH. Diversity of vaginal lactic acid bacterial microbiota in 15 algerian pregnant women with and without bacterial vaginosis by using culture independent method. J Clin Diagn Res. 2016;10(9):DC23–DC27. doi:10.7860/jcdr/2016/21621.8546
9. Mokoena MP. Lactic acid bacteria and their bacteriocins: classification, biosynthesis and applications against uropathogens: a mini-Review. Molecules. 2017;22(8):1255. doi:10.3390/molecules22081255
10. Hatti-Kaul R, Chen L, Dishisha T, Enshasy HE. Lactic. acid bacteria: from starter cultures to producers of chemicals. FEMS Microbiol Lett. 2018;365(20). doi:10.1093/femsle/fny213
11. Nami Y, Haghshenas B, Khosroushahi AY. Molecular identification and probiotic potential characterization of lactic acid bacteria isolated from human vaginal microbiota. Adv Pharm Bull. 2018;8(4):83–95. doi:10.15171/apb.2018.077
12. Khan A, Thakur R. Formulation and evaluation of mucoadhesive microspheres of tenofovir disoproxil fumarate for intravaginal use. Curr Drug Deliv. 2014;11(1):112–122. doi:10.2174/156720181000131028120709
13. Velmurugan S, Ali MA. Formulation and evaluation of Maraviroc mucoadhesive microspheres by Ionotropic gelation method. Int J Pharm Pharm Sci. 2013;5:294–302.
14. Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59(2):91–95. doi:10.1016/s0010-7824(99)00010-4
15. Owen DH. A Review of the physical and chemical properties of human semen and the formulation of a semen simulant. J Androl. 2005;26(4):459–469. doi:10.2164/jandrol.04104
16. Zhang T, Zhang C, Agrahari V, Murowchick JB, Oyler NA, Youan BC. Spray drying tenofovir loaded mucoadhesive and pH-sensitive microspheres intended for HIV prevention. Antiviral Res. 2013;97(3):334–346. doi:10.1016/j.antiviral.2012.12.019
17. Agrahari V, Zhang C, Zhang T, et al. Hyaluronidase-sensitive nanoparticle templates for triggered release of HIV/AIDS microbicide in vitro. AAPS J. 2013;16(2):181–193. doi:10.1208/s12248-013-9546-7
18. Gouda R, Baishya H, Qing Z. Application of mathematical models in drug release kinetics of carbidopa and levodopa ER tablets. Drug Dev Res. 2017;6:171. doi:10.4172/2329-6631.1000171
19. Ilomuanya MO, Elesho RF, Amenaghawon AN, Adetuyi AO, Velusamy V, Akanmu AS. Development of trigger sensitive hyaluronic acid/palm oil-based organogel for in vitro release of HIV/AIDS microbicides using artificial neural networks. Future J Pharm Sci. 2020;6(1):1. doi:10.1186/s43094-019-0015-8
20. Beer BE, Doncel GF, Krebs FC, et al. In-vitro preclinical testing of nonoxynol-9 as potential anti-human immunodeficiency virus microbicide: a retrospective analysis of results from five laboratories. Antimicrob Agents Chemother. 2006;50(2):713–723. doi:10.1128/AAC.50.2.713-723.200.6
21. Coates J. Interpretation of infrared spectra, a practical approach. Encycl Anal Chem. 2006;10815–10837. doi:10.1002/9780470.027318.a5606
22. Ways TM, Lau WM, Khutoryanskiy VV. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers. 2018;10(3):267. doi:10.3390/polym10030267
23. AbdoolKarim Q, AbdoolKarim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–1174. doi:10.1126/science.1193748
24. Notario-Pérez F, Ruiz-Caro R, Veiga M. Historical development of vaginal microbicides to prevent sexual transmission of HIV in women: from past failures to future hopes. Drug Des Devel Ther. 2017;11:1767–1787. doi:10.2147/dddt.s133170
25. Mayer BT, Srinivasan S, Fiedler TL, Marrazzo JM, Fredricks DN, Schiffer JT. Rapid and profound shifts in the vaginal microbiota following antibiotic treatment for bacterial vaginosis. J Infect Dis. 2015;212(5):793–802. doi:10.1093/infdis/jiv079
26. Gautam R, Borgdorff H, Jespers V, et al. Correlates of the molecular vaginal microbiota composition of African women. BMC Infect Dis. 2015;15(1):1–14. doi:10.1186/s12879-015-0831-1
27. Purkayastha SD, Bhattacharya MK, Prasad HK, et al. Contrasting diversity of vaginal lactobacilli among the females of Northeast India. BMC Microbiol. 2019;19(1):1–10. doi:10.1186/s12866-019-1568-6
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.