Back to Journals » Journal of Pain Research » Volume 15
MRI for in vivo Analysis of Ablation Zones Formed by Cooled Radiofrequency Neurotomy to Treat Chronic Joint Pain Across Multiple Axial Spine Sites
Received 5 October 2021
Accepted for publication 18 January 2022
Published 9 February 2022 Volume 2022:15 Pages 423—430
DOI https://doi.org/10.2147/JPR.S342795
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Dawood Sayed
Mehul J Desai,1,2 Yair Safriel3,4
1International Spine, Pain & Performance Center, Washington, DC, USA; 2Department of Anesthesiology and Critical Care, George Washington University, School of Medicine & Health Sciences, Washington, DC, USA; 3Pharmascan, Wilmington, DE, USA; 4University of South Florida Affiliated Programs, Clearwater, FL, USA
Correspondence: Mehul J Desai, International Spine, Pain & Performance Center, Washington, DC, 20006, USA, Tel +1 202 808 8295, Email [email protected]
Purpose: Radiofrequency (RF) ablation is the targeted damage of neural tissues to disrupt pain transmission in sensory nerves using thermal energy generated in situ by an RF probe. The present study aims to evaluate the utility of magnetic resonance imaging (MRI) for in vivo quantitative assessment of ablation zones in human subjects following cooled radiofrequency neurotomy for chronic pain at spinal facet or sacroiliac joints. Ablation zone size and shape have been shown in animal models to be influenced by size and type of RF probe – with cooled RF probes typically forming larger, more spherical ablation zones. To date, MRI of RF ablation zones in humans has been limited to two single retrospective case reports.
Patients and Methods: A prospective, open-label pilot study of MRI for evaluation of cooled radiofrequency ablation zones following standard of care procedures in adult outpatients was conducted. Adult subjects (n=13) received monopolar cooled RF (CRF) ablation (COOLIEF™, Avanos Medical) of sensory nerves at spinal facet or sacroiliac joints, followed by an MRI 2– 7 days after the procedure. MRI data were acquired using both Short Tau Inversion Recovery (STIR) and contrast-enhanced T1-weighted (T1C) protocols. T1C MRI was used to calculate 3-dimensional ellipsoid ablation zone volumes (V), where well-defined regions of signal hyperintensity were used to identify three orthogonal diameters (T, D, L) and apply the formula V=π/6×T×D×L.
Results: Among 13 patients, 96 CRF ablation zones were created at 4 different anatomic sites (sacroiliac, lumbar, thoracic and cervical). CRF ablation zone morphology varied by anatomical location and structural features of surrounding tissues. In some cases, proximity to bone and striations of surrounding musculature obscured ablation zone borders. The volumes of 75 of the 96 ablation zones were measurable from MRI, with values (mean±SD) ranging from 0.4679 (± 0.29) cm3 to 2.735 (± 2.62) cm3 for the cervical and thoracic sites, respectively.
Conclusion: In vivo T1C MRI analysis of cooled RF ablation zones at spinal facet and sacroiliac joints demonstrated variable effects of local tissues on ablation zone morphology. Placement of the CRFA probe very close to bone alters the ablation zone in a negative way, causing non-spherical and incomplete lesioning. These new data may serve to inform practicing physicians about optimal cooled RF probe placement in clinical procedures.
Keywords: RF lesion size, lesion geometry, magnetic resonance imaging, facet joints, sacroiliac joints, RF probe placement
Introduction
Radiofrequency ablation (RFA) is well established1 as a treatment modality across multiple clinical indications, including a range of painful conditions of the spine.2 Monopolar RFA generates thermal energy in situ by delivering high-frequency alternating current through an insulated needle-sized radiofrequency probe, causing ionic agitation, ambient heating through friction and cell death through coagulative necrosis.3,4 Because the RFA probe may be inserted into the body percutaneously, the procedure is relatively non-invasive.
Cooled radiofrequency ablation (CRFA) probes differ from standard RFA probes in that water circulated through the CRFA probe tip draws heat away from the tissue–tip interface to prevent tissue charring or desiccation, which have been shown to greatly increase resistance to further energy flow.5 CRFA probes deliver more energy than standard RF probes and form larger, more spherical ablation zones. It is believed that larger lesions can increase the chances of interventional success, as larger lesions improve the odds of successful capture of the target nerve within the lesion zone. To date, however, most ablation zone measurements of both cooled and standard RFA probes have been made using ex vivo chicken breast models.6 Chicken breast is non-perfused and relatively homogeneous tissue, so it is not well suited as a surrogate for understanding in vivo response. In animal and ex vivo models, the size and type of RFA probe tip used influence the size and shape of the ablation zone formed, characteristics which are believed to be critical in determining procedural success.7
With its unique ability to safely resolve normal soft tissues as well as pathological changes, magnetic resonance imaging (MRI) has been used to examine in vivo effects of RFA lesioning in the cardiac, CNS and tumor ablation spaces in both humans and animal models.8–11 MRI has been used to characterize in vivo effects of pain management RFA neurotomy procedures in animals, including rodents and pigs prior to sacrifice and verification through histological analysis.12,13 The need for human clinical studies using MRI to characterize in vivo RF lesions for pain management was emphasized in a 2018 report on porcine RF lesion characterization.12
Some MRI studies of CRFA ablation zones in vivo in humans have been conducted, including two retrospective case reports, which inspired the present work.14,15 To our knowledge, and based upon literature searches (eg, PubMed with search filters such as “cooled AND radiofrequency AND (ablation OR neurotomy) AND MRI”), the present study is the first prospective quantitative analysis of CRFA ablation zones using MRI. Broader literature searches to include standard RF neurotomy also fail to find prospective clinical studies using MRI to quantify lesion size.
Materials and Methods
The goal of this study was to confirm the utility of MRI technology to identify, obtain and categorize CRFA ablation zones in vivo under normal clinical conditions. Study approval was obtained by the Sterling Institutional Review Board (Atlanta, GA). All patients were properly consented prior to initiating screening activities. This prospective, single-center, pilot study was conducted in accordance with the Declaration of Helsinki. The study enrolled 13 patients over the age 21 who were diagnosed with chronic joint pain (≥3 months), and were eligible to receive CRFA (based on dual, comparative diagnostic nerve blocks) as part of their standard of care within a single practice.
The treatment algorithm for joint pain often begins with physical therapy and oral-non-steroidal anti-inflammatory drugs. If pain persists, the next steps may involve minimally invasive treatments, such as CFRA. The ideal CFRA patient is anyone ready to move beyond the first line treatments based upon the physician’s judgments and patient preference. Because the goal of this study was to characterize CRFA lesions, only patients selected for CRFA treatment were considered.
Areas originally targeted for treatment included: cervical, thoracic and lumbar spinal facet joints; the sacroiliac joint (SIJ) region; the hip and the knee. Limited data collection in the hip and knee (3 subjects total) were ultimately excluded from analysis to focus on the axial spine. The study physician provided Standard of Care treatment for all subjects across two outpatient office visits: a screening visit and a CRFA procedure visit. CRFA in the cervical region was performed using a 2-mm exposed active tip, thoracic, a 5.5-mm active tip, while lumbar and sacral RF utilized 4-mm active tips. MRI was performed at an outpatient imaging center 2–7 days later using both STIR and contrast-enhanced (gadolinium) T1-weighted (T1C) protocols. MRI was performed within the first week post procedure based upon the anticipated time course for tissue response: it was hoped edema and inflammation would be resolved leaving CRFA ablation zones more clearly visible. The ablation zone was defined as the area of coagulative necrosis that is well marginated as defined in previous work.10
The methodology for calculating ablation zone size was informed by the significant precedent of similar work previously published. Imaging conventions developed to assess tumor size have influenced quantitative assessment of ablation zones.8 The current study employed a simple diameter-based ablation zone size and volume calculation assuming a 3-dimensional ellipsoid shape (V=π/6×T×D×L or alternatively, V=π/6×Dcc×Dap×Dl) and involving simple measurement of three orthogonal diameters that can be performed on standard radiology images.16 Cranial–caudal diameter Dcc and anterior–posterior diameter Dap are from the largest slice in the sagittal plane, and the lateral diameter Dl is from largest slice in the axial plane.
CRFA ablation zone volumes were calculated from MR images by an independent board-certified radiologist, subspecialized in neuroradiology.
Results
A total of 13 adult subjects (10 females: 3 males) underwent CRFA for chronic joint pain at spinal facet or sacroiliac joints for a total of 96 ablation zones, as summarized in Table 1. MRI data acquired using the STIR protocol were generally indicative of the edema present in and around the ablation zone site, whereas T1C images were judged to be more indicative of the inflammation/necrosis present at the ablation zone site. Thus, T1C images were judged to be most representative of true CRFA ablation zone size in the first week post procedure. Of all the T1C images acquired, 75 were analyzable, based on radiologist impressions of well-defined lesion area borders.
 |
Table 1 Summary of Cooled Radiofrequency Ablation Zone Measurements by T1C MRI |
T1C Ablation zone volumes were affected by CRFA probe size as well as anatomical location (Table 1). As expected, smaller probe tip sizes correlated with smaller ablation zone volumes. The smallest tip size of 2 mm was used on the cervical sites, yielding mean volume 0.4679 (±0.29) cm3, in contrast to 2.735 (±2.62) cm3 volume resulting from the largest tip size of 5.5 mm used on the thoracic sites. Interestingly, ablation zone volume in SIJ and lumbar regions were noticeably different from each other (0.6915 (±1.08) versus 1.685 (±2.51) cm3), despite using the same size probe tip (4 mm). The large standard deviations show that significant variations exist among lesions delivered to the same anatomic site with the same needle size. Image analysis was conducted to gain insight into this variation.
Ablation zone borders were affected by the impedance of surrounding tissues, including the surrounding musculature as well as adjacent bone structures. Figure 1 shows a well-defined CRFA ablation zone with the characteristic spheroidal shape. Striations in musculature appeared to influence the size and shape of areas of hyperintensity in T1C images (Figure 2).
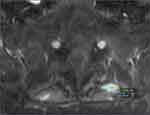 |
Figure 1 T1C image demonstrating well-defined ablation zone with measurements marked by the blue and green line segments. |
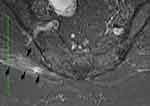 |
Figure 2 Sagittal T1C image from a SI joint ablation with increased signal spreading along the paraspinal sacral musculature. Arrows are placed around the border of the elongated lesion zone. |
Placement of the CRFA probe very close to bone alters the shape of the ablation zone which deviated from the expected rounded shape. This is notably demonstrated within Figure 3A and B, where very minor differences in probe placement within the same patient at the same treatment level resulted in drastic differences in ablation zone morphology on MRI.
Discussion
Previous ex vivo work has demonstrated the influence of probe size and probe type on ablation zone size and shape. Internally cooled probes create larger, more spherical lesions than standard probes in homogeneous tissues (ie, chicken breast). Additionally, lesion size increases expectedly with larger gauge and active tip sizes.7 When identifiable ablation zones were visible within this MRI dataset, this spherical shape remained and proved this concept is transferable from the ex vivo modeling. However, this work suggests that environmental factors (tissue composition, probe proximity to bone and tissue structural features) may play a more important role than previously appreciated.
The impact of tissue heterogeneity on ablation zone size can be explained by the conductivity of various tissues. Bone has one of the lowest electrical conductivities, followed by fat. Skeletal muscle has the highest electrical conductivity of tissues related to RF ablation.3 Tissues with higher conductivities have higher current flows, which results in greater induced temperature when subjected to radiofrequency energy. As such, ablation to tissues with higher conductivity will result in larger ablation zones than tissues with low conductivity.
Previous computational work has shown that ablation volumes in heterogeneous models vary more than that of homogeneous models.17 In this computational model, homogeneous domains were comprised of only muscle tissue, one heterogeneous domain consisted of muscle and nervous tissue, and another heterogeneous domain comprised bone, nerve and muscle tissues. Investigators uncovered that heterogeneity resulted in distorted electric field distribution, which significantly reduced ablation volume.
When looking at ablation zone volumes reported in this manuscript across anatomical locations, it is clear that tissue heterogeneity plays a critical role in influencing size and shape of ablation zone creation. Consider the mean ablation volumes in the SI joint and the lumbar facet joint, each made with the same size probe tip. The ablation volumes within SI joint are noticeably smaller. When looking into the tissue heterogeneity of the joints, the SI joint has more bone structure, tendons, ligaments, cartilage and other connective tissues,18 whereas the area surrounding the lumbar facet joint consists of more skeletal muscle. The differences in conductivity of these various tissues may help explain why the same cooled radiofrequency probe size can create different ablation zone volumes.
Proximity to bone also appears to impact the size and shape of ablation zones measured by MRI. Previous ex vivo modeling showed that RF probe placement directly against the bone resulted in the projection of the lesion outward from the bone and perpendicular to the needle axis in the vertical plane.19 It is suggested that the bone, with its higher electrical impedance, acts as an insulator that directs the current return path further outward into the surrounding soft tissue.
The results of our data mirror this phenomenon, in that ablation zone size and shape were directly impacted by close proximity (or placement against) bone. The probes in the previously described ex vivo model were placed directly against bone, resulting in the perpendicular distribution of RF energy,19 the probes within this study had a more varied placement, enabled by the distal projection of cooled radiofrequency probes. As such, deflection of RF energy could not be determined to be directly perpendicular to the probe/bone interface. Likely, because of the differences in distribution of RF energy of cooled probes, the deflection of RF energy was much more disperse. It is hypothesized that this dispersion of RF energy results in less thorough lesioning, as reflected by the different imaging characteristics of these ablation zones as viewed in the corresponding MRI images (Figure 3A and B). It is noted that the majority of ablation zones with irregular or atypical borders were seemingly impacted by the probe’s proximity to bone structure.
In addition to probe type, surrounding musculature and proximity to bone, there are yet still other factors that can affect ablation zone size. Previous studies have demonstrated the effects of pre-injectate solutions on in vivo lesion size.12 Injection of a hypertonic sodium chloride solution significantly increased lesion volume, as measured by both histological analysis and MRI analysis. This study also demonstrated that a 1 mL 1% lidocaine injection did not alter the overall size of a lesion (compared with no fluid injection), but the 1% lidocaine injection did result in a less symmetrical shape of lesion, suggesting that local anesthetic can influence lesion geometry.
While this is not a direct comparison with the work presented herein (eg, different animal model, different tissues, different probes), it does serve to highlight the impact of local anesthetic on lesion geometry. It is suggested that the in vivo MRI analysis of ablation zones is demonstrating a similar phenomenon, wherein tissue structural features enabled local anesthetic to infiltrate the striations of musculature. RF energy is believed to have been distributed preferentially through these striations and, due to the low conductivity of the infiltrating fluid, resulted in the formation of a more diffuse, non-concentrated ablation zone.
Clinically, these results providing compelling evidence for the role of impedance of tissues adjacent to the radiofrequency probe. Specifically, the heterogeneity of adjacent structures may have a significant and meaningful impact on lesion geometry, thereby influencing the placement of cooled RF probes. Based on radiological examination, ablation zones with less defined borders are hypothesized to be indicative of incomplete lesioning. These ablation zones lack the necrosis and coagulation that are believed to be associated with a successful lesioning and denervation. Proximity to bone appeared to be the biggest factor driving incomplete lesioning.
Current manufacturer-endorsed protocols for CRFA involve inserting the introducer into the relevant anatomical area until the practitioner finds the bone, utilizing it as a backstop. Upon retraction of the introducer, the cooled probe is inserted in its place. Given the differences in the lengths of the introducer and the probe, this should leave a 2 mm gap between the probe tip and the bone. However, it is possible that micro-adjustments made during either introduction of the probe or during the ablation procedure, can result in the probe eventually being directly on the bone. It is suggested that extra precautions be taken when conducting micro-adjustments to ensure that there is space between the probe tip and the bone to allow for more thorough and predictable lesioning.
Furthermore, these results likely provide the first tangible contributor to inconsistent results or failures of standard RFA following positive response to diagnostic blocks. Given the size limitations associated with standard RF probes, current recommendations/protocols for standard radiofrequency probes involve placing probes directly on bone structure within the SIJ and facet joints. As seen within these data, bone disperses the radiofrequency energy in such a manner as to prevent the creation of a concentrated ablation zone, which may impede ability to successfully ablate nervous tissue.
Probe designs and needle placement techniques have historically been guided by findings taken from chicken breast models. Based on the present human MRI findings, however, it would seem reasonable to revisit the current placement recommendations in the spine. A revisitation of probe placement and trajectory in genicular ablations has been recommended by the authors who first discovered the ablation zone in the knee, underscoring the ability of MRI imaging in assisting practitioners with procedural insights.15 Another case study made use of post-treatment MRI, not originally intended for ablation zone analysis, to correlate the large, spherical ablation zone with successful genicular pain reduction.14 Although serendipity led to some of these previous insights, the present study represents the proactive use of human MRI ablation zone characterization. It is aimed at informing future work to improve CRFA techniques for facet and sacroiliac joint pain treatment, and immediately shows that the use of the homogeneous chicken breast model should be laid to rest as a surrogate for expectations of use in vivo.
While the information provided within this study is novel, there is a relatively small sample size. Variance in MRI collection dates post CRFA (2–7 days) could also have altered measurements given differences in inflammatory response. Specimens collected at the start of the window would show a greater impact of the inflammatory response on the imaging findings (especially the STIR images) and measurements, which could skew the data toward larger numbers. It is also important that the ablation zone sizes do not formally measure lesion size, as lesion sizes are typically calculated through histological staining, a process not suited for clinical practice. This exploration was also limited to monopolar lesioning utilizing CRFA. This methodology should be repeated using various other products and techniques (ie, standard RF, tined probes, bipolar lesioning) and placement techniques to guide procedural optimization. Given the dramatic impact of tissues and proximity to bone, some current placement techniques may need to be revisited. Finally, as the goal of this study was to primarily understand whether ablation zones could even be visualized in vivo, no clinical follow up was collected on these patients, so no connection with associated outcomes could be made. Future studies should attempt to link clinical response to ablation zone formation and placement.
Conclusion
This is the first work of its kind to evaluate in-vivo human response to CRFA ablation in pain management conditions using MRI imaging and to describe a model that can be used for future exploration. Probe placement, proximity to bone, surrounding tissues and striations in musculature all impacted ablation zone size and shape. These contextual factors may need to be considered when placing RFA probes, regardless of technology. Given the multiple variables to consider when utilizing this treatment, it is truly amazing this procedure works as well as it does. While size varied of CRFA ablation zones across each anatomical location (and across different active tip sizes), CRFA ablation zones were consistent within each anatomic location.
Acknowledgments
Clinical trial approved by the Sterling Institutional Review Board, 6300 Powers Ferry Rd, Suite 600-351, Atlanta, GA 30339, USA. This work was funded by Avanos Medical, 5405 Windward Parkway Alpharetta, GA 30004, USA.
Disclosure
Dr Mehul J Desai reports research support to organization from Avanos, Abbott and SPR Therapeutics, owned stock options from SPR Therapeutics, Virdio and Smart Implant Systems, and also reports advisory board royalties from Camber IPT, outside the submitted work. Dr Yair Safriel is an employee of Pharmascan. The authors report no other conflicts of interest in this work.
References
1. Kirschner M. Zur elektrochirurgie.[Electrosurgery]. Langenbecks Arch Kiln Chir. 1931;167:761–768.
2. Maas ET, Ostelo RW, Niemisto L, et al. Radiofrequency denervation for chronic low back pain. Cochrane Database Syst Rev. 2015;10:CD008572.
3. Ball RD. The science of conventional and water-cooled monopolar lumbar radiofrequency rhizotomy: an electrical engineering point of view. Pain Physician. 2014;17(2):E175–E211. doi:10.36076/ppj.2014/17/E175
4. Finelli A, Rewcastle JC, Jewett MA. Cryotherapy and radiofrequency ablation: pathophysiologic basis and laboratory studies. Curr Opin Urol. 2003;13:187–191. doi:10.1097/00042307-200305000-00003
5. Kapural L, Deering JP. A technological overview of cooled radiofrequency ablation and its effectiveness in the management of chronic knee pain. Pain Manag. 2020;10(3):133–140. doi:10.2217/pmt-2019-0066
6. Vallejo R, Benyamin R, Tilley DM, et al. An ex vivo comparison of cooled-radiofrequency and bipolar-radiofrequency ablation zone size and the effect of injected fluids regional. Anesthes Pain Med. 2014;39:312–321.
7. Cedeno D, Vallejo A, Kelley C, Tilley D, Kumar N. Comparisons of ablation zone volumes and shapes produced by a radiofrequency system with a cooled, a protruding, or a monopolar probe. Pain Physician. 2017;20(6):915–922. doi:10.36076/ppj.20.5.E915
8. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi:10.1093/jnci/92.3.205
9. Vedantam A, Hou P, Chi TL, et al. Postoperative MRI evaluation of a radiofrequency cordotomy ablation zone for intractable cancer pain. AJNR Am J Neuroradiol. 2017;38(4):835–839. doi:10.3174/ajnr.A5100
10. Sainani NI, Gervais DA, Mueller PR, Arellano RS. Imaging after percutaneous radiofrequency ablation of hepatic tumors: part 1, normal findings. AJR Am J Roentgenol. 2013;200(1):184–193. doi:10.2214/AJR.12.8478
11. Ghafoori E, Kholmovski EG, Thomas S, et al. Characterization of gadolinium contrast enhancement of radiofrequency ablation zones in predicting edema and chronic ablation zone size. Circ Arrhythm Electrophysiol. 2017;10(11). doi:10.1161/CIRCEP.117.005599
12. Provenzano D, Cosman E, Wilsey J. Hypertonic sodium chloride preinjectate increases in vivo radiofrequency ablation size histological and magnetic resonance imaging findings. Reg Anesth Pain Med. 2018;43:776–778. doi:10.1097/AAP.0000000000000814
13. Zachariah C, Mayeux J, Alas G, et al. Physiological and functional responses of water-cooled versus traditional radiofrequency ablation of peripheral nerves in rats. Reg Anesth Pain Med. 2020;45(10):792–798. doi:10.1136/rapm-2020-101361
14. Farrell ME, Gutierrez G, Desai MJ. Demonstration of ablation zones produced by cooled radiofrequency neurotomy for chronic osteoarthritic knee pain: a case report. Pm&r. 2017;9(3):314–317. doi:10.1016/j.pmrj.2016.09.001
15. Conger A, McCormick ZL, Henrie AM. Pes anserine tendon injury resulting from cooled radiofrequency ablation of the inferior medial genicular nerve. PM R. 2019;Nov;11(11):1244–1247. doi:10.1002/pmrj.12155
16. Kim JK, Kim W. Method of tumor volume evaluation using magnetic resonance imaging for outcome prediction in cervical cancer treated with concurrent chemotherapy and radiotherapy. Radiat Oncol J. 2012;30(2):70–77. doi:10.3857/roj.2012.30.2.70
17. Singh S, Melnik R. Domain heterogeneity in radiofrequency therapies for pain relief: a computational study with coupled models. Bioengineering. 2020;7(2):35. doi:10.3390/bioengineering7020035
18. Foley BS, Buschbacher RM. Sacroiliac joint pain: anatomy, biomechanics, diagnosis, and treatment. Am J Phys Med Rehabil. 2006;85:997–1006. doi:10.1097/01.phm.0000247633.68694.c1
19. Eckmann MS, Martinez MA, Lindauer S, Khan A, Ramamurthy S. Radiofrequency ablation near the bone-muscle interface alters soft tissue ablation zone dimensions. Reg Anesth Pain Med. 2015;40:270–275. doi:10.1097/AAP.0000000000000221
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.