Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
MRI Evaluation of the Relationship Between Abnormalities in Vision-Related Brain Networks and Quality of Life in Patients with Migraine without Aura
Authors Cui W , Zhang J, Xu F, Zhi H, Li H, Li B, Zhang S, Peng W, Wu H
Received 28 September 2021
Accepted for publication 22 November 2021
Published 8 December 2021 Volume 2021:17 Pages 3569—3579
DOI https://doi.org/10.2147/NDT.S341667
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Wenqiang Cui,1,* Jiwei Zhang,2,* Fei Xu,3 Hongwei Zhi,1 Haitao Li,1 Baopeng Li,4 Sishuo Zhang,1 Wei Peng,1 Hongyun Wu1
1Department of Neurology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, Shandong, People’s Republic of China; 2College of Acumox and Tuina, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, People’s Republic of China; 3Department of Geriatric Medicine, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, Shandong, People’s Republic of China; 4Department of Medical Imaging, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongyun Wu; Wei Peng
Affiliated Hospital of Shandong University of Traditional Chinese Medicine, 16769 Jingshi Road, Jinan, Shandong, People’s Republic of China
Tel +86 13853148590
; +86 13953187058
Email [email protected]; [email protected]
Objective: To evaluate whether migraine without aura (MwoA) can be partly attributed to abnormalities of vision-related brain networks (VBN) and whether these specific regional abnormalities affect the patients’ quality of life (QoL).
Methods: A total of 40 participants, including 20 MwoA patients and 20 healthy control volunteers, were enrolled. There were no significant differences in sex, age, educational qualifications and dominant hand between the two groups. Headache intensity and QoL were assessed by the Pain Number Evaluation Scale (NRS) and the Migraine-Specific Quality of Life Questionnaire (MSQ 2.1), respectively. Resting state functional magnetic resonance imaging (rs-fMRI) and independent component analysis (ICA) were performed to determine and evaluate the VBN.
Results: Three components were identified as consistent with the VBN in the template and recorded as N1, N2 and N3, respectively. The functional activity of the left primary visual cortex (N1), left culmen of cerebellum (N1), left lingual gyrus (N2), superior frontal gyrus (N2) and left posterior lateral prefrontal cortex (N3) in the MwoA group enhanced compared with the healthy control group. However, the functional activity of right middle occipital gyrus, left fusiform gyrus, right lingual gyrus, and right primary motor cortex in the N3 network weakened. Pearson correlation analysis showed that decline of attention to work and life (MSQ5) was positively associated with the functional activity of left primary visual cortex and left lingual gyrus. Canceling from work and daily life (MSQ8) was inversely associated with the functional activity of right primary motor cortex. The burden of feeling like others (MSQ13) and the overall decrease in QoL were both positively associated with the functional activity of right lingual gyrus.
Conclusion: MwoA patients showed abnormal VBN function, which was moderately correlated with decreased QoL. This study provides evidence for the precise prevention and treatment of migraine by neural regulation.
Keywords: migraine, rs-fMRI, neural regulation, vision-related brain networks, quality of life
Introduction
Migraine is a common neurobiological headache caused by changes in neuronal excitability of the central nervous system, characterized by hypersensitivity to light and sound.1,2 A study of 162,576 people aged 12 years and older found that the annual prevalence of migraine in the United States was 11.7%, 17.1% for women and 5.6% for men.3 The annual prevalence of migraine in Japan is 8.4%, of which 5.8% corresponds to migraine without aura (MwoA) and 2.6% to migraine with aura (MA).4 Migraine is one of the most disabling diseases, and seriously affects the QoL of its sufferers, affecting their ability to work, social activities and family life. Moreover, it also interferes with the ability to function in school and the academic performance of students with migraine.5
Visual function is one of the focuses of migraine research. Patients with MwoA are all suffering from visual impairment, and that impacts the QoL of patients even more than the headache itself.6,7 The visual networks are known to be involved in migraine pathophysiology. Many neuroimaging studies have reported visual network abnormalities in patients with MwoA. One study indicated that visual cortical excitability was increased in patients with MwoA by using proton magnetic resonance spectroscopy and diffusion weighted spectroscopy.8 One study about visual motion processing network showed that the thickness of area V3A in the transverse occipital sulcus was abnormal in MwoA patients.9 With the help of advanced imaging techniques, we have gained a deeper understanding of the visual network-related pathological mechanisms of migraine. However, this mechanism of MwoA is still incomplete and needs to be improved by more imaging studies. To enrich the visual network-related mechanisms of migraine, we further analyzed the visual network abnormalities in MwoA using fMRI. Further, it is unclear whether the visual network abnormalities have an effect on the QoL of MwoA patients. To determine the relationship, we conducted a migraine-specific quality of life questionnaire and an analysis of the association between QoL and abnormal visual networks.
The purpose of this study was to evaluate whether MwoA can be partly attributed to abnormalities in vision-related brain networks (VBN) and whether these specific regional abnormalities affect patients’ quality of life (QoL). We hope that this study can help improve MwoA patients’ QoL by identifying the potential specific brain networks that affect MwoA patients’ QoL and by guiding targeted clinical interventions.
Methods
To test our hypothesis, we recruited 20 individuals with MwoA and 20 healthy volunteers for rs-fMRI imaging. The rs-fMRI results were analyzed by independent component analysis (ICA, a data-driven analysis method that decomposes a signal into multiple independent components without prior model assumptions) and Regional Homogeneity (ReHo, an analysis method based on region of interest [ROI]) to explore the dysfunction of vision-related brain networks in MwoA. Meanwhile, Pearson’s correlation was used to analyze the effect of vision-related brain networks’ dysfunction on the QoL of patients.
Study Population and Protocol
Participants were enrolled in the study from December 2020 to March 2021. The study population consisted of graduate students aged 25 to 30 years diagnosed with MwoA according to the diagnostic criteria for migraine without aura of the International Classification of Headache Disorders, 3rd Edition (ICHD-3. Code 1.1, 2013).10 Patients with MwoA were assessed by Professor Wu and Peng who are neurologists focusing on diagnosing and treating migraine. All subjects were right-handed and had a disease duration of more than 6 months and with migraine attacks in the last month. MwoA patients enrolled were allowed to use pain killers for prevention or treatment when recommended by physicians and when necessary (NRS > 7). Healthy controls were recruited who had no headache attack in the past year and had not been ill and taken no drugs for a month and whose family members did not suffer from migraine or other headaches. In the MwoA group, resting-state fMRI was taken in the interictal stage, at least 3 days after a migraine attack. Participants were forbidden to take any drugs, alcohol, nicotine, caffeine, and other chemically similar substances for at least 3 days before MRI examination.
Exclusion criteria for all participants included: 1) use of opioid analgesics, antipsychotics or ergotamine; 2) alcoholism or other drug abuse; 3) chronic systemic diseases and illness affecting central nervous system function, such as heart, brain, liver, kidney and hematopoietic system primary diseases and mental diseases; and 4) pregnancy or lactation. None of the participants had contraindications for fMRI. All subjects provided written informed consent, and the study was approved by the Ethics Committee of the Affiliated Hospital of Shandong University of Chinese Medicine (approval number 2014-028).
Data Collection
NRS Score
The NRS score with a single 11-point (0 to 10) scale was used to assess the participant’s pain intensity. A score of 0 marked “no pain”, whereas a score of 10 indicated “the worst pain imaginable”. NRS-based assessments have been shown to provide valid and reliable assessments in patients with migraine.11 At the time of assessment, patients were asked to report their perceived pain level of the last attack by marking the NRS score.
MSQ Score
The QoL of participants was measured by using the MSQ version 2.1 which was translated into traditional Chinese.12 The MSQ questionnaire includes 14 items and 3 domains: 7 items, Role Function-Restrictive (RFR), measure the functional impact of migraine through limitations on daily social and work activities; 4 items, Role Function-Preventive (RFP), measure the impact of migraine through prevention of daily work and social activities; 3 items, Emotional Function (EF), assess the emotional impact of migraine. The sum of relevant item scores is the raw score for each domain, and the sum of all item scores is the raw total score. These scores are rescaled from 0–100, with a higher score indicating better QoL.
rs-fMRI
Blood oxygen level dependent RS-fMRI data was collected by Philips Achieva 3.0T MRI scanner and 12-channel matrix head coil. Subjects remained stationary, eyes closed, and breathing evenly throughout the scan. Structural images were acquired with using a high-resolution T1-weighted 3-dimensional magnetization-prepared acquisition gradient-echo pulse sequence, The parameters were as follows: Repetition Time (TR) = 8.0 ms, Echo Time (TE) = 3.8 ms, Thickness = 1 mm, Gap = 0 mm, Flip angle = 12°, Matrix = 512 × 512, and Field of View (FOV) = 250 mm × 250 mm. The structural sequence took 5 min and 30 s. Functional imaging was performed using GRE-EPI sequence, scanning parameters: Repetition Time (TR) = 3000 ms, Echo Time (TE) = 35 ms, Matrix = 128 × 128, Field of View (FOV) = 230 mm × 230 mm, Flip Angle = 90°, Thickness = 5mm, Gap = 0 mm, interlayer scanning. Functional imaging was captured by continuous scanning for 10 minutes. The scanning range included brain, cerebellum, and brain stem.
rs-fMRI Data Analysis
Data Preprocessing
After converting the DICOM format raw data obtained from MRI scan into NIFTI format commonly used for data analysis of functional brain images, the preprocessing was performed to eliminate noise, error and other factors that influence the results of ICA analysis. Based on the Matlab 2017a platform, dPABI V4.3 software was used to preprocess RS-fMRI data,13 including removing the first 10 time points of data, correcting time layer and head movements, spatial standardization, smoothing space, removing linear drift and filtering. The signals in the range of 0.01Hz~0.08Hz were selected, and the Gaussian kernel function of 4×4×4 mm3 with half height and full width was used to perform spatial smoothing of RS-fMRI images.
Determination of Visual Networks
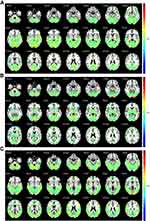
Based on the Matlab 2017a platform, ICA was performed on the preprocessed data of the two groups respectively, by using the GIFT software (Group ICA/IVA of fMRI Toolbox) V3.0b (https://trendscenter.org/software/). The number of components was set at 20, and the GIG-ICA algorithm was used to obtain the number of independent components respectively. Rand Init and Bootstrap were used to repeat the calculation 50 times.14 Based on the resting state brain network template produced by Smith et al15 with a large number of healthy subjects, three resting state visual networks related to MwoA were selected in this study to guide the generation of resting state visual networks in new subjects. The preprocessed fMRI data and the selected resting state visual networks were input into GIG-ICA algorithm at the same time to generate the 3D spatial components of the subjects and the corresponding 1D time series. No threshold was set in the above calculation process, so the resting state brain network of all subjects was retained. Three selected resting state visual networks were showed in Figure 1.
Diagnosis of Abnormal Brain Regions in Visual Networks
Based on the Matlab 2017a platform, REST (Resting State fMRI Data Analysis Toolkit) V1.8 was used to conduct an inter-group t-test for the visual networks of the two groups.16 P < 0.01 was considered statistically significant. A voxel threshold of 256 and a connection rule of RMM = 4 were set for Alphasim correction to obtain brain regions with differences in the visual networks of the two groups.
ReHo Analyses
After the MRI data was preprocessed, the ReHo was conducted by DPABI v4.3 software. We calculated the concordance of KCC of the time series of a given voxel with those of its 26 nearest neighbours17 and then generated an individual ReHo map. To reduce the effect of individual diversification, we converted the ReHo value of each voxel into a z-score by subtracting and dividing by the standard deviation of the whole-brain ReHo map. The brain regions for which ReHo was significantly altered were set as the center of the sphere, and the sphere region formed with a radius of 6mm was set as ROI. In accordance with the sequence, the ROIs were recorded as ROI1, ROI2, ROI3, ROI4, ROIn, etc.
Statistical Analysis
All statistical analyses were performed in GraphPad Prism 6 (GraphPad Software Inc., San Diego, CA, USA). The comparison of measurement data between two groups was analyzed with the Student’s t-test. The comparison of enumeration data between two groups was analyzed with the chi-square test. All data were shown as the mean ± standard deviation of the mean. Pearson’s correlation was used for the linear correlation analysis between ROIs and MSQ scores of each item. The correlation coefficient R represents the correlation strength, 0.1 < │R│ < 0.3 represents a weak correlation, 0.3 < │R│ < 0.5 medium correlation, and │R│ > 0.5 strong correlation. P < 0.05 was used as the threshold of significance in all analyses.
Results
Participants and Baseline Characteristics
After the screening of 78 participants (35 healthy control volunteers and 43 MwoA patients), 20 patients were included in this study with a disease duration of more than 6 months (13.45 ± 4.54 months), an average attack frequency of more than 1 time/month (3.10 ± 1.45 times/month) and a average attack duration of more than or equal to 4 hours (13.85 ± 9.37 hours). Twenty age-, sex-, educational qualification- and dominant hand-matched healthy controls were included in the study. The demographic characteristics, including sex, age, educational qualifications and dominant hand are shown in Table 1; no significant differences were observed between the groups (P = 1.00, 0.58, 1.00 and 1.00 for sex, age, educational qualifications and dominant hand, respectively).
 |
Table 1 Baseline Characteristics and NRS Scores of Participants |
NRS Outcomes
The NRS scores of patients with MwoA (5.9 ± 1.45) were significantly higher compared with healthy volunteers (0.2 ± 0.52) (Student’s t-test, P < 0.0001, Table 1), indicating a higher pain intensity.
QoL Outcomes
MSQv2.1 scale scores from the study sample can be found in Table 2. The scores for MSQ-RFR, MSQ-RFP and MSQ-EF were fairly low. The low MSQ-RER score indicated that patients’ performance of normal activities was limited by migraine. The low MSQ-RFP score demonstrated that patients’ performance of normal activities was interrupted by migraines. The low MSQ-EF score demonstrated that patients’ emotions were affected by migraines. The low total score indicated an overall poor quality of life.
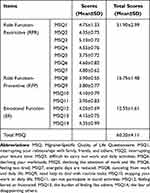 |
Table 2 The MSQ Scores of MwoA Patients |
rs-fMRI Outcomes
Among the 20 components obtained by ICA analysis, three were identified as consistent with the vision-related brain networks in the template and were recorded as Network1 (N1), Network2 (N2), and Network3 (N3).
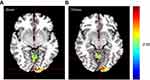
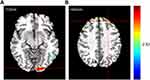
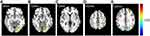
In the N1 network, the functional activity of the left primary visual cortex and left culmen of cerebellum of patients with migraine were significantly stronger compared with the healthy control (HC) group (Figure 2). Further, in the N2 network, the functional activity of the left lingual gyrus and left superior frontal gyrus was significantly higher than in the HC group (Figure 3). Finally, in the N3 network, the functional activity of the right middle occipital gyrus, left fusiform gyrus, right lingual gyrus and right primary motor cortex significantly weakened, while the that of the left posterolateral prefrontal cortex significantly enhanced compared to the HC group (Figure 4).
Linear Correlation Analysis Outcomes
The results of Pearson correlation analysis showed that the decline of attention in work and life (MSQ5) was positively associated with the functional activity of the left primary visual cortex and left lingual gyrus (r = 0.533 and 0.496, P = 0.016 and 0.026, respectively Table 3). Cancelling from work and daily life (MSQ8) was inversely associated with the functional activity of right primary motor cortex (r = −0.501, P = 0.024, Table 3), while the burden of feeling like others (MSQ13) was positively associated with the functional activity of right lingual gyrus (r = 0.534, P = 0.015, Table 3). Further, overall decrease in quality of life was positively associated with the functional activity of right lingual gyrus (r = 0.549, P = 0.012, Table 3).
 |
Table 3 The Correlation Analysis of the ReHo-Value of ROIs and MSQ Scores |
Discussion
In this study, we enrolled a group of MwoA patients and a healthy control group matched for sex, age, and education. RS-fMRI scans and ICA methods were used to evaluate the abnormalities of vision-related brain networks in the interictal phase of MwoA. Our results support our hypothesis that the MwoA patients have abnormalities in their vision-related brain networks. Headache and visual impairment are two key factors in the decreased QoL of migraine patients.6 The higher NRS scores indicated that patients with MwoA suffered from headache. Even though we did not assess the degree of visual impairment in the patients, but we recorded their visual impairment that happened during and for some time after the attack. It indicated that 18 patients had photophobia, 14 patients had blurring of vision, 5 patients had diplopia, 2 patients abnormal visual-spatial attention, and 1 patient had color vision. To evaluate patients’ QoL, we used the MSQ Chinese version which has great reliability and validity.12 The correlation analysis results showed that there was connection between MSQ scores and the function activity of visual networks. These suggest that regulating the neural functions of the corresponding brain regions may improve the QoL of MwoA patients, improving their ability to engage with work. Long-term lower QoL can trigger nervous anxiety and depression that enhance the development of migraine and lead to chronicity.18 Thus, the improvement of patients’ QoL should be given the same emphasis as pain management.
A previous study showed that interictal glutamate levels were increased in the visual cortex of patients with MwoA, indicating the visual cortical hyperexcitability in migraine.8 Consistent with these results, we found that the functional activity of left primary visual cortex was higher than controls. However, the functional activity of right visual cortex was weakened. The human visual cortex includes the primary visual cortex (V1, also known as the striate cortex) and the extrastriate cortex (V2, V3, V4, V5, etc.).19,20 We analyzed them separately and got different results. A recent study indicated that the left V3/V3A of visual cortex was both sensitive and specific for migraine atrophy coordinates.21 These results illustrate that enhanced functional activity of the left visual cortex is one of the important pathogenesis of migraine. However, previous studies have focused less on visual networks of reduced functional activity and there’s no study about the functional activity of the right primary visual cortex to refer to. Thus, our results of weakened functional activity of the right visual cortex need to be verified by more neuroimaging experiments.
In MA patients, the functional activity of lingual gyrus and cerebellum were significantly higher than controls with traumatic thermal stimulation of the trigeminal nerve.22 Our results of the left lingual gyrus and parietal cerebellum were consistent with this study, but the results of the right lingual gyrus were opposite. The difference in functional activity of left and right lingual gyrus may be due to the functional asymmetry between the two hemispheres. Interestingly, the right lingual gyrus was not only associated with the burden of feeling like someone else but also associated with the overall decrease of patients’ QoL. The cerebellum is a key component of the sensory processing circuit. Since granulosa cells (GCs) and Golgi cells (GOCs) receive a wide range of afferent information from the mossy fiber (MF) system, the cerebellum plays an important role in the integration and processing of visual, auditory, tactile and even olfactory information.23 The functional activity of the culmen of cerebellum was stronger in MwoA patients during the interictal period, partly explaining why MwoA sufferers are hypersensitive to visual and auditory stimuli.
It deserved our attention that the functional activity of the posterolateral prefrontal cortex was higher than HCs in our study. The lateral prefrontal cortex receives information about object vision from the visual areas of the temporal cortex and is strongly connected to the sensory cortex.24 Thus, it may be associated with pain and visual impairment of MWOA, although it was not directly associated with decreased QoL in patients our correlation analysis. Interestingly, the pain networks trigger an adaptive visual networks functional plasticity that might help migraine patients to overcome impaired visuospatial skills.25 These suggest that the interaction between the visual networks and the pain networks is strong in migraine. In order to better understand the pathological mechanism of visual network abnormalities in CM, we should investigate the correlation between visual networks and pain networks.
Although our MRI results did not show the abnormal activity of thalamus nucleus in migraine, some of them may also contribute to the headache and visual impairment in MwoA, the pulvinar in particular. Pulvinar, lateral posterior nucleus of thalamus, is the largest nucleus of the thalamus. Its lateral and inferior areas have rich connections with the visual- and dorsolateral parietal cortices and the medial and upper connect with the anterior cingulum and the premotor and prefrontal association areas. Thus, pulvinar was considered to play an important role in the visual and multisensory processing.26–29 Moreover, previous studies have shown abnormal activity of the pulvinar nucleus in patients with migraine.30,31 The abnormal dynamic functional network connectivity of pulvinar with the visual cortex and the precuneus were significantly correlated with headache frequency of migraine.30 Therefore, the pulvinar is well positioned to serve as a key subcortical node in the neural circuits underlying the sensory and visual symptoms of migraine. Next, we will enroll more patients and furtherly study its role in MwoA by using MRI scans and dynamic functional network connectivity state analysis.
The decrease of visual recognition ability is related to age, sex, and educational qualifications, along with spatial visual and cognitive states.32 In this study, the age, sex and educational qualifications of the subjects in the two groups matched, and interference factors were excluded. However, It should also be noted that 5 patients used migraine treatments during their MwoA attack in 1 month before MRI experiment, including 2 patients used ibuprofen and 3 Zolmitriptan. Among them, 2 patients took migraine preventives in the interictal stage, and they took traditional Chinese medicine in 2 month before MRI experiment. Although they stopped treatments 3 days and stopped preventives 1 month before the MRI, it may also affect the MRI results.
It has been reported that neural connections between the visual networks and other regions are significantly enhanced in patients with CM, and that their strength is proportional to the severity of migraine.33 However, ICA analysis can obtain vision-related brain network spatial distribution but cannot measure the strength of the connections between related brain regions. Moreover, RS-fMRI cannot judge if activated and weakened brain regions are really the result of enhanced and decreased self-function or of the negative feedback of network. In future, task-based functional MRI will be designed to verify these questions. The combined analysis of RS-MRI and task-based functional MRI will provide theoretical basis for precise treatment strategies of clinical manifestations of abnormal brain regions through neuro-regulation. Furthermore, neuro-regulation techniques can modulate the functions of the cerebral cortex and deep nuclei without the burden of serious side effects and drug interactions.34 Several neuro-regulation devices have been approved by the Food and Drug Administration (FDA) for the treatment of MA.35 This provides us with the possibility of clinical treatment transformation of this study. According to the clinical manifestations of MwoA patients such as decreased QoL and attention, limitation of social and work activity, and poor mood, the corresponding brain regions could be selected as targets, and different neuro-regulatory techniques could be used to prevent and treat MwoA.
Conclusion
This study reveals that, in between headache attacks, MwoA induced abnormalities in vision-related brain networks, including an enhanced functional activity in the left primary visual cortex, left culmen of cerebellum, left lingual gyrus, superior frontal gyrus, and left posterior lateral prefrontal cortex, as well as a weakened functional activity in right middle occipital gyrus, left fusiform gyrus, right lingual gyrus, and right primary motor cortex. The enhanced functional activity of the left primary visual cortex and left lingual gyrus triggered the decline of attention in work and life, while the weakened functional activity of the right primary motor cortex induced a withdrawal from work and daily life. Further, the enhanced functional activity in the right lingual gyrus was associated with the burden of feeling like others and with a decrease in the overall QoL of MwoA patients. These specific cortical areas may become key targets for the neuro-regulation treatment of MwoA, generating an improvement of patients’ QoL.
We hope that this study can help improve MwoA patients’ QoL by identifying the potential specific brain networks that affect MwoA patients’ QoL and by guiding targeted clinical interventions. However, additional studies including long-term clinical trials and animal experiments investigating the underlying neural mechanisms, are eagerly expected in order to achieve a better understanding of the important role of vision-related brain networks in MwoA.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Ethical Approval
This study was approved by the Ethics Board of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine (2014-028).
Consent
All participants signed informed consent form prior to participation.
Acknowledgments
This work was supported by Shandong Medical and Health Science and Technology Development Program (grant number 2017WS072), Shandong Provincial Hospital of Traditional Chinese Medicine Science and Technology Development Program (grant number 2019-0165), Shandong Natural Science Foundation of China (grant number ZR2014CL013), and the National Natural Science Foundation of China (grant numbers 82001190 and 82004281).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work and declare that there are no conflicts of interest regarding the publication of this paper. Each of the authors conformed to the Helsinki Declaration concerning human rights and informed consent and has followed correct procedures concerning treatment of humans in research.
References
1. Royal P, Andres-Bilbe A, Avalos PP, et al. Migraine-associated TRESK mutations increase neuronal excitability through alternative translation initiation and inhibition of TREK. Neuron. 2018;101(2):232–245. doi:10.1016/j.neuron.2018.11.039
2. Tian Z, Yin T, Xiao Q, et al. The altered functional connectivity with pain features integration and interaction in migraine without aura. Front Neurosci. 2021;15:646538. doi:10.3389/fnins.2021.646538
3. Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343–349. doi:10.1212/01.wnl.0000252808.97649.21
4. Sakai F, Igarashi H. Prevalence of migraine in Japan: a nationwide survey. Cephalalgia. 1997;17(1):15–22. doi:10.1046/j.1468-2982.1997.1701015.x
5. Koller LS, Diesner SC, Voitl P. Quality of life in children and adolescents with migraine: an Austrian monocentric, cross-sectional questionnaire study. BMC Pediatr. 2019;19(1):164. doi:10.1186/s12887-019-1537-0
6. Hanson LL, Ahmed Z, Katz BJ, et al. Patients with migraine have substantial reductions in measures of visual quality of life. Headache. 2018;58(7):1007–1013. doi:10.1111/.head.13330
7. Jurgens TP, Schulte LH, May A. Migraine trait symptoms in migraine with and without aura. Neurology. 2014;82(16):1416–1424. doi:10.1212/WNL.0000000000000337
8. Zielman R, Wijnen JP, Webb A, et al. Cortical glutamate in migraine. Brain. 2017;140(7):1859–1871. doi:10.1093/.brain/awx130
9. Granziera C, Dasilva AF, Snyder J, Tuch DS, Hadjikhani N. Anatomical alterations of the visual motion processing network in migraine with and without aura. PLoS Med. 2006;3(10):e402. doi:10.1371/journal.pmed.0030402
10. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629–808. doi:10.1177/0333102413485658
11. De Matteis E, Affaitati G, Frattale I, et al. Early outcomes of migraine after erenumab discontinuation: data from a real-life setting. Neurol Sci. 2021;42:3297–3303. doi:10.1007/s10072-020-05022-z
12. Chang HY, Jensen MP, Yang CC, Lai YH. Migraine-specific quality of life questionnaire Chinese version 2.1 (MSQv2.1-C): psychometric evaluation in patients with migraine. Health Qual Life Outcomes. 2019;17(1):108. doi:10.1186/s12955-019-1169-y
13. Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics. 2016;14(3):339–351. doi:10.1007/.s12021-016-9299-4
14. Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7(6):1129–1159. doi:10.1162/neco.1995.7.6.1129
15. Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106(31):13040–13045. doi:10.1073/.pnas.0905267106
16. Song XW, Dong ZY, Long XY, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6(9):e25031. doi:10.1371/journal.pone.0025031
17. Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22(1):394–400. doi:10.1016/j.neuroimage.2003.12.030
18. May A, Schulte LH. Chronic migraine: risk factors, mechanisms and treatment. Nat Rev Neurol. 2016;12(8):455–464. doi:10.1038/nrneurol.2016.93
19. Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron. 2012;75(2):230–249. doi:10.1016/j.neuron.2012.06.009
20. Schmiedt JT, Maier A, Fries P, Saunders RC, Leopold DA, Schmid MC. Beta oscillation dynamics in extrastriate cortex after removal of primary visual cortex. J Neurosci. 2014;34(35):11857–11864. doi:10.1523/JNEUROSCI.0509-14.2014
21. Burke MJ, Joutsa J, Cohen AL, et al. Mapping migraine to a common brain network. Brain. 2020;143(2):541–553. doi:10.1093/brain/awz405
22. Russo A, Tessitore A, Silvestro M, et al. Advanced visual network and cerebellar hyperresponsiveness to trigeminal nociception in migraine with aura. J Headache Pain. 2019;20(1):46. doi:10.1186/s10194-019-1002-3
23. Sathyanesan A, Zhou J, Scafidi J, Heck DH, Sillitoe RV, Gallo V. Emerging connections between cerebellar development, behaviour and complex brain disorders. Nat Rev Neurosci. 2019;20(5):298–313. doi:10.1038/s41583-019-0152-2
24. Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55(1):11–29. doi:10.1016/S0278-2626(03)00277-X
25. Messina R, Meani A, Riccitelli GC, Colombo B, Filippi M, Rocca MA. Neural correlates of visuospatial processing in migraine: does the pain network help? Mol Psychiatry. 2021. doi:10.1038/s41380-021-01085-2
26. Fang Q, Chou XL, Peng B, Zhong W, Zhang LI, Tao HW. A differential circuit via retino-colliculo-pulvinar pathway enhances feature selectivity in visual cortex through surround suppression. Neuron. 2020;105(2):355–369.e6. doi:10.1016/j.neuron.2019.10.027
27. Chou XL, Fang Q, Yan L, et al. Contextual and cross-modality modulation of auditory cortical processing through pulvinar mediated suppression. Elife. 2020;9. doi:10.7554/eLife.54157
28. Ibrahim LA, Mesik L, Ji XY, et al. Cross-modality sharpening of visual cortical processing through layer-1-mediated inhibition and disinhibition. Neuron. 2016;89(5):1031–1045. doi:10.1016/j.neuron.2016.01.027
29. Zhou H, Schafer RJ, Desimone R. Pulvinar-cortex interactions in vision and attention. Neuron. 2016;89(1):209–220. doi:10.1016/j.neuron.2015.11.034
30. Tu Y, Fu Z, Zeng F, et al. Abnormal thalamocortical network dynamics in migraine. Neurology. 2019;92(23):e2706–e2716. doi:10.1212/WNL.0000000000007607
31. Dumkrieger G, Chong CD, Ross K, Berisha V, Schwedt TJ. Static and dynamic functional connectivity differences between migraine and persistent post-traumatic headache: a resting-state magnetic resonance imaging study. Cephalalgia. 2019;39(11):1366–1381. doi:10.1177/0333102419847728
32. Lott LA, Haegerstrom-Portnoy G, Schneck ME, Brabyn JA. Face recognition in the elderly. Optom Vis Sci. 2005;82(10):874–881. doi:10.1097/01.opx.0000180764.68737.91
33. Coppola G, Di Renzo A, Petolicchio B, et al. Increased neural connectivity between the hypothalamus and cortical resting-state functional networks in chronic migraine. J Neurol. 2020;267(1):185–191. doi:10.1007/s00415-019-09571-y
34. Vigano A, Toscano M, Puledda F, Di Piero V. Treating chronic migraine with neuromodulation: the role of neurophysiological abnormalities and maladaptive plasticity. Front Pharmacol. 2019;10:32. doi:10.3389/fphar.2019.00032
35. Halker SR, Ailani J, Robbins MS. Neuromodulation for the acute and preventive therapy of migraine and cluster headache. Headache. 2019;59(Suppl 2):33–49. doi:10.1111/head.13586
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.