Back to Archived Journals » Reports in Parasitology » Volume 5
Mosquito-borne diseases in Europe: an emerging public health threat
Authors Calzolari M
Received 9 June 2015
Accepted for publication 10 November 2015
Published 19 February 2016 Volume 2016:5 Pages 1—12
DOI https://doi.org/10.2147/RIP.S56780
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Manuel Rodriguez Valle
Mattia Calzolari
Medical and Veterinary Entomology Laboratory, Istituto Zooprofilattico Sperimentale della Lombardia e dell'Emilia Romagna “B. Ubertini”, Reggio Emilia, Italy
Abstract: Mosquito-borne pathogens cause some of the more deadly worldwide diseases, such as malaria and dengue. Tropical countries, characterized by poor socioeconomic conditions, are more exposed to these diseases, but Europe is experiencing an increasing number of human cases of mosquito-borne diseases, both imported and indigenous. Some of these cases are due to recrudescence of pathogens already present in the territory, particularly the West Nile virus. However, other neglected mosquito-borne pathogens remain present in Europe, and could produce human cases sustained by local mosquitoes (such as the Tahyna and Sindbis viruses). Native mosquitoes are still able to transmit pathogens eliminated from Europe and reimported by the sick (such as malaria plasmodia), as well as new imported pathogens. An increasing number of large epidemics involving arboviruses, for which humans could be reservoir hosts (eg, Dengue virus, Chikungunya virus, and Zika virus), seasonally concordant with the activity period of European vectors, poses an expanding risk for potential introduction of these viruses. More autochthonous cases of exotic diseases were reported in Europe, including dengue and chikungunya, raising the potential for the establishment of those pathogens which can be transmitted vertically in vectors. These episodes were often responsible for the establishment of exotic mosquitoes, such as tiger mosquito, imported into Europe by trade and now present in adequate numbers to transmit these pathogens. This actually occurred for chikungunya in Italy in 2007, with more than 200 cases of this disease. Other mosquitoes, potentially vectors of pathogens, can use the same means of entry into Europe, posing new potential risks for health. Dealing with mosquito-borne pathogens, characterized by a complex cycle, will require the establishment of interdisciplinary measures and an internationally coordinated approach, since these diseases do not recognize borders.
Keywords: malaria, arbovirus, Tahyna virus, Sindbis virus, West Nile virus, Dengue virus, Chikungunya virus, Zika virus
Introduction
Mosquitoes are insects known for their annoying habit of biting animals to obtain blood as the protein source to mature their eggs. During their blood meal, mosquitoes can transmit a wide variety of pathogenic agents, ranging from viruses to parasites, to their animal hosts. Several mosquito-borne diseases (MBDs) have been recognized for centuries and are associated to specific environmental conditions; for example, malaria was linked to the swamps by Roman authors as early as the third century BC.1 However, the ability of mosquitoes to transmit diseases has only been demonstrated relatively recently. Evidence of the ability of the Wuchereria bancrofti worm (the agent of human lymphatic filariasis) to develop in mosquitoes was provided in 1877, while in 1898, the Anopheles mosquitoes were proved to transmit malaria plasmodia to humans, and in 1900, Aedes aegypti was confirmed to transmit yellow fever.1 The ability of these diseases to travel in humans was recorded in historical time by the inadvertent export of some of these diseases, including malaria,2 yellow fever, and dengue,3,4 from the Old World to the Americas by the slave trade.
Europe was endemic for malaria, a disease known for centuries causing underdevelopment in endemic areas, and finally eradicated in 1975.5,6 Nevertheless, Europe experienced epidemics of hemorrhagic fever transmitted by mosquitoes in its port cities in the 18th and 20th centuries (see “Historically reported diseases” section).
Although the MBDs are more prevalent in the tropics and in counties with diffused poor socioeconomic conditions,7 the health problems related to these diseases are now increasingly reported in Europe; for example, the chikungunya outbreak in Italy in 2007, or several episodes of locally acquired dengue and chikungunya in France and Croatia (see “Diseases at risk of introduction” section and Table 1).
  | Table 1 Autochthonous confirmed cases of exotic diseases reported in Europe |
The increasing incidence of such episodes in last 10 years demonstrated that Europe is not immune from MBDs, and that the continent is increasingly exposed to the return of old pathogens and to the importation of new ones, due to the modern social scenarios associated with greater movement of people and products and with changes in environmental conditions, such as climatic and landscape modifications.
Mosquito bionomics
Mosquitoes are dipterans of the Nematocera suborder, all placed within the Culicidae family. Approximately 3,200 species and subspecies of mosquitoes are recognized, and divided into 42 genera.6,8 In Europe, the most relevant species from a medical point of view belong to the genera Anopheles, Culex, and Aedes5,6,9 (in this review, Ochlerotatus and Stegomyia taxa were considered as subgenera of the Aedes genus) (Table 2). The definition of the composition of the mosquito fauna in a particular area is not always simple because of the presence of grouped complex, morphologically indistinguishable or hardly distinguishable sibling species.10 This is particularly true for the Anopheles mosquito: among the 460 species within the genus, 70 are known to transmit malaria and approximately 40 are important vectors. Several malaria vectors are grouped in closely related species that are morphologically similar but differ in biology, and hence in their capacity to transmit malaria (Table 2).6,8
  | Table 2 The most relevant vector species of mosquito in Europe |
Regarding the host preference, mosquitoes can be more generalists or bait preferentially a particular group of animals, such as mammals or birds.8 Some species have a strict preference for one species only, such as the yellow fever mosquito (Ae. aegypti) which is an anthropophilic mosquito.8,11,12 The host preference is an important mosquito characteristic that strongly influences the vector capacity of the species (see “Mosquito–pathogen interactions” section).13
These insects have great adaptability to different environments. Since the immature stages are aquatic, they are linked to fresh or brackish water. Depending on the species, mosquitoes are capable of breeding in a wide variety of aquatic environments: man-made containers of variable dimensions (eg, used cans, plastic containers, flowerpot saucers, etc), manholes, cesspits, catch basins, snow-melt ponds, tree holes, leaf axils, ditches, flooded basements, flooded meadows, fountains, marshes, peat bogs, ponds, rice fields, river banks, springs, wells, and marshes.6,8,9,14 Mosquito larvae usually do not occur in running or open waters; they prefer edges that provide shelter and minimal running water.6,8
Some mosquitoes, such as Culex or Anopheles species, deposit eggs directly onto the water surface of a preferably permanent environment, while other mosquitoes, such as Aedes species, deposit eggs in mud, leaf litter, or damp substrates near the water. These latter species breed in more transient environments and usually show population peaks after increases in the water level; for example, after flooding or rains. Eggs of these species are often very resistant to desiccation and can remain viable for years in a dry state.6,8,10,14
All mosquito larvae breathe atmospheric air by accessing the water surface; those of the Coquillettidia genus have a modified siphon and breathe by exploiting the immersed parts of plants. This characteristic allows the larvae to occupy water that is polluted or rich in organic material – an environment with fewer competitors and predators. Larvae feed on detritus, algae, and aquatic microorganisms, and after three molts, they become pupae. The larval duration is variable, depending primarily on temperature and food sources, and, in good conditions, it could last 7–10 days;6 the larvae can also survive as an overwintering stage; the duration of the pupae, a stage unable to feed, can last from 2 to 3 days up to a week.6,10 The total development time, from egg to adult, can last 10 days for certain Culex and Aedes mosquitoes,8,10 while Anopheles species have longest development time, and Mansonia and Coquillettidia show protracted life cycle.8
Adult mosquitoes feed on sugary liquids (eg, nectar, damaged fruits, vegetative tissues, and honey dew);15 only the females need a blood meal to mature their eggs. These insects can deposit hundreds of eggs per oviposition. The time between the blood meal and the maturation of the eggs ranges from 2 to 3 days in hot conditions to up to 1–2 weeks at lower temperatures;6,8 this interval is known as gonotrophic cycle, a mosquito may have different gonotrophic cycles during her life.13,14 Some mosquito species do not fly away from their larval breeding sites (hundreds of meters for Aedes albopictus16,17 and Ae. aegypti),8,18 while other species may travel over great distances, such as Culex mosquitoes, which can fly approximately 2 km away from its breeding sites,19 and even greater distances, especially if carried by the wind (distances over 20 km are reported for Aedes vexans).14 Adult females live 1–2 weeks in the tropics, but in temperate countries they may live 1–2 months.6
Some mosquitoes overwinter as adults (such as Anopheles and Culex species); this provides a possible way of overwintering for pathogens, through the infected female. In other species (Aedes genus), the eggs are the stage that survive the unfavorable season; the production of diapausing eggs can allow the overwintering of pathogens through vertical transmission.6,8,10
Mosquitoes can be transported for long distances by cars, trains, ships, and airplanes. If these specimens are infected, then the pathogen could travel with the mosquito. Eggs or immature stages of mosquitoes could be also transported with commercial products. Several invasive species within the Aedes genus (eg, Ae. albopictus, Ae. japonicas, Ae. atropalpus, Ae. koreicus, Ae. triseriatus, and Ae. aegypti) are transported primarily by humans, and have been increasingly reported in Europe since 1990.12 The more impressive case is the tiger mosquito (Ae. albopictus), whose eggs were transported in used tires, a good breeding site for this mosquito, and traded worldwide, likely arising from infested deposits in Japan.20 Tiger mosquito eggs were also transported with ornamental plants such as lucky bamboos.21 Today, this species is diffused throughout several counties outside its native area (Asia) and is expanding its home range to Europe and North and South America. That this species could have such expansion and be so often regarded with indifference by people and authorities is surprising, given that Ae. albopictus is a competent vector of several arboviruses.6,7,17,21
MBDs
Human MBDs
Human vector-borne diseases account for 17% of the estimated global burden of all infectious diseases.22 The major part and most widely distributed of these diseases are transmitted by mosquitoes.7 The more relevant MBDs, with an estimate of their global burden are reported in Table 3.23
  | Table 3 The more relevant MBDs worldwide, and relative DALYs estimation (in thousands) |
Among the known MBDs, the one that is the greatest cause of mortality in human is malaria, the disease caused by Plasmodium parasites. The World Health Organization estimates that 198 million cases of malaria occurred in 2013, causing 584,000 deaths,24 especially in children. Four Plasmodium species cause disease in humans: Plasmodium falciparum, Plasmodium ovale, Plasmodium malariae, and Plasmodium vivax.6 These parasites circulate between humans and mosquitoes. Plasmodium knowlesi, a species typical of monkeys, is an important human pathogen in particular areas of Southeast Asia.25 Malaria is endemic in 97 countries; more than 90% of malaria cases occur in sub-Saharan Africa.24
Lymphatic filariasis is a neglected disease transmitted by mosquitoes, with an impressive estimation of 120 million human infections worldwide, distributed in endemic counties in sub-Saharan Africa, Southern Asia, the Eastern Pacific Islands, and, to a lesser extent, in South America.6,26 This disease is caused by three species of nematode: W. bancrofti, Brugia malayi, and Brugia timori; the first species accounts for approximately 90% of the cases.26 The role of other vertebrates as reservoir hosts of this disease is marginal, recorded only for B. malayi.8,27 Other filarial worms of the genus Dirofilaria, which primarily cause disease in animals, could accidentally infect humans by encysting in subcutaneous tissues.5 Mosquitoes were also suspected to have a more than merely mechanical role in the transmission of tularemia, the disease caused by the gram-negative bacterium Francisella tularensis.28
Arthropod-borne viruses, known as arboviruses, are a group composed of viruses from different families, without a taxonomic significance but with an epidemiological relevance (Table 4). Many arboviruses transmitted by mosquitoes are recognized as disease agents, and cause mainly systemic febrile disease, hemorrhagic fever, encephalitis, and polyarthralgia.6,29 Some of these can cause serious illness, while others just provoke just mild disease. Other viruses are not definitively linked to human disease, such as viruses isolated from a few sick persons or only serologically detected in humans (as Arumowot virus, Kedougou virus, Nyando virus, Shuni virus, and Tanga virus).
  | Table 4 Arboviruses transmitted by mosquitoes causing (or suspected to cause) disease in humans |
Many mosquito-borne viruses belong to the Flavivirus genus, which includes some of the more pathogenic viruses; these may provoke hemorrhagic fevers or encephalitis. Yellow fever and dengue are two of the major world diseases caused by flaviviruses, with 200,000 cases estimated of yellow fever (30,000 deaths, 90% occurring in Africa),30 and 2.35 million cases of dengue in the Americas alone in 2013, of which 37,687 cases were severe.31 While dengue is spread in tropics and subtropics worldwide, yellow fever show a tropical distribution but does not occur in Asia.6 Both diseases are principally transmitted by the invasive mosquito Ae. aegypti; while Ae. albopictus has a secondary, but increasingly important, role in dengue transmission (see “Mosquito–pathogen interactions” section). WHO reported approximately 68,000 clinical cases of Japanese encephalitis every year (the first viral encephalitis in Asia);32 it is transmitted by several species of mosquitoes, principally rice-field mosquitoes.6 The globally widespread West Nile virus (WNV) causes symptoms in approximately 20% of infected persons, of which approximately 1% could develop encephalitis;33 however, the presence of that virus raises problems in the safety of transfusions and transplants.
Another virus related to WNV, but with no clear pathogenic potential for humans, is the Usutu virus (USUV).34 Other flaviviral diseases are St Louis encephalitis, endemic in the USA and Canada; the Rocio encephalitis, reported in Brazil; and the Murray Valley encephalitis typical of Australia.6 An emerging flavivirus is the Zika virus, isolated in Africa and subsequently in Asia;6 this virus caused an outbreak in Western Pacific countries in 2013–2014, with an estimation of 19,000 suspected cases in 2013,35 and in 2015 it was detected in Brazil.36 At the beginning of 2016 circulation of Zika virus has been recorded in 20 American countries, and seems linked with congenital malformations, particularly microcephaly.36
Some arboviruses belong to the Alphavirus genus and produce symptoms that include hemorrhagic fever, arthritis, and encephalitis.6,29 Chikungunya virus (CHIKV) is known as one of the main emerging pathogens, sustained outbreaks of CHIKV spread in many counties, departing from the 2005 outbreak in Indian ocean, to the ongoing outbreak in the Caribbean (which started in 2013), and also resulted in an outbreak in Italy in 2007.37
The O’nyong-nyong virus is closely related to CHIKV; it circulates in Africa, where it also caused large epidemics. Interestingly, this virus is transmitted by Anopheles mosquitoes.6 This genus includes the most common agents of encephalitis in the Americas: Venezuelan equine encephalitis virus, Eastern equine encephalitis virus, and Western equine encephalitis virus.6 Several alphaviruses have primates as reservoir hosts, and then become potentially dangerous for human (such as the Mayaro virus, reported in South America, CHIKV, O’nyong-nyong virus) or can develop a human viremia suitable for vector transmission (Venezuelan equine encephalitis virus) (Table 4).3,6 Another alphavirus present in Europe, but also found in Asia and Australia, is the Sindbis virus (SINV), while the Ross River virus and Barmath Forest virus are particularly dispersed in Australia.6
Several arboviruses fall in the Orthobunyavirus genus (Table 4), and at least four are present in Europe (see “Emerging and endemic pathogens” section). Orthobunyaviruses cause febrile illness in humans, were isolated from forest environments in South America, and their detections were linked to human activities such as deforestation, mining, urbanization, and dam and highway construction.38
Although a large part of the pathogenic phleboviruses is transmitted by sandflies, some of them were isolated from mosquitoes.5,6,29 Rift Valley fever virus (RVFV) is the more dangerous phlebovirus transmitted by mosquitoes in terms of its pathogenic potential: it circulates between domestic ruminants and can be transmitted to humans through contact or ingestion of organic fluids.6 Several outbreaks of Rift Valley fever have been recorded in Africa: an outbreak affecting more than 89,000 persons was recorded between Somalia and Kenya in 1997–1998, but this virus also affected the Arabian Peninsula and Isles in Indian Ocean.6,29 Other phleboviruses isolated from mosquitoes have not been associated with disease, but Arumowot virus was serologically detected in populations of Africa and Asia39 and was also serologically detected in Southern Italy.40
Other viruses within the Reoviridae family were mainly isolated from mosquitoes and have been suspected to have pathogenic capacity: the Banna virus (genus Seadornavirus) spread in Asia is a likely causative of encephalitis,41 and the two orbiviruses, the Lebombo and Orungo viruses primarily isolated from mosquitoes, are also linked with human disease but their cycle is largely unknown.29
Sometime mosquitoes are also suspected to play a secondary role in transmission of arboviruses primarily spread by other arthropods; for example, the Oropouche virus, a causative agent of encephalitis and primarily transmitted by culicoids.6
Mosquito–pathogen interactions
Vector-borne diseases are illnesses transmitted by an infected person, or animal (reservoir host), to another person, or animal, by another living organism (vector).13 Mosquitoes are the best-known disease vectors.7 Unlike mechanical transmission, vector-borne disease transmission involves the biological transmission of the pathogen, and its reproduction, or development, in the vector before transmission to the next vertebrate host.13 A pathogenic agent must cross several physical barriers inside the vector to infect it (ie, midgut barriers), and then be transmitted via saliva (ie, salivary gland barriers);42 once infected, a biological vector usually remains infected for life.13
Parasites infect mosquitoes by an infected blood meal. In addition to this mechanism, arboviruses can be transmitted by other routes, such as horizontal transmission during mating, transovarian transmission, cofeeding transmission, and vertical transmission to the eggs during oviposition.13 While a single bite of an infective mosquito can transmit malaria or arbovirus, several bites are necessary to transmit filarial infection.26
The MBD cycles comprise a dynamic interaction between pathogen, vertebrate host, vector, and environment,13 and could involve various vectors and hosts that have different weight in the transmission of the pathogen and can vary in distinct geographical areas. While the four main Plasmodium causes of malaria in humans circulate between mosquito and human,6,8 they harbor a sexual and asexual phase respectively; the virus cycles usually involve a wider range of species.
The agent of mosquito-transmitted disease could circulate in a sylvatic cycle (or enzootic cycle) between the mosquito and wild reservoir hosts, which often act as amplification hosts, and rarely affect humans by the occasional spillover that occurs under certain conditions.13
Human can be infected tangentially by “bridge” mosquitoes that occasionally bite humans but usually feed on other animals, such as birds in the case of WNV and SINV.6,13 The involvement of migratory birds as hosts can facilitate the pathogen spread over great distances.29 Some arbovirus cycles involve domestic animals as amplifying hosts, exposing humans to the risk of infection (eg, Venezuelan equine encephalitis, Japanese encephalitis, and Rift Valley Fever).6
The agent for which humans represent a potential amplification host could also trigger an epidemic cycle (Table 4).13 These agents have high pathogenic potential and can be exported from one country to another via a sick person. Some of these are particularly dangerous because they can develop urban cycles upon reaching a densely populated area occupied by a competent vector that is adapted to feeding on humans (eg, urban dengue, chikungunya, and yellow fever).3 A more complex situation can also involve a rural cycle that links sylvatic and urban cycles, such as yellow fever and dengue.6
Vector competence refers to the intrinsic capability of a vector to transmit a pathogenic agent. It results from a series of biological characteristics, such as the permissiveness of host barriers to penetration of the agent,13 since the pathogenic agents have to cross the gut and reach the salivary glands of the mosquito to be transmitted to another host. Every mosquito has a particular competence for a pathogen; sometimes, distinct populations of the same mosquito have a different vector competence. Other than the biological ability to transmit a virus, which could be tested under controlled conditions, other criteria are necessary to incriminate a species as a vector, such as a natural infection and the seasonal association with the reservoir hosts.13
Vector capacity is the overall ability of a vector species in a given location and specific time to transmit a pathogen.13 It is influenced by factors such as vector population abundance, longevity, feeding behavior, and gonotrophic cycle lengths and numbers.13 The most competent vector in laboratory conditions is not always the principal vector in field conditions. Experimental studies indicated that Culex modestus is a better WNV vector than Culex pipiens,43 but the latter mosquito seems to play the main vector role in different areas based on its abundance.44 An important consideration is that the absence of a proven vector of a pathogen in an area does not automatically mean that this area is free of risks since the autochthonous mosquitoes, not yet tested for this pathogen, could be potential vectors. This is particularly true for viruses that have different potential vectors, such as RVFV, which could be transmitted by several species of mosquitoes in different geographical areas in Africa, and is linked to the environments in which mosquitoes can proliferate.45 The entrance of Rift Valley fever is a risk for Europe, and it would be incorrect to exclude from risk, a priori, areas suitable for proliferations of mosquitoes with uncharacterized vector competences for RVFV.
Nevertheless, the association between a vector and a virus is not a paradigm; adaptation of a pathogen to other mosquitoes is possible, particularly in the transmission of local pathogens by exotic species. The capacity of CHIKV to shift from Ae. aegypti to Ae. albopictus was already reported, and linked with a single amino acid mutation (from alanine to valine at position 226 of the envelope glycoprotein) recorded in strains which caused epidemics in Indian Ocean, Asia, and also in strain which caused the Italian outbreak. Other mutations of CHIKV, linked with enhanced capacity to be transmitted from Ae. albopictus, were also recorded in Asian lineage.46 A similar phenomenon could be hypothesized for Dengue virus (DENV).12 Dengue outbreaks were linked to the presence of Ae. aegypti, and epidemics sustained by Ae. albopictus are rare, but reported.12,47,48 The adaptation of DENV to the mosquito Ae. aegypti, was driven by the circulation of the virus in areas with high density of the reservoir host (humans) and this mosquito.4 However, DENV circulated between sylvatic Aedes mosquitoes (including the Asian tiger mosquito) in the original habitats in Asia, before its adaptation to Ae. aegypti.4 A possible “readaptation” of the virus to Ae. albopictus is not unlikely. Recent epidemic events sustained by Ae. albopictus, particularly the outbreak in Tokyo with at least 100 cases,49 indicate that this “readaptation” is ongoing.
MBDs in Europe
Historically reported diseases
Europe was endemic for malaria, and this disease reached the northern limit of Central England, Southern Norway, Central Sweden and Finland, and Northern Russia, but the Mediterranean and Eastern Europe were the most exposed areas to the disease.5
The first noticeable decline of malaria was seen during the 19th century due to new agricultural practices, land drainage, and changed social conditions (such as housing improvement). The final disappearance was probably due to the changed ecological conditions linked to elimination of larval breeding sites of Anopheles species, to the improvement in health systems, and ultimately to large-scale eradication campaigns.5,6
Europe experienced hemorrhagic fever outbreaks due to mosquito-borne viruses in the 18th century. Although the diagnosis of the pathogen causing historical outbreaks is difficult, at least 20 epidemics, recorded in Europe in the 19th century, were attributable to yellow fever. In the 1800, epidemics occurred in Cadiz, Seville, and Gibraltar, with an estimated number of 51,000 victims.50 Furthermore, epidemics ascribable to dengue were recorded in Europe from the second half of the 18th century until 1927–1928 in Greece, with an outbreak that caused more than 1,000 deaths.51 These outbreaks occurred in ports after the docking of a ship with human cases. Ancient ships had different breeding sites for Ae. aegypti (as barrels for water and bilge water),11 and the virus could circulate between mosquitoes and passengers. Mosquitoes arriving with ships proliferated in port cities, usually characterized by abundant breeding sites (such as containers for water storage),11,50,51 thereby initiating epidemics in naive populations. Despite these episodes, the Ae. aegypti mosquito was unable to establish itself permanently in Europe,12 probably because of the inability to produce dormant eggs capable to survive the temperate winters.11,14,52
Emerging and endemic pathogens
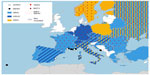
In recent years, a recrudescence of WNV was recorded in Europe. This virus was first serologically detected in Europe in two patients in Albania, in 1958.5 Subsequently, the virus was recorded discontinuously, with sporadic cases in several countries, until the 1996 outbreak in Romania with 683 suspected cases (Figure 1).6 An increasing number of cases was reported in the EU over the years, as reported by European Centre for Disease Prevention and Control (ECDC) between 2014 and 2011, with 672 autochthonous cases (128 in 2011, 242 in 2012, 228 in 2013, and 74 in 2014).53 This virus shows a complex cycle, which could involve distinct species of mosquito and wild birds in different areas. Different Culex mosquitoes can be the main vectors of WNV in distinct areas, depending on ecological conditions;6 in Europe, this role is often played by Culex pipiens.9,34 Due to this ecological plasticity, WNV can assume complex circulation patterns, as were recorded in several European areas, with alternating periods of circulation or cocirculation of different strains.54 Complex phenomena, such as herd immunity in wild birds, are likely to be involved in these patterns.
Another flavivirus widely detected in Europe is the USUV; this virus is closely related to WNV and shows a similar cycle, circulating between mosquitoes and wild birds.5,34 The USUV was first detected in Europe in 2001 in Vienna, where it caused an abnormal mortality in blackbirds,34 but it was retrospectively detected in birds that died in 1996 in Italy.55 The health implications of this virus are not well defined; it seems that this virus could cause diseases in humans, since two cases of neurologic disease due to this virus were reported in immunesuppressed persons in Italy and three cases of meningitis were attributed to this virus in Croatia.55 This virus, however, seems less pathogenic than WNV, as the minor number of USUV human cases recorded in area of sympatric circulation of both viruses,56,57 as Northern Italy, in which USUV was persistently detected over the years, conversely to WNV.57
Different arboviruses are autochthonous in Europe, such as the SINV (Alphavirus), known as the causative agent of a human disease most often recorded in Scandinavia and South Africa,5,6 but this virus is also found sporadically in Central Europe.34 The disease is characterized by fever, rash, and arthritis and is named Ockelbo disease in Sweden, Kerelian fever in Russia, and Pogosta disease in Finland.6 In Scandinavian Peninsula, disease linked to SINV represents an important health issue, with a rough estimation of approximately 170 annual cases (30 in Sweden [Ockelbo disease] and 140 in Finland [Pogosta disease]).58 Three orthobunyaviruses are also present in Europe (Figure 1): the Tahyna virus (TAHV), which causes Valtice fever (principally transmitted by Aedes vexans),6 and two viruses transmitted by several Aedes species: Snowshoe Hare virus (isolated in European Russia) and Inkoo virus (Figure 1).5,34 These viruses cause influenza-like symptoms, with possible involvement of the central nervous system, but mortality has not been definitively associated with them.34 They infect different wild animals, particularly lagomorphs. Recrudescence of human disease due to TAHV was linked to floods in Eastern Europe and the subsequent proliferation of mosquitoes.34 Another pathogenic orthobunyavirus present in Europe is the Batai virus (Figure 1), the causative agent of influenza-like symptoms, but it is not associated with clinical disease.5
Several of these viruses were detected through serological survey or by biomolecular methods in vectors. An example is the TAHV that was detected or isolated from different sources in the 70s in European countries (Figure 1) but was also recently directly detected in mosquitoes in Italy59 and Austria.60 These results are consistent with the continuous circulation of the arboviruses within the European territory, which could result in several undiagnosed human cases of mild or sporadic disease. The possible recrudescence of these viruses may be due to the significant warming recorded in recent decades in Europe, which could change the distribution and abundance of mosquitoes or increase virus replication in mosquito vectors.34
Mosquitoes are also recorded as important vectors of tularemia in particular areas of Scandinavia. Tularemia can be transmitted from host to host by a variety of routes, including arthropods.8 Ticks are usually recognized as the primary vector of this disease, but mosquitoes are involved in the transmission in Scandinavia,28 possibly by trans-stadial transmission.61
The two agents of pulmonary and subcutaneous dirofilariasis, Dirofilaria immitis and Dirofilaria repens, are widespread in Europe, but although the incidence of this disease is probably underestimated, no fatalities directly linked to these nematodes were recorded in Europe.5
Diseases at risk of introduction
After the eradication of malaria, several human cases with a probable autochthonous origin were reported over the years, demonstrating that a malarogenic potential is still present in Europe. Infected mosquitoes could be transported directly from endemic countries, especially by airplanes, causing airport malaria; approximately 90 cases of malaria due to this phenomenon were reported between 1969 and 2001 in Europe.5 The phenomenon of airport malaria confirms the possible importation of an MBD by infected mosquitoes and highlights the need for disinfection of aircraft coming from airports situated in endemic countries.
Local populations of Anopheles mosquitoes can transmit plasmodia imported by travelers (as migrants, infected tourists, business travelers) and cause autochthonous malaria; malaria was the first imported disease in Europe.5 Several sporadic cases were reported in Europe after the eradication of the disease;5 the most significant episode was recorded in Greece. From 2009 to 2013, the Greek Health Authority reported 76 cases (seven in 2009, four in 2010, 42 in 2011, 20 in 2012, and three in 2013) without a travel history in endemic countries.62 Another localized episode of local transmission of malaria, with 18 cases, was recorded in Bulgaria in 1995–1996.5 This is an impressive discovery, but due to today’s European life habits and health system, the reintroduction of malaria seems to be low.63,64 Deterioration of these parameters may raise this potential, but the importation of a number of plasmodia adequate to reintroduce the disease seems unlikely, episodes of local transmission shall be possible, and malaria surveillance should remain a priority.5,64
Different arboviruses for which humans are reservoirs cause wide outbreaks worldwide (see “Human MBDs” section), and several infected travelers arriving infected in Europe. In the period from 2008 to 2012, the ECDC reported a total of 5,054 dengue fever cases (530 in 2008, 577 in 2009, 1,622 in 2010, 1,118 in 2011, and 1,207 in 2012), in 15 of 24 reporting countries, and 516 chikungunya cases (41 in 2008, 149 in 2009, 179 in 2010, 55 in 2011, and 51 in 2012) in seven of 22 reporting countries;65 the largest part of these cases were imported cases. Since the possibility of local transmission was first hypothesized for the establishment of the exotic tiger mosquitoes – and in 2007 this was unfortunately demonstrated by the outbreak of chikungunya in Italy, in which the virus arrived with an infected person coming from India – the already present Asian tiger mosquito transmitted the virus, causing more than 250 cases.66 Autochthonous cases of dengue and chikungunya were reported over the years in Europe mainland in Croatia, France, and Spain (Table 1 and Figure 1).12,65–72 Another episode was recorded in Madeira, a European territory near to Africa, in which the established Ae. aegypti population sustained a dengue outbreak with 2,168 probable and 1,080 confirmed dengue cases.65
These episodes testify to the concrete risk of the introduction of one of these mosquito-borne viruses into Europe. Crucial aspects regarding the possible introduction of imported cases are the outbreak strength and seasonality, which influence the frequency of the infected persons arriving in Europe during vector season. Outbreaks concordant with the European seasons are more dangerous, as shown by the recent chikungunya outbreak in the Caribbean, or the 2010 worldwide increase of dengue cases that improved the number of sick travelers arriving in Europe.7,65 Moreover, emerging viruses with a few diagnosed cases in Europe, such as the Zika virus, must carefully be surveyed.73
The duration of viremia or parasitemia in affected persons is another important parameter that must be taken into account when considering potentially importable mosquito-borne pathogens. Furthermore, infected but asymptomatic persons can infect mosquitoes, at least for DENV.74 The arrival of exotic pathogens in areas with competent mosquito populations raises concerns about their possible establishment. Despite initial concerns, CHIKV did not become endemic in Italy due to its poor vertical transmission in the vector.75 For other viruses, such as flaviviruses like DENV, vertical passage in mosquito vectors was reported, and this raises the possibility of an establishment of these viruses.13,27,34,48
The main route of introduction of exotic MBD is then not only represented by sick travelers, but also in other human activities such as animal trade (livestock, pets, zoo animals, and illegal trade) or direct transportation of infected mosquitoes by car, plane, or ship, could result in the import of mosquito-borne pathogens into Europe.5,34 Moreover, the importation of a disease by the trade of infected animal products is conceivable for some agents, as in the case of RVFV.76 Natural expansion of MBD is more improbable, but possible, as seen with infection in migratory birds.5 Characteristics of some of these pathogens raise the possibility of the use of some of these as agents of biological terrorism.
Surveillance
Surveillance is a fundamental tool that helps health authorities and decision makers by providing essential information. The knowledge of the mosquitoes present in a territory and of population densities reached are indispensable data to determine if the transmission of a pathogen can occur. Detailed ECDC guidelines for surveying exotic and native mosquitoes are available.9,21
Environmental surveillance, based on the fast laboratory screening of field-collected mosquitoes, is more suitable for detecting pathogens already present in the environment. Encouraging results were obtained in WNV surveillance in Northern Italy, which demonstrated that, with an adequate effort of sampling, circulation of the virus is recorded before human cases are documented.77 Moreover, with this kind of surveillance, other nontarget viruses can also be detected and important information collected about the species of mosquitoes present.
Surveillance of syndromic cases is more useful for viruses that could be imported via infected travelers, and it is already active for several diseases in the EU.78 The syndromic surveillance can also involve vertebrates, for diseases that cause detectable symptoms in animals (such as Rift Valley fever or West Nile disease), taking in account eventual vaccine applications. Particular attention must be paid to the seasons in which possible vectors are present in high density in a specific area. An integrated approach of both systems is necessary to obtain a valid surveillance system.12,79 The combination of obtained information and weather and environmental data could produce good models that could explain fundamental factors involving the disease appearance and circulation.13
The efficacy of disease surveillance will enable the early detection of cases and the prompt implementation of control measures.
Conclusion
Europe could be affected by MBDs, both by the recrudescence of pathogens already present on the territory and for the importation of exotic agents, particularly by sick travelers.
The increasing numbers of travelers and traded products amplify the risk that a pathogen can arrive in Europe; worldwide epidemics of MBDs in the period of mosquito activities augment the risk of introduction. High populations of exotic mosquitoes, able to transmit these viruses, are now established in Europe. The Italian epidemic of chikungunya, in which an Asian mosquito transmitted a virus of African origin in a European country, reflects the paradigm of globalization.
A potentially pathogenic agent can appear unexpectedly in a territory, as occurred for the USUV in Europe, or for the Schmallenberg virus, a virus transmitted by culicoids and pathogen for ruminants that suddenly appeared and rapidly spread in Central Europe,80 and was probably introduced into the affected area from another country in an as yet unknown way.
Correct prophylaxis and personal protection for travelers in endemic countries can reduce the potential for introduction of MBDs, but these measures are not always undertaken, and are available only for some pathogens, such as the vaccines for yellow fever and Japanese encephalitis, or prophylaxis for malaria. Socioeconomic factors, which are not easily manageable, are also involved in the diffusion of MBDs, due to illegal immigration or armed conflicts that start mass movements of refugees and deterioration of health services.
The presence of MBD represents a direct health problem for the affected area, but often poses indirect problems for the necessity of screenings of blood for transfusions and solid organs for transplants in order to exclude the presence of pathogens, with relevant costs for Health Systems, as is seen for the case of WNV.65,77,78 This raises the necessity of preparedness for the possible introduction or recrudescence of MBDs. This implies that a series of actions needs to be undertaken: the training and alerting of physicians to guarantee a correct diagnosis of these diseases and rapid detection of possibly imported cases; the supplying of diagnostic laboratories with all the necessary tests to detect these pathogens; the proper management of affected patients to avoid infection, such as allowing recovery in mosquito-free places during the period of viremia; information campaigns on personal protection against mosquito bites and for containment of peridomestic mosquitoes; larval control to contain mosquito populations or adulticide action in areas with risk of transmission, such as domiciles of imported cases of dengue and chikungunya. These actions could be provided by the establishment of a risk assessment plan as a relative measure to adopt in different scenarios, such as provided by the ECDC for WNV.79
The interconnection of multiple factors in the emergence of these pathogens is evident; for example, the decrease in the natural environment in developing countries, caused by the exploitation of natural resources, can prompt pathogens out from their sylvatic cycle. These pathogens can travel with people and reach territories thousands of kilometers far, where the conditions are suitable for causing an epidemic.
Clearly, only a coordinated approach between countries – possibly on a global scale – and an interdisciplinary approach can effectively deal with MBDs, which do not recognize borders.
Disclosure
The author reports no conflicts of interest in this work.
References
Service MW. A short history of early medical entomology. J Med Entomol. 1978;14(6):603–626. | |
Yalcindag E, Elguero E, Arnathau C, et al. Multiple independent introductions of Plasmodium falciparum in South America. PNAS. 2012; 109(2):511–516. | |
Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2010;85(2):328–345. | |
Vasilakis N, Cardosa J, Hanley KA, Holmes EC, Weaver SC. Fever from the forest: prospects for the continued emergence of sylvatic dengue virus and its impact on public health. Nat Rev Microbiol. 2011; 9(7):532–541. | |
Gratz N. Vector- and Rodent-borne Diseases in Europe and North America: Distribution, Public Health Burden and Control. New York, NY: Cambridge University Press; 2006. | |
Service MV, editor. The Encyclopedia of Arthropod-transmitted Infections of Man and Domesticated Animals. Wallingford, CT: CABI Publishing; 2001. | |
World Health Organization. A Global Brief on Vector-borne Diseases [Document number: WHO/DCO/WHD/2014.1]. Geneva, Switzerland: WHO; 2014. | |
Kettle DS, editor. Medical and Veterinary Entomology. 2nd ed. Wallingford, CT: CABI Publishing; 1995. | |
European Centre for Disease Prevention and Control. Guidelines for the Surveillance of Native Mosquitoes in Europe. Stockholm, Sweden: ECDC; 2014. | |
Busvine JR, editor. Insects and Hygiene: The Biology and Control of Insects Pests of Medical and Domestic Importance. 3rd ed. London, England: Chapman and Hall; 1980. | |
Christopher SR. Aedes aegypti (L.), the Yellow Fever Mosquito. London, England: Cambridge University Press; 1960. | |
Schaffner F, Medlock JM, Van Bortel W. Public health significance of invasive mosquitoes in Europe. Clin Microbiol Infect. 2013; 19(8):685–692. | |
Marquardt W, editor. Biology of Disease Vectors. 2nd ed. Burlington, Canada: Academic Press; 2004. | |
Becker N, Petric D, Zgomba M, et al. Mosquito and their Control. 2nd ed. Berlin, Germany: Springer; 2010. | |
Clements AN. The Biology of Mosquitoes. Volume 1: Development, Nutrition and Reproduction. Wallingford, CT: CABI Publishing; 2000. | |
Silver JB. Mosquito Ecology: Field Sampling Methods. Berlin, Germany: Springer; 2008. | |
Hawley WA. The biology of Aedes albopictus. J Am Mosq Control Assoc Suppl. 1988;1:1–39. | |
Clements AN. The Biology of Mosquitoes. Volume 2: Sensory Reception and Behavior. Wallingford, CT: CABI Publishing; 1999. | |
Ciota AT, Drummond CL, Ruby MA, Drobnack J, Ebel GD, Kramer LD. Dispersal of Culex mosquitoes (Diptera: Culicidae) from a wastewater treatment facility. J Med Entomol. 2012;49(1):35–42. | |
Hawley WA, Reiter P, Copeland RS, Pumpuni CB, Craig GB Jr. Aedes albopictus in North America: probable introduction in used tires from northern Asia. Science. 1987;236(4805):1114–1116. | |
European Centre for Disease Prevention and Control. Guidelines for the Surveillance of Invasive Mosquitoes In Europe. Stockholm, Sweden: ECDC; 2012. | |
WHO. World Health Day 2014: vector-borne diseases. Available from: http://www.who.int/campaigns/world-health-day/2014/vector-borne-diseases/en/. Accessed November 13, 2015. | |
GBD 2013 DALYs and HALE Collaborators, Murray CJ, Barber RM, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015;386(10009):2145–2191. | |
World Health Organization. World Malaria Report 2014. Geneva, Switzerland: WHO; 2014. | |
Millar SB, Cox-Singh J. Human infections with Plasmodium knowlesi-zoonotic malaria. Clin Microbiol Infect. 2015;21(7):640–648. | |
World Health Organization. Lymphatic filariasis. Fact sheet No 102 [updated May 2015]. Available from: http://www.who.int/mediacentre/factsheets/fs102/en/. Accessed November 13, 2015. | |
World Health Organization. Geographical Distribution of Arthropod-borne Diseases and their Principal Vectors [WHO/VBC/89.967]. Geneva, Switzerland: WHO; 1989. | |
Eliasson H, Broman T, Forsman M, Bäck E. Tularemia: current epidemiology and disease management. Infect Dis Clin North Am. 2006;20(2):289–311. | |
Farrar J, Hotez P, Junghanss T, Kang G, Lalloo D, White NJ, editors. Manson’s Tropical Diseases. 23rd ed. China: Saunders Ltd. 2014. | |
World Health Organization. Yellow fever. Fact sheet No 100 [updated March 2014]. Available from: http://www.who.int/mediacentre/factsheets/fs100/en/. Accessed November 13, 2015. | |
World Health Organization. Dengue and severe dengue. Fact sheet No 117 [updated May 2015]. Available from: http://www.who.int/mediacentre/factsheets/fs117/en/. Accessed November 13, 2015. | |
World Health Organization. Japanese encephalitis. Fact sheet No 386 [updated March 2014]. Available from: http://www.who.int/mediacentre/factsheets/fs386/en/. Accessed November 13, 2015. | |
Sambri V, Capobianchi M, Charrel R, et al. West Nile virus in Europe: emergence, epidemiology, diagnosis, treatment, and prevention. Clin Microbiol Infect. 2013;19(8):699–704. | |
Hubálek Z. Mosquito-borne viruses in Europe. Parasitol Res. 2008; 103(Suppl 1):S29–S43. | |
Cao-Lormeau VM, Roche C, Teissier A, et al. Zika virus, French polynesia, South Pacific, 2013. Emerg Infect Dis. 2014;20(6):1085–1086. | |
European Centre for Disease Prevention and Control. Zika virus epidemic in the Americas: potential association with microcephaly and Guillain-Barré syndrome (first update) 21 January 2016. Stockholm: ECDC; 2016. | |
World Health Organization. Chikungunya. Fact sheet No 327 [updated May 2015]. Available from: http://www.who.int/mediacentre/factsheets/fs327/en/. Accessed November 13, 2015. | |
Vasconcelos PF, Travassos da Rosa AP, Rodrigues SG, Travassos da Rosa ES, Dégallier N, Travassos da Rosa JF. Inadequate management of natural ecosystem in the Brazilian Amazon region results in the emergence and reemergence of arboviruses. Cad Saude Publica. 2001; 17(Suppl):155–164. | |
Tesh RB, Saidi S, Gajdamovic SJ, Rodhain F, Vesenjak-Hirjan J. Serological studies on the epidemiology of sandfly fever in the Old World. Bull World Health Organ. 1976;54(6):663–674. | |
Le Lay-Roguès G, Valle M, Chastel C, Beaucournu JC. Small wild mammals and arboviruses in Italy. Bull Soc Pathol Exot Filiales. 1983; 76(4):333–345. | |
Attoui H, Mohd Jaafar F, de Micco P, de Lamballerie X. Coltiviruses and seadornaviruses in North America, Europe, and Asia. Emerg Infect Dis. 2005;11(11):1673–1679. | |
Franz AWE, Kantor AM, Passarelli AL, Clem RJ. Tissue barriers to arbovirus infection in mosquitoes. Viruses. 2015;7(7):3741–3767. | |
Balenghien T, Vazeille M, Grandadam M, et al. Vector competence of some French Culex and Aedes mosquitoes for West Nile virus. Vector Borne Zoonotic Dis. 2008;8(5):589–595. | |
Engler O, Savini G, Papa A, et al. European surveillance for West Nile virus in mosquito populations. Int J Environ Res Public Health. 2013; 10(10):4869–4895. | |
FAO, WHO. Rift Valley Fever Outbreaks Forecasting Models. Rome, Italy: FAO; 2008. | |
Weaver SC, Forrester NL. Chikungunya: evolutionary history and recent epidemic spread. Antiviral Res. 2015;120:32–39. | |
Reiter P. Yellow fever and dengue: a threat to Europe? Euro Surveill. 2010;15(10):19509. | |
Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004;18(3):215–227. | |
Kutsuna S, Kato Y, Moi ML, et al. Autochthonous dengue fever, Tokyo, Japan, 2014. Emerg Infect Dis. 2015;21(3):517–520. | |
Morillon M, Mafart B, Matton T. Yellow fever in Europe during 19th century. In: Bennike P, Bodzsar EB, Suzanne C, editors. Ecological Aspects of Past Settlement in Europe. Budapest, Hungary: European Anthropological Association, Eötvös University Press; 2002. | |
Louis C. Daily newspaper view of dengue fever epidemic, Athens, Greece, 1927–1931. Emerg Infect Dis. 2012;18(1):78–82. | |
Eisen L, Monaghan AJ, Lozano-Fuentes S, Steinhoff DF, Hayden MH, Bieringer PE. The impact of temperature on the bionomics of Aedes (Stegomyia) aegypti, with special reference to the cool geographic range margins. J Med Entomol. 2014;51(3):496–516. | |
European Centre for Disease Prevention and Control. West Nile fever maps – historical data. Available from: http://ecdc.europa.eu/en/healthtopics/west_nile_fever/West-Nile-fever-maps/Pages/historical-data.aspx. Accessed November 13, 2015. | |
Barzon L, Pacenti M, Franchin E, et al. The complex epidemiological scenario of West Nile virus in Italy. Int J Environ Res Public Health. 2013;10(10):4669–4689. | |
Nikolay B. A review of West Nile and Usutu virus co-circulation in Europe: how much do transmission cycles overlap? Trans R Soc Trop Med Hyg. 2015;109(10):609–618. | |
Calzolari M, Bonilauri P, Bellini R, et al. Evidence of simultaneous circulation of West Nile and Usutu viruses in mosquitoes sampled in Emilia-Romagna region (Italy) in 2009. PLoS One. 2010; 5(12):e14324. | |
Calzolari M, Bonilauri P, Bellini R, et al. Usutu virus persistence and West Nile virus inactivity in the Emilia-Romagna region (Italy) in 2011. PLoS One. 2013;8(5):e63978. | |
Suhrbier A, Jaffar-Bandjee MC, Gasque P. Arthritogenic alphaviruses – an overview. Nat Rev Rheumatol. 2012;8(7):420–429. | |
Calzolari M, Bonilauri P, Bellini R, et al. Arboviral survey of mosquitoes in two northern Italian regions in 2007 and 2008. Vector Borne Zoonotic Dis. 2010;10(9):875–884. | |
Sonnleitner ST, Lundström J, Baumgartner R, et al. Investigations on California serogroup orthobunyaviruses in the Tyrols: first description of Tahyna virus in the Alps. Vector Borne Zoonotic Dis. 2014; 14(4):272–277. | |
Lundström JO, Andersson AC, Bäckman S, Schäfer ML, Forsman M, Thelaus J. Transstadial transmission of Francisella tularensis holarctica in mosquitoes, Sweden. Emerg Infect Dis. 2011;17(5):794–799. | |
Hellenic Center for Disease Control and Prevention. Epidemiological surveillance report, malaria in Greece, 2014. Available from: http://www.keelpno.gr/Portals/0/%CE%91%CF%81%CF%87%CE%B5%CE%AF%CE%B1/%CE%95%CE%BB%CE%BF%CE%BD%CE%BF%CF%83%CE%AF%CE%B1/2015/Malaria%20annual%20report_2014_EN_final.pdf. Accessed November 13, 2015. | |
Romi R, Sabatinelli G, Majori G. Could malaria reappear in Italy? Emerg Infect Dis. 2001;7(6):915–919. | |
Petersen E, Severini C, Picot S. Plasmodium vivax malaria: a re-emerging threat for temperate climate zones? Travel Med Infect Dis. 2013;11(1):51–59. | |
European Centre for Disease Prevention and Control. Annual Epidemiological Report 2014 – Emerging and Vector-borne Diseases. Stockholm, Sweden: ECDC; 2014. | |
Rezza G, Nicoletti L, Angelini R, et al; CHIKV study group. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370(9602):1840–1846. | |
Grandadam M, Caro V, Plumet S, et al. Chikungunya virus, southeastern France. Emerg Infect Dis. 2011;17(5):910–913. | |
Institut de Veille Sanitaire. Chikungunya et dengue – Données de la surveillance renforcée en 2014. Available from: http://www.invs.sante.fr/Dossiers-thematiques/Maladies-infectieuses/Maladies-a-transmission-vectorielle/Chikungunya/Donnees-epidemiologiques. Accessed December 26, 2016. | |
La Ruche G, Souarès Y, Armengaud A, et al. First two autochthonous dengue virus infections in metropolitan France, September 2010. Euro Surveill. 2010;15(39):19676. | |
Gjenero-Margan I1, Aleraj B, Krajcar D, et al. Autochthonous dengue fever in Croatia, August–September 2010. Euro Surveill. 2011;16(9). pii: 19805. | |
Schmidt-Chanasit J, Haditsch M, Schoneberg I, Gunther S, Stark K, Frank C. Dengue virus infection in a traveller returning from Croatia to Germany. Euro Surveill. 2010;15(40). pii: 19677. | |
European Centre for Disease Prevention and Control. Chikungunya Case in Spain without Travel History to Endemic Areas. Stockholm, Sweden: ECDC; 2015. | |
Zammarchi L, Stella G, Mantella A, et al. Zika virus infections imported to Italy: clinical, immunological and virological findings, and public health implications. J Clin Virol. 2015;63:32–35. | |
Duong V, Lambrechts L, Paul RE, et al. Asymptomatic humans transmit dengue virus to mosquitoes. Proc Natl Acad Sci U S A. 2015; 112(47):14688–14693. | |
Bellini R, Medici A, Calzolari M, et al. Impact of Chikungunya virus on Aedes albopictus females and possibility of vertical transmission using the actors of the 2007 outbreak in Italy. PLoS One. 2012; 7(2):e28360. | |
EFSA Opinion. The Risk of a Rift Valley Fever Incursion and its Persistence within the Community [Question number: EFSA-Q-2004-050]; 2005. | |
Bellini R, Calzolari M, Mattivi A, et al. The experience of West Nile virus integrated surveillance system in the Emilia-Romagna region: five years of implementation, Italy, 2009 to 2013. Euro Surveill. 2014; 19(44). pii: 20953. | |
Zeller H, Marrama L, Sudre B, Van Bortel W, Warns-Petit E. Mosquito-borne disease surveillance by the European Centre for Disease Prevention and Control. Clin Microbiol Infect. 2013;19(8):693–698. | |
European Centre for Disease Prevention and Control. West Nile virus risk assessment tool. Available from: http://ecdc.europa.eu/en/healthtopics/west_nile_fever/risk-assessment-tool/Pages/risk-assessment-tool.aspx. Accessed June 1, 2015. | |
Beer M, Conraths FJ, van der Poel WH. ‘Schmallenberg virus’ – a novel orthobunyavirus emerging in Europe. Epidemiol Infect. 2013; 141(1):1–8. | |
King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses. San Diego, CA: Elsevier Academic Press; 2012. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.