Back to Journals » Cancer Management and Research » Volume 12
Moxibustion Enhances Chemotherapy of Breast Cancer by Affecting Tumor Microenvironment
Authors Xue N, Fu X, Zhu Y, Da N, Zhang J
Received 14 February 2020
Accepted for publication 4 August 2020
Published 4 September 2020 Volume 2020:12 Pages 8015—8022
DOI https://doi.org/10.2147/CMAR.S249797
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Ning Xue,1,2 Xingli Fu,3 Yin Zhu,4 Nili Da,2 Jianbin Zhang1
1Department of Acupuncture, Second Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing 210017, People’s Republic of China; 2Department of Acupuncture, Jurong Hospital Affiliated to Jiangsu University, Zhenjiang 212400, People’s Republic of China; 3Jiangsu University Health Science Center, Zhenjiang 212001, People’s Republic of China; 4Department of Oncology, Jurong Hospital Affiliated to Jiangsu University, Zhenjiang 212400, People’s Republic of China
Correspondence: Jianbin Zhang Email [email protected]
Introduction: Chemotherapeutic drugs often cause obvious toxicity and side effects. Moxibustion can improve the immunity of cancer patients, enhance cellular immunity, and reduce the toxicity and adverse effects of radiotherapy and chemotherapy. In this study, the efficacy of moxibustion combined with paclitaxel on breast cancer was evaluated.
Methods: A breast cancer mouse model was established. Hematoxylin and eosin staining was used to analyze tumor necrosis in mouse tumors. Immunohistochemistry, Western blot, and qPCR were used to detect the expression of CD34, hypoxia-inducible factor-1α (HIF-1α), vascular endothelial growth factor A(VEGFA), programmed death-1 (PD-1), and programmed death-1 ligand (PD-L1) in tumor tissues.
Results: Moxibustion combined with paclitaxel significantly inhibited weight loss in breast cancer-burdened mice and increased the survival rate. Moxibustion combined with paclitaxel increased the number of white blood cells, thymus index, and spleen index, and enhanced immune function by upregulating interferon-gamma and interleukin-2 and downregulating interleukin-10 and transforming growth factor-β 1. Notably, moxibustion combined with paclitaxel inhibited the angiogenesis of tumors through the downregulation of CD34, HIF-1α, and VEGFA, and overcame the immunosuppressive microenvironment by inhibiting the PD-1/PD-L1 signaling pathway.
Conclusion: Moxibustion improves the body’s immune function and enhances the efficacy of chemotherapy by overcoming the immunosuppressive microenvironment.
Keywords: moxibustion, breast cancer, PD-L1, chemotherapy, tumor microenvironment
Introduction
Cancer immunotherapy could alleviate the side effects of and resistance to anticancer options such as radiotherapy and chemotherapy. Immunotherapy has gradually become an important method for the clinical treatment of tumors, with high expectations among the medical community.1 The cells and molecules in the tumor microenvironment (TME) change dynamically, resulting in the accumulation of a large number of immunosuppressive cells (MDSCs, regulatory T cells, tumor-related macrophages) and inflammatory-related factors in the TME to promote immune escape and metastasis of tumors.2 Programmed death-1 (PD-1) is highly expressed in activated T cells, while PD-1 ligand (PD-L1) is widely expressed in activated T cells and many kinds of cancer cells. When PD-1 binds to PD-L1, immunoreceptor tyrosine-based inhibitory motif (ITIM) and immunoreceptor tyrosine-based activation motif (ITAM) are activated and recruit anti-Src homology phosphatase protein tyrosine phosphatase-1 (SHP-1) and protein tyrosine phosphatase-2 (SHP-2) to inhibit cytokine secretion and T-lymphocyte proliferation.3 The activation of PD-1/PD-L1 signaling can promote immunosuppressive TME, while blocking this pathway can enhance antitumor therapy.4,5
Moxibustion is a traditional Chinese medicine therapy that enhances the blood circulation and relieves swelling and pain.6 In recent years, moxibustion has been applied in the treatment of several diseases, such as temporomandibular joint disturbance syndrome, malposition, colitis, dysmenorrhea, herpes zoster, knee osteoarthritis, diarrhea, heel pain, asthma, soft tissue injury, urinary retention, and gastrointestinal disorders.7–9 More importantly, moxibustion has been used to improve the quality of life and alleviate the adverse effects of conventional treatment options for various diseases.10
The incidence of breast cancer has rapidly increased worldwide, with a total incidence rising nearly 10 times in just 10 years.11 At present, the main treatment strategy for breast cancer is the use of conventional anticancer drugs. Nevertheless, these chemotherapeutic drugs have toxicity and side effects. It was suggested that moxibustion can promote blood circulation, increase drug concentration in tumors, inhibit inflammation, and improve immune function, which might relieve adverse effects and potentiate the therapeutic effects of chemotherapeutic drugs.12 However, the effect of moxibustion combined with conventional anticancer drug on breast tumor has not been reported yet. Therefore, in this study we aimed to evaluate the efficacy of moxibustion combined with paclitaxel on breast cancer and investigate the underlying signaling mechanism.
Materials and Methods
Animals
Female BALB/c nude mice (20±2 g, 4 weeks old) were provided by the Laboratory Animal Center of Jiangsu University (Zhenjiang, China) and kept in the specific-pathogen-free (SPF) facility with controlled temperature (22±2°C), a 12:12-h light–dark cycle, and free access to water and food. All animal protocols were approval by the Animal Care and Use Committee of Jiangsu University of China and followed Chinese National Guidelines for Experimental Animal Welfare. Mouse mammary tumor 4T1 cells were suspended in PBS (1×107 cells in 50 μL) and injected into the fourth left inguinal mammary gland fat pad, and successful establishment of the models was observed when the tumors became palpable (100–200 mm3). The mice were randomized into five groups (n=6): control group (CG), tumor group (TG), moxibustion + tumor group (MTG), paclitaxel + tumor group (PTG), and moxibustion + paclitaxel + tumor group (MPTG). Mice in the PTG and MPTG groups were intravenously injected with 0.1 mL cy7-paclitaxel (1 mg/mL).
For moxibustion, the mice were shaved at the Housanli acupoint on both sides to expose the skin, fixed at the Sanli acupoint, and smeared with appropriate baicalein ointment. Next, moxa was rubbed into the size of a wheat grain (about 6 mg), placed on the acupoints, and the moxa ash was directly pressed on the moxibustion site after the moxa burned out. The mice were treated with three pillars at each acupoint once a day.
Hematoxylin and Eosin (H&E) Staining of Mouse Tumors
The dissected breast cancer was fixed in 10% formalin, dehydrated from low to high concentration of ethanol, and embedded in paraffin. The embedded tissues were cut into sections and stained with H&E.
Routine Blood Test
Eyeball blood was withdrawn into tubes containing EDTA anticoagulant. The hematology analyzer was used to determine the total white blood cells (WBCs), and the ratio of lymphocytes (LY), neutrophils (NEUT), and mononuclear cells (MONO) in the blood.
Western Blot Analysis
The tumor samples were lysed in lysis buffer containing protease inhibitor cocktail (Pierce). Next, the obtained protein lysates were separated via 10% SDS-PAGE and then electro-transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore Corp., Bedford, MA). Subsequently, the membrane was blocked with 5% nonfat milk for 1 h at room temperature, incubated with antibodies for CD34 (ab81289), hypoxia inducible factor-1α (HIF-1α, ab51608), vascular endothelial growth factor A (VEGFA, ab1316), PD-1 (ab252192), and PD-L1 (ab205921) (all from Abcam, Cambridge, MA) overnight at 4°C, and then incubated with secondary antibody. Subsequently, an appropriate HRP-coupled secondary antibody was added. The protein bands were visualized using a Western blotting detection system in accordance with the instructions of the manufacturer (Cell Signaling Technology, MA).
ELISA
The levels of transforming growth factor-β1 (TGF-β1), interferon-γ (IFN-γ), interleukin 2 (IL-2), and IL-10 in the serum were quantified using ELISA kits (Multi Science Co., Hangzhou, China) according to the manufacturer’s specifications.
Quantitative PCR
Total RNA was isolated from the tissues with Trizol (Invitrogen, Carlsbad, CA) and reverse transcribed to cDNA. Quantitative PCR was performed with the ABI 7500 Detection System (Applied Biosystems, CA) using ChamQ Universal SYBR qPCR Master Mix (Vazyme, China). The program for amplification was one cycle of 95°C for 2 min followed by 40 cycles of 95°C for 10 s, 60°C for 30 s, and 72°C for 30 s. The primer sequences used in this study were as follows: 5ʹ- GGAGCCACCAGAGCTATTCC-3ʹ (forward) and 5ʹ-TCCCAACAGCCATCAAGGTT-3ʹ (reverse) for CD34; 5ʹ-CGCCTCTGGACTTGTCTCTT-3ʹ (forward) and 5ʹ-TCGACGTTCAGAACTCATCCT-3ʹ (reverse) for HIF-1α; 5ʹ-TATTCAGCGGACTCACCAGC-3ʹ (forward) and 5ʹ-AACCAACCTCCTCAAACCGT-3ʹ (reverse) for VEGFA; 5ʹ-TTACCACAAGCTGGGAGCAG-3ʹ (forward) and 5ʹ-AGACATAGGCTTAGGCCCCA-3ʹ (reverse) for PD-1; 5ʹ-TTCACAGCCTGCTGTCACTT-3ʹ (forward) and 5ʹ-TAAGGTCCTCCTCTCCTGCC-3ʹ (reverse) for PD-L1; and 5ʹ-AATGGGCAGCCGTTAGGAAA-3ʹ (forward) and 5ʹ-GCGCCCAATACGACCAAATC-3ʹ (reverse) for GAPDH. GAPDH was used as an endogenous control to normalize relative gene expression calculated using the 2−ΔΔCT method.
Immunohistochemical Analysis
Immunohistochemical analysis was performed on paraffin-embedded tissue sections (4–5 μm). The sections were deparaffinized, rehydrated, blocked, and incubated with antibodies for CD34 (ab81289), HIF-1α (ab51608), VEGFA (ab1316), PD-1 (ab252192), and PD-L1 (ab205921) overnight at 4°C. Next, the slides were incubated with streptavidin-HRP prior to staining with DAB substrate (Santa Cruz Biotechnology, CA) and counter-staining with hematoxylin. The images were obtained under an Olympus IX81 microscope.
Statistical Analysis
Data are presented as mean ± SD, with the experiments performed in triplicate. One-way ANOVA and Student’s t-test were used for the comparison of the various experimental and control groups. Student’s t-test was used to compare the difference between two groups. A P-value less than 0.05 was considered to be statistically significant.
Results
Moxibustion Enhanced the Efficacy of Paclitaxel on Breast Cancer in BALB/c Nude Mice
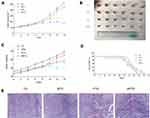
The ability of moxibustion to enhance the antitumor efficacy of paclitaxel on breast cancer was investigated in BALB/c nude mice. The moxibustion group had no strong anticancer effect, while the mice in the paclitaxel group showed certain antitumor effects. Compared with the paclitaxel group, the mice in the moxibustion–paclitaxel group had significantly smaller tumor volume (Figure 1A). The size of tumor dissected from mice in each group differed (Figure 1B). While the paclitaxel group had reduced body weight, the combined moxibustion–paclitaxel group showed better body weight (Figure 1C). Compared with the paclitaxel group, the combination of moxibustion with paclitaxel significantly prolonged the survival of breast cancer-burdened mice (Figure 1D). Finally, we performed histological analysis on tumor tissues. In the control group, tumor cells were round, with dark and round nuclei. In MTG, tumor cells were slightly necrotic, with a small number of blood vessels in the stroma and mild to moderate hemorrhage. However, in PTG and MPTG, tumor cells were loosely arranged, with serious disorder of structure and hierarchy, nuclear pyknosis, fragmentation, and large necrotic masses (Figure 1E).
Moxibustion Improved the Immunity of Tumor-Bearing Mice
To analyze the effect of moxibustion on the immunity of mice, the thymus index was examined. The results showed that paclitaxel significantly inhibited the thymus index, indicating its immunotoxicity. However, moxibustion improved the thymus index in PTG (Figure 2A). Similarly, the spleen index of PTG was significantly decreased compared to CG, but moxibustion improved the spleen index in PTG (Figure 2B).
 |
Figure 2 Moxibustion improved the inhibition effects of paclitaxel on the thymus (A) and spleen (B) of tumor-burdened mice *P<0.05 (n=6). |
Compared with the controls, the total number of WBCs in TG mice significantly decreased (P<0.05). Compared with TG, the total number of WBCs in MTG increased (P<0.05), while the total number of WBCs in PTG decreased. Compared with TG, no significant difference in the total number of WBCs in MPTG was observed (Table 1).
 |
Table 1 Distribution of White Blood Cells in Peripheral Blood of Mice |
Effect of Moxibustion and Paclitaxel on Serum Levels of Cytokines in Mice
Next we analyzed the levels of IL-2, IL-10, TGF-β, and IFN-γ in the serum of mice. Compared with control, the levels of IL-2 and IFN-γ in TG decreased significantly (P<0.01), while the levels of IL-10 and TGF-β increased significantly (P<0.01). Compared with TG, the levels of IL-2 and IFN-γ in MTG, PTG, and MPTG increased significantly (P<0.05), while the levels of IL-10 and TGF-β in MTG, PTG, and MPTG decreased significantly (P<0.05) (Figure 3).
 |
Figure 3 Levels of IL-2, IL-10, TGF-β, and IFN-γ in serum of tumor-burdened mice. Compared with control, **P<0.01; compared with TG, #P<0.05, ##P<0.01 (n=6). |
Moxibustion and Paclitaxel Stimulate the Microvasculature
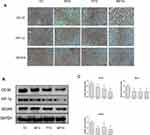
Tumor cells normally acquire nutrients and oxygen for growth through neovascularization. CD34 is highly expressed in microvascular endothelial cells of tumors, and the HIF-1α–VEGF signaling pathway can promote neovascularization. Therefore, we detected HIF-1α and VEGFA in tumor tissues. Immunohistochemical staining showed that CD34, HIF-1α, and VEGFA were significantly downregulated in MPTG and PTG compared with TG (Figure 4A). Similar results were observed by Western blot analysis (Figure 4B) and PCR analysis (Figure 4C). These data suggest that moxibustion could suppress tumor progression via the inhibition of tumor angiogenesis.
Effect of Moxibustion on the Expression of PD-1 and PD-L1 in Tumors
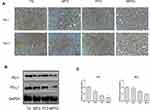
Compared with TG, the expression of PD-1 and PD-L1 decreased significantly in tumors in MTG and MPTG (P<0.05), but showed no significant difference in PTG (Figure 5A). The protein levels of PD-1 and PD-L1 were significantly downregulated in MPTG and MTG compared to TG and PTGby Western blot analysis (Figure 5B). Moreover, the mRNA levels of PD-1 and PD-L1 were significantly downregulated in MPTG and MTG compared to TG and PTG by PCR analysis (Figure 5C). Collectively, these results suggest that moxibustion may inhibit breast cancer through the PD-1/PD-L1 signaling pathway.
Discussion
In this study, we established the MDB-MB-231 breast cancer nude mouse model to assess the ability of moxibustion to enhance the antitumor effects of paclitaxel. We found that paclitaxel caused weight loss in the mice, and decreased the thymus and spleen indices of the immune organs. However, moxibustion-treated mice did not lose weight and had no decrease in the thymus and spleen indices. More importantly, the prolonged survival of tumor-burdened mice in MPTG in comparison with PTG suggested that moxibustion combined with chemotherapy may sustain the life of cancer patients. These results are consistent with a previous study which found that moxibustion could improve the total number of leukocytes in 23 patients with malignant tumors of the respiratory and digestive systems after chemotherapy.13 In this study, we found that moxibustion could improve the antitumor effect of paclitaxel by reducing the total number of WBCs.
The growth and metastasis of a tumor depend on the formation of neovascularization. Usually, tumor cells obtain nutrients and oxygen for growth through neovascularization. CD34 is highly expressed in the cytoskeleton of splenoma microvascular endothelial cells. Therefore, positive expression of CD34 has been regarded as a marker of microangiogenesis in tumor tissues. Importantly, HIF-1α is highly expressed in many tumors, and it is involved in angiogenesis and affects the biological behavior of tumors.14 In tumors, the hypoxic tumor microenvironment interacts with VEGFA to promote the development of tumors.15 Previous research has shown that HIF-1α is highly expressed in breast cancer and is associated with tumor angiogenesis.16 HIF-1α can increase the angiogenesis of tumors by supplying oxygen and energy for tumors and promoting the infiltration and dissemination of tumors.17 In this study, we showed that a combination of moxibustion and paclitaxel could inhibit the expression of CD34 in tumors of nude mice.
In tumor microenvironment, the inhibition of T-lymphocyte function by PD-1 is considered to be one of the main mechanisms of tumor immune escape. Usually, PD-1 is expressed in immune cells such as T lymphocytes, while its ligand, PD-L1, is expressed in tumor cells. The interaction of PD-1 and PD-L1 promotes immunosuppression and ultimately culminates in tumor immune escape.18 In support of this notion, we showed that moxibustion could inhibit the expression of PD-1 and PD-L1 in tumor cells, thereby preventing the immune escape of tumors.
Conclusion
Moxibustion combined with paclitaxel could inhibit the growth of breast cancer in mice by regulating the microvascular system and immunosuppression. However, the specific signaling pathway is still unclear, and needs further study. Moxibustion has obvious advantages, such as simple operation, remarkable curative effect, and low price, and it improves the body’s immune function and alleviates the toxicity and side effects of chemotherapy. Moxibustion has potential as a long-term adjuvant therapy of chemotherapy to improve the quality of life of cancer patients.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Liang H, Wang M. Prospect of immunotherapy combined with anti-angiogenic agents in patients with advanced non-small cell lung cancer. Cancer Manag Res. 2019;11:7707–7719. doi:10.2147/CMAR.S212238
2. Aqbi HF, Wallace M, Sappal S, Payne KK, Manjili MH. IFN-γ orchestrates tumor elimination, tumor dormancy, tumor escape, and progression. J Leukoc Biol. 2018;103(6):1219–1223. doi:10.1002/JLB.5MIR0917-351R
3. Gros A, Robbins PF, Yao X, et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J Clin Invest. 2014;124(5):2246–2259. doi:10.1172/JCI73639
4. Xu-Monette ZY, Zhang M, Li J, Young KH. PD-1/PD-L1 blockade: have we found the key to unleash the antitumor immune response. Front Immunol. 2017;8:1597.
5. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi:10.1038/nrc3239
6. Jia Y, Yang SY, Guo LM, Bai X, Wang YN, Meng FJ. Effects of moxibustion on cancer-related fatigue: a meta-analysis. TMR Non Drug Ther. 2019;2(2):62–70.
7. Wang C, Yang M, Fan Y, Pei X. Moxibustion as a therapy for breast cancer-related lymphedema in female adults: a preliminary randomized controlled trial. Integr Cancer Ther. 2019;18:1–9. doi:10.1177/1534735419866919
8. Deng H, Shen X. The mechanism of moxibustion: ancient theory and modern research. Evid Based Complement Alternat. 2013;2013(6):379291. doi:10.1155/2013/379291
9. Mao H, Mao JJ, Guo M, et al. Effects of infrared laser moxibustion on cancer-related fatigue: a randomized, double-blind, placebo-controlled trial. Cancer. 2016;122(23):3667–3672. doi:10.1002/cncr.30189
10. Zhang HW, Lin ZX, Cheung F, Cho WC, Tang JL. Moxibustion for alleviating side effects of chemotherapy or radiotherapy in people with cancer. Cochrane Database Syst Rev. 2018;11.
11. Boudier J, Oldrini G, Barlier C, Lesur A. Axillary staging in daily practice in the diagnosis of breast cancer. Oncologie. 2019;21:11–16. doi:10.3166/onco-2019-0034
12. Yang M, Wan Y, Jiang X, et al. Electro-acupuncture promotes accumulation of paclitaxel by altering tumor microvasculature and microenvironment in breast cancer of mice. Front Oncol. 2019;9:576. doi:10.3389/fonc.2019.00576
13. Fan Y, Yang Z, Wan M, Wu X, Yan J. Effects of moxibustion therapy on preventing and treating side effects from chemotherapy of malignant tumor patients. J Acupunct Tuina Sci. 2011;9(6):351–353. doi:10.1007/s11726-011-0549-6
14. Lu X, Kang Y. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin Cancer Res. 2010;16(24):5928–5935. doi:10.1158/1078-0432.CCR-10-1360
15. Clara CA, Marie SKN, de Almeida JRW, et al. Angiogenesis and expression of PDGF-C, VEGF, CD105 and HIF-1α in human glioblastoma. Neuropathology. 2014;34(4):343–352. doi:10.1111/neup.12111
16. de Nonneville A, Goncalves A. Triple-negative breast cancer: current data and future prospects. Oncologie. 2019;21:33–39. doi:10.3166/onco-2019-0039
17. Saponaro C, Malfettone A, Ranieri G, et al. VEGF, HIF-1α expression and MVD as an angiogenic network in familial breast cancer. PLoS One. 2013;8(1):e53070. doi:10.1371/journal.pone.0053070
18. Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015–3029. doi:10.1084/jem.20090847
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.