Back to Journals » Infection and Drug Resistance » Volume 15
Mortality Risk Factors of Early Neonatal Sepsis During COVID-19 Pandemic
Authors Kolesnichenko SI , Kadyrova IA, Lavrinenko AV , Zhumadilova ZA , Avdienko OV, Vinogradskaya YV, Fominykh YA, Panibratec LG, Akhmaltdinova LL
Received 21 September 2022
Accepted for publication 22 October 2022
Published 31 October 2022 Volume 2022:15 Pages 6307—6316
DOI https://doi.org/10.2147/IDR.S390723
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Svetlana I Kolesnichenko,1,* Irina A Kadyrova,1 Alyona V Lavrinenko,1 Zhibek A Zhumadilova,1 Olga V Avdienko,1 Yelena V Vinogradskaya,2 Yevgeniy A Fominykh,3 Lyudmila G Panibratec,3 Lyudmila L Akhmaltdinova1,4,*
1Shared Resource Laboratory, Research Centre, Karaganda Medical University, Karaganda, Kazakhstan; 2Regional Clinical Hospital of Karaganda, Perinatal Center No. 1, Karaganda, Kazakhstan; 3Regional Clinical Hospital of Karaganda, Perinatal Center No. 2, Karaganda, Kazakhstan; 4National Scientific Cardiac Surgery Center, Nur-Sultan, Kazakhstan
*These authors contributed equally to this work
Correspondence: Svetlana I Kolesnichenko, Shared Resource Laboratory, Research Centre, Karaganda Medical University, 40 Gogol St, Karaganda, 100008, Kazakhstan, Tel +7 702 599 0225, Email [email protected]
Purpose: This study aimed to determine predisposing factors for negative outcome in infants with early neonatal sepsis during COVID-19.
Patients and Methods: A prospective cohort study of 172 newborns up to 4 days diagnosed with neonatal sepsis was carried out in Karaganda (Kazakhstan). The microbiological examination was used to identify a causative agent of bloodstream infection. ELISA was performed to determine the total anti-SARS-CoV-2 antibodies. Gestational age, mode of delivery, birth weight, C-reactive protein and procalcitonin levels, comorbidities, type of pathogen, duration of hospitalization and mother’s infection diseases were used for statistical analysis.
Results: Mortality in infants with neonatal sepsis was 22% (38/172). Anti-SARS-CoV-2 antibodies were detected in 68.3% of the newborns. Culture-negative ELBW infants have a 5.3-fold higher risk of death (p< 0.001). Low gestational age and a shorter period of hospitalization were statistically associated with fatality. CRP is generally higher in deceased children (p=0.002). Necrotizing enterocolitis (p< 0.001), pneumonia (p=0.009) and anemia (p=0.016) were significantly associated with negative outcome. And, 31.4% of the infants with sepsis had positive blood cultures. The leading cause of sepsis in newborns was CoNS – 57%.
Conclusion: During COVID-19 pandemic neonatal sepsis mortality was associated with low birth weight, gestational age, and comorbidities as in non-pandemic time. The relationship between COVID-19 in the mother and neonatal mortality was not found. However, anti-SARS-CoV-2 antibodies were detected in more than half of newborns.
Keywords: neonatal sepsis, preterm infants, mortality, coagulase-negative staphylococci, COVID-19
Introduction
One of the crucial and pivotal stages in life is the neonatal period. The increased vulnerability during the neonatal period lies in the susceptibility to various infections. One of the most dangerous and serious problems that can occur in infants is neonatal sepsis. Studies estimated that between 1.3 million and 3.9 million annual neonatal sepsis cases and between 400,000 and 700,000 annual deaths occur worldwide.1 The global incidence of neonatal sepsis is 2824 per 100,000 live births according to data estimated in a recent meta-analysis.2
The COVID-19 pandemic had an effect on all areas of medicine and the lives of humanity. For the period of 2021, a 7.0% increase in infant mortality was noted in Kazakhstan and amounted to 8.35 per 1000 live births against 7.79 for the same period in 2020, according to the report of the Ministry of Health of the Republic of Kazakhstan for 2021.3
It has been established that during the COVID-19 pandemic, pregnant women disrupted their regular antenatal care, which led to an increase in the incidence of preterm and hypoxic-ischemic encephalopathy in neonates.4
The epidemiology of neonatal sepsis ultimately varies across geographies. In Africa and South Asia, gram-positive pathogens are the most common in infants,5 while in the USA and Europe (high-income countries) we can see both gram-positive and gram-negative sepsis and it changes through time.6 According to the last systematic reviews, sepsis caused by gram-negative pathogens is associated with higher mortality rates,7,8 on the contrary, coagulase-negative staphylococci (CoNS) correlate with neurodevelopmental impairment and cerebral palsy in sepsis survivors.9
Previous studies have identified lower weight and gestational age, type of pathogen, and higher base deficit as factors associated with mortality.10,11
During the coronavirus infection pandemic, there were cases of severe complications and death of newborns whose mothers suffered COVID-19 disease during pregnancy or childbirth. It was also noted that pregnant women with COVID-19 disease often gave birth prematurely, which also increased the risk of morbidity and death.12
Assumedly, the COVID-19 pandemic contributed to additional factors that raised infant mortality in the Republic of Kazakhstan.
In this study, we attempted to assess the predisposing factors for negative outcomes among infants with early neonatal sepsis during COVID-19 pandemic.
Materials and Methods
Study Participants
A prospective cohort study was conducted among infants diagnosed with neonatal sepsis in the period from October 2020 to December 2021. The Bioethics Committee of the Karaganda Medical University No. 19 dated 08/05/2019 approved the study. Parents gave written informed consent to participate in the study. All infants, who were admitted to intensive care units (ICU) and met including criteria, were involved in the study (Figure 1). The research included (n=172) newborns up to 4 days of life from the intensive care units of regional maternity hospitals in Karaganda city (Kazakhstan). Infants with one or more of clinical features of sepsis according to the protocol of diagnosis of the Republic of Kazakhstan have been selected13: fever >38.3°C or hypothermia <36.0°C, tachycardia >90 bpm or tachypnea >20 breaths/min or PaCO2 <4.3 kPa (32 mmHg), leukocytosis (white blood cells >12×109/L) or leukopenia (white blood cells <4×10^9/L), normal white blood cell count with >10% immature forms, hyperglycemia (blood glucose >7.7 mmol/L) in the absence of diabetes.
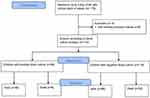 |
Figure 1 Patient flow chart for infants included in the research. |
The exception criteria were as follows: patients born to HIV, high-dose glucocorticosteroid therapy, primary immunodeficiency state, bleeding, severe malformations, acute hemolytic disease of the newborn, refusal of the parent(s) or legal representative of the patient to participate in the study. In total, four patients were excluded according to exception criteria.
Negative outcome was assessed by the endpoint of mortality at the end of hospitalization. The following factors were considered as factors influencing the fatal outcome: gestational age, mode of delivery, birth weight, C-reactive protein (CRP) and procalcitonin (PCT) levels, comorbidities (pneumonia, anemia, necrotizing enterocolitis, CNS involvement, mild congenital malformations), type of pathogen, mother’s infection diseases (urinary tract infection, skin and soft tissue infection, COVID-19, chorioamnionitis, other infection) and duration of infant’s hospitalization were evaluated. Infectious diseases at the time of birth were taken into account to assess the impact of maternal infections on outcomes in infants. For COVID-19, a PCR-verified positive test throughout pregnancy was taken into account. Other infections included non-COVID-19 respiratory viral infections and sexually transmitted infections (viral and bacterial).
Microbiological Testing
All infants were tested for bloodstream infections. A microbiological study was conducted on the base of the shared resource laboratory of the Medical University of Karaganda (Kazakhstan). Peripheral blood sampling in a volume of 1–2 mL was carried out directly in intensive care units from patients with aseptic technique in BD BACTEC™ Peds Plus vials before the beginning of antibiotic therapy. The vials were delivered to the laboratory for further incubation using the BD BACTEC ™ FX blood culture system. Express diagnosing of positive blood culture was performed according to the previously described protocol.14,15 Simultaneously, positive blood culture samples were cultured on blood agar for 18–20 hours to exclude mixed cultures and to get isolated colonies. Identification of pathogens was performed using the matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) Microflex mass spectrometer (Bruker Daltonics, Germany).16
Quantitation of Anti-SARS-CoV-2 Ab via ELISA
Anti-SARS-CoV-2 total antibodies enzyme-linked immunosorbent assay (ELISA) was determined in 101 patients using the Diagnostic Kit for Total Antibody to SARS-CoV-2 (Wantai, China) on the Evolis 100 ELISA reader (Bio-Rad) according to the manufacturers’ protocols. The cut-off value was taken according to the manufacturer’s instructions = negative control (Nc) + 0.16. The result was expressed as a positivity coefficient, the ratio of the optical density of the sample to the cut-off value.
Statistical Analysis
Statistical analysis was carried out in SPSS Statistics 20 software, using logistic regression. Logistic regression was conducted according to convenient conditions considering the independence of errors, absence of multicollinearity, and lack of influential outliers. Results for independent variables are reported as odds ratio (ORs) with p-value. Outcomes such as mortality and survival were presented as binary value (0 and 1).
Comparison between groups was performed using the χ2 test (Pearson’s chi-squared test), Mann–Whitney U-test for quantitating variables.
Results
Among 176 infants admitted to the ICU, 172 met including criteria. During the entire period of hospitalization 22% of all newborns who did not survive. Among patients diagnosed with sepsis 54/172 (31.4%) infants had positive blood cultures. Newborns were divided into culture-positive and culture-negative groups according to blood culture analysis (Figure 1).
There were 11% (6/54) of deceased infants in the culture-positive sepsis group, and 27.1% (32/118) in the negative blood culture group. The mortality odds is 2.97-fold greater in culture-negative sepsis group. In general, the chance of death in the group with culture-negative sepsis was 2.97 ± 0.48 times higher.
Analysis of the contribution of the parameter to the fatal outcome by groups is shown in Table 1.
 |
Table 1 Effect of Parameter on Fatal Outcome in Culture-Proven and Culture-Negative Sepsis Groups |
There was not found a statistical significant association between COVID-19 disease in the mother and a negative outcome in the fetus (p>0.05). Most infants from mothers with COVID-19 disease were in the culture-negative sepsis group (13/16) and 38.5% (5/13) of them had a negative outcome. Separately, the presence of anti-SARS-CoV-2 Ab in the part of the cohort for which it became available was tested (Table 2). Surprisingly, 68.3% (69/101) of newborns had a positive analysis for total anti-SARS-CoV-2 Ab, which did not match the information provided by mothers about the disease or vaccination. The percentage of positive samples was approximately equal in the subgroups of the dead and survivors. Although the median coefficient was significantly lower in the group of dead infants; however, this was not statistically significant.
 |
Table 2 Results of Anti-SARS-CoV-2 Total Antibody in All Newborns with Sepsis |
As a result of the analysis, it was found that birth weight was strongly associated with fatal outcomes in infants with both positive (p=0.044) and negative blood cultures (p<0.001). Moreover, in children with sepsis, newborns with extremely low birth weight (ELBW) died more often (<0.001). Interestingly, culture-negative ELBW infants have a 5.3±0.77 fold higher risk of death (p<0.001) than blood culture-positive infants, and even newborns of normal weight (>2500 g) had a higher risk of death in the culture-negative group, OR 7.5 ± 1.57 (Figure 2).
Gestational age was statistically associated with poor outcomes in culture-positive sepsis (p=0.035) and culture-negative sepsis (p<0.001) groups, but prematurity did not affect the outcome.
We did not find the impact of PCT level on mortality odds in both groups. However, we observed that CRP is generally higher in deceased infants (p=0.002), but, for reasons that can be hardly explained, this is more true for culture-negative sepsis (p=0.003).
Newborns had met negative outcome in a shorter period. In the culture-proven sepsis group, dead patients had a longer period of treatment (median, IQR: 11.5 days (7.5–17.75)), while in culture-negative infants this period was shorter (median, IQR: 5 days (2–12.25) with p<0.001).
Of the total neonatal sepsis patients, 170 (98%) had comorbidities. The most common comorbid diagnosis was central nervous system (CNS) involvement, followed by pneumonia and mild congenital malformations (mostly non-surgical heart defects). Necrotizing enterocolitis (NE), (p<0.001), pneumonia (p=0.009) and anemia (p=0.016) were found to be significantly associated with negative outcome in all sepsis cohort and in the culture-negative sepsis group. The share of infants with concomitant NE was two times higher (13%) in the culture-positive group, than in the culture-negative group (7.6%) (p<0.001). Also, poor outcome in culture-proven sepsis group was associated with next comorbidities: NE, (p<0.001), CNS involvement (p<0.001), and mild congenital malformations (p<0.001).
According to our data, mode of delivery, mother’s infection and type of pathogen was not associated with a negative outcome. However, in the assessment of the mother’s infection, parameter chorioamnionitis did not get a significant level (p= 0.057).
A total of 56 pathogens were isolated from positive blood cultures. There were two cases of mixed culture that was presented by two types of bacteria. The most common cause of sepsis in newborns was CoNS – 57%, followed by Klebsiella pneumoniae - 14% and Enterococcus faecium - 7%. Other pathogens met in individual cases (Figure 3). Staphylococcus epidermidis (30%) was the most frequently isolated pathogen among CoNS. There was no influence of pathogen type on the outcome of sepsis in culture-proven sepsis group.
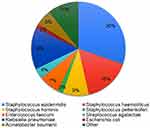 |
Figure 3 The etiology of neonatal sepsis. |
Discussion
Considering that in terms of pathophysiology and epidemiology, fundamentally sepsis may differ as culture-positive and culture-negative sepsis, infants were divided into two groups according to this aspect.17 All infants included in cohort presented with clinical signs of sepsis; however, pathogen was determined just in 31.4% cases.
The findings from this analysis showed rather high mortality of newborns with neonatal sepsis of 22%. Data is similar on such cases in the countries of South-East Asia,18 where the percentage of negative outcome among newborns is between 19% and 22.6%. Nonetheless, global mortality ranges from 1% to 5% in sepsis and 9% to 20% in severe sepsis.7 The possible results can be explained by the difference in the level of care and diagnostic capabilities provided to pregnant women in high-income (HIC) and low- and middle-income countries (LMIC).7
Noteworthy, that most infants from mothers with COVID-19 disease were in the culture-negative sepsis group and 38.5% of them had a fatal outcome. Significant results have been observed among preterm infants and mothers with comorbidities or complicated coronavirus infection19,20 although evidence on this is ambiguous and outcome likely depends on the maternal condition and gestational age.
Additionally, quantification of anti-SARS-CoV-2 Ab and found an unusually high level of total antibodies in newborns (68.3%). During the cohort recruitment period, vaccination for pregnant women was not available, and general vaccination was only gaining momentum, so we believe this level is related to maternal infection before or, in a lesser extent, during pregnancy. However, we did not find significant differences that could be associated with fatality.
Birth weight remains the main important parameter for poor outcome of sepsis, as described in previous studies.6,21 In assessment of all cohort, high mortality odds was noted in infants with ELBW (<1000g) – 65.8% in comparison to other weight categories. Preterm newborns less than 1000g of weight and <29 weeks’ gestation had a significantly higher risk of early-onset sepsis, respiratory distress syndrome, severe intraventricular hemorrhage or periventricular leukomalacia and bronchopulmonary dysplasia – conditions, that are associated with high mortality rate.22 Considering the immaturity of many body systems, lower gestational age infants require special monitoring of vital signs and longer hospital stays, which entails additional risks such as sepsis, NE and neurological disorders, and therefore the survival rate of this category is low.23
As for inflammatory markers in different ages preterm infants have a less pronounced response compared to term infants.24 In our study, CRP behaved atypically. We obtained CRP values close to normal in our patients; the median level was higher in deceased patients, but mostly in the culture-negative sepsis group. Normal CRP at the onset of clinical sepsis inherent for low birth weight neonates, very low gestational age infants and can be a result of antibiotic therapy.25 Infants that are born extremely prematurely or with a very low birth weight are likely to have fewer immunomodulatory or pro-inflammatory proteins after bacterial infection, because CRP is the final product of a cytokine response after tissue injury.23 Causative pathogens affect CRP level; thus, Lai et al presented that CoNS episodes of BSI were associated with normal CRP, while in cases with gram-negative sepsis, CRP level was >10 mg/L.25
It should be noted PCT level was insignificant in our cohort. According to earlier data, PCT level is variable in first 72 h, and its sensitivity ranged from 47.4% (95% CI 27.3–68.3) to 100% (95% CI 67.6–100) and specificity from 35.3% (95% CI 17.3–58.7) to 100% (95% CI 96.8–100).26
Consistent with previous data analyses, younger age, presence of comorbidities, and multiple organ dysfunction conferred increased mortality risk in sepsis patients.27 As in our research, children with NE, pneumonia and anemia had a significant role in mortality in the culture-negative group and NE, CNS involvement and mild congenital malformations in the culture-proven group. Moreover, in other studies, the authors highlighted other comorbidities associated with death: asphyxia, respiratory distress syndrome, hypothermia, congenital surgical anomalies, hypoglycemia.28
When studying the contribution of maternal infections to the development of sepsis and negative outcome, we were attracted by the significance of chorioamnionitis in the development of sepsis, but the rates did not reach a statistically significant level (p=0.057). Nevertheless, previous studies have identified the impact of maternal chorioamnionitis on the development of neonatal sepsis.29
Concerning hospitalization time, it was shorter in infants with negative outcome in the studied research; this is can be explained as a critical period of newborns life and part of infants did not overcome it. As reported in past studies, survivors of neonatal sepsis often require prolonged hospitalization; however, it puts them in a risk group of long-term sequelae including chronic lung disease and adverse neurodevelopmental outcomes.23 The duration of hospitalization for EOS is shorter than for late-onset sepsis (LOS) according to studies, which is logically comparable to naked results.30
Positive blood cultures, although they are the gold standard, do not always confirm the clinical picture and determine the course of the disease, as in our case. Among infants with positive blood cultures, there are more survivors (88.8%) than in the group with negative blood growth (72.8%). The presence of a pathogen in the blood helps the doctor to adjust the therapy adequately, which may be associated with better survival in the culture-proven sepsis group. In the course of our study, it was found that in the culture-negative sepsis group, the condition tends to develop according to the worst-case scenario. The reason for the high number of culture-negative cases is not clear, and diagnostic criteria used in the different publications vary substantially.31 This may have a number of reasons, such as a small volume of blood samples to study, disruption of transportation steps, or the mother’s antibiotic therapy before and during childbirth. As it was found out, antibiotic treatment within 4 days of a bacteremic episode has been associated with false-negative blood cultures and low-level bacteremia.32 According to Piantino et al, systemic inflammatory response syndrome (SIRS) among very low birth weight (VLBW) and ELBW infants frequently had a negative blood cultures because of vary aggravating conditions, like viral infections, congenital infections, cardiovascular, metabolic and neurological disorders.33 There is increasing evidence for viral pathogens associated with sepsis-like syndrome in preterm infants: cytomegalovirus, enterovirus, parechovirus, coxsackie, adenovirus, parainfluenza, rhinovirus, coronavirus.33 However, other authors have been noted opposite results, where children with culture-proven sepsis had higher mortality rate.34
The etiological agents of neonatal sepsis differ by region and time of seeding (EOS or LOS). For example, in a study in the Canadian study, the most common bacterial isolates were Escherichia coli, group B Streptococcus, and Staphylococcus aureus.11 In contrast, in a Korean35 study assessing medical records over 20 years, the main causative agents of neonatal sepsis were CoNS (53%), S. aureus (12.5%) and K. pneumoniae (7%). Also in the retrospective analysis of 13-year period in north Italy hospitals, leading pathogen of EOS and LOS were CoNS,36 which is consistent with the findings in this study. It is important to note, in the current study, S. aureus was not isolated from the blood of newborns, and as the latest data collected in Kazakhstan, shows that S. aureus fades into the background.37 The results of this study are comparable with the data obtained earlier on the predominant role of CoNS in the development of sepsis.14 Interestingly, CoNS are more likely to cause bloodstream infections in neonates, but are less likely to result in death and such cases more likely to end up as recovery.38
Our research had some limitations due to the vulnerabilities of the studied samples volume; short duration of the study could not assess the long-term effects and outcomes; lack of confirmed maternal COVID-19 disease. Although in our work, we did not find an association between prematurity and mortality, other researchers, when evaluating larger samples, claim the opposite.
Conclusion
During the pandemic, an increase in infant mortality was noted in the Republic of Kazakhstan, and it is logical to assume that COVID-19 affects mortality; however, we did not find a relationship between COVID-19 disease in the mother and neonatal mortality. Even so, we found the presence of antibodies in more than half of the examined newborns. Our research allowed to illustrate the complications of the pandemic period – insufficiency of resources and problems in efficient distribution of them, a general panic that in some cases was a barrier for pregnant women to seek healthcare, and overall information deficiency. Considering all these factors, the pandemic period affected the increase in infant mortality more indirectly than directly. The leading causes of infant mortality are still low birth weight and low gestational age.
On the other hand, the frequency of negative outcomes was more often noted in the group with negative sepsis. Also, children in this group had higher mortality rate due to the immaturity of the body systems and the development of concomitant pathology (necrotizing enterocolitis, pneumonia, and anemia), the complexity of infectious process diagnosing and weak immune response.
CoNS was the most common causative agent of sepsis and its early detection contributed to a favorable outcome. PCT levels were uninformative in low birth weight preterm infants.
Ethics Approval and Informed Consent
The Bioethics Committee of the Karaganda Medical University No. 19 dated 08/05/2019 approved the study. All patients provided informed consent, and all procedures were conducted according to the Declaration of Helsinki.
Funding
This work was supported by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan (grant no. AP08857386).
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Global report on the epidemiology and burden of sepsis: current evidence, identifying gaps and future directions. World Health Organization; 2020. Available from: https://apps.who.int/iris/handle/10665/334216.
2. Sands K, Spiller OB, Thomson K, Portal EA, Iregbu KC, Walsh TR. Early-onset neonatal sepsis in low- and middle-income countries: current challenges and future opportunities. IDR. 2022;15:933–946. doi:10.2147/IDR.S294156
3. Report on the implementation of the development plan of the state authority for the period 2021. The Ministry of Health of the Republic of Kazakhstan; 2021. Available from: https://www.gov.kz/memleket/entities/dsm/documents/details/269952?lang=ru.
4. Hekimoğlu B, Aktürk Acar F. Effects of COVID-19 pandemic period on neonatal mortality and morbidity. Pediatr Neonatol. 2022;63:78–83. doi:10.1016/j.pedneo.2021.08.019
5. Hamer DH, Darmstadt GL, Carlin JB, et al. Etiology of bacteremia in young infants in six countries. Pediatr Infect Dis J. 2015;34(1):e1–8. doi:10.1097/INF.0000000000000549
6. Bizzarro MJ, Shabanova V, Baltimore RS, Dembry L-M, Ehrenkranz RA, Gallagher PG. Neonatal sepsis 2004–2013: the rise and fall of coagulase-negative staphylococci. J Pediatr. 2015;166(5):1193–1199. doi:10.1016/j.jpeds.2015.02.009
7. Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. 2018;6(3):223–230. doi:10.1016/S2213-2600(18)30063-8
8. Dong Y, Glaser K, Speer CP. Late-onset sepsis caused by Gram-negative bacteria in very low birth weight infants: a systematic review. Expert Rev Anti Infect Ther. 2019;17(3):177–188. doi:10.1080/14787210.2019.1568871
9. Dong Y, Speer CP, Glaser K. Beyond sepsis: staphylococcus epidermidis is an underestimated but significant contributor to neonatal morbidity. Virulence. 2018;9(1):621–633. doi:10.1080/21505594.2017.1419117
10. Shah J, Jefferies A, Yoon E, Lee S, Shah P; on behalf of the Canadian Neonatal Network. Risk factors and outcomes of late-onset bacterial sepsis in preterm neonates born at < 32 weeks’ gestation. Amer J Perinatol. 2014;32(07):675–682.
11. Baczynski M, Kharrat A, Zhu F, et al. Bloodstream infections in preterm neonates and mortality-associated risk factors. J Pediatr. 2021;237:206–212.e1. doi:10.1016/j.jpeds.2021.06.031
12. Mirbeyk M, Saghazadeh A, Rezaei N. A systematic review of pregnant women with COVID-19 and their neonates. Arch Gynecol Obstet. 2021;2021:1–34.
13. Ministry of Health of the Republic of Kazakhstan. Bacterial Sepsis of the Newborn. Clinical Protocol of Diagnosis and Treatment No. 10 Dated July 4, 2014. Ministry of Health of the Republic of Kazakhstan; 2014.
14. Kolesnichenko SI, Lavrinenko AV, Akhmaltdinova LL. Bloodstream infection etiology among children and adults. Int J Microbiol. 2021;2021:e6657134. doi:10.1155/2021/6657134
15. Lin J-F, Ge M-C, Liu T-P, Chang S-C, Lu -J-J. A simple method for rapid microbial identification from positive monomicrobial blood culture bottles through matrix-assisted laser desorption ionization time-of-flight mass spectrometry. J Microbiol Immunol Infect. 2018;51(5):659–665. doi:10.1016/j.jmii.2017.03.005
16. Croxatto A, Prod’hom G, Greub G. Applications of MALDI-TOF mass spectrometry in clinical diagnostic microbiology. FEMS Microbiol Rev. 2012;36(2):380–407. doi:10.1111/j.1574-6976.2011.00298.x
17. Li Y, Guo J, Yang H, et al. Comparison of culture-negative and culture-positive sepsis or septic shock: a systematic review and meta-analysis. Crit Care. 2021;25(1):167. doi:10.1186/s13054-021-03592-8
18. Jiang S, Hong L, Gai J, et al. Early-onset sepsis among preterm neonates in China, 2015 to 2018. Pediatr Infect Dis J. 2019;38(12):1236–1241. doi:10.1097/INF.0000000000002492
19. Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9(1):51–60. doi:10.21037/tp.2020.02.06
20. Estrada-Chiroque LM, Orostegui-Arenas M, Burgos-Guanilo MDP, Amau-Chiroque JM. Clinical characteristics and maternal perinatal outcome in women with a confirmed diagnosis of COVID-19 in a hospital in Peru. Retrospective cohort study. Rev Colomb Obstet Ginecol. 2022;73(1):28–38. doi:10.18597/rcog.3776
21. Sung T-J, Sohn JA, Oh S, Lee JA. The influence of the variation in sepsis rate between neonatal intensive care units on neonatal outcomes in very-low-birth-weight infants. Sci Rep. 2020;10:6687. doi:10.1038/s41598-020-63762-6
22. Stoll BJ, Hansen N, Fanaroff AA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med. 2002;347(4):240–247. doi:10.1056/NEJMoa012657
23. Skogstrand K, Hougaard DM, Schendel DE, Bent N-P, Sværke C, Thorsen P. Association of preterm birth with sustained postnatal inflammatory response. Obstet Gynecol. 2008;111(5):1118–1128. doi:10.1097/AOG.0b013e31817057fb
24. Turner MA, Power S, Emmerson AJB. Gestational age and the C reactive protein response. Arch Dis Child. 2004;89(3):F272–F273. doi:10.1136/adc.2002.011288
25. Lai M-Y, Tsai M-H, Lee C-W, et al. Characteristics of neonates with culture-proven bloodstream infection who have low levels of C-reactive protein (≦10 mg/L). BMC Infect Dis. 2015;15(1):320. doi:10.1186/s12879-015-1069-7
26. Chiesa C, Lucia OJF, Bonci E, Hofer N, Resch B, Resch B. Early-onset neonatal sepsis: still room for improvement in procalcitonin diagnostic accuracy studies. Medicine. 2015;94(30):e1230. doi:10.1097/MD.0000000000001230
27. Cook A, Hsia Y, Russell N, et al. Association of empiric antibiotic regimen discordance with 30-day mortality in neonatal and pediatric bloodstream infection—a global retrospective cohort study. Pediatr Infect Dis J. 2021;40(2):137–143. doi:10.1097/INF.0000000000002910
28. Schmatz M, Srinivasan L, Grundmeier RW, et al. Surviving sepsis in a referral neonatal intensive care unit: association between time to antibiotic administration and in-hospital outcomes. J Pediatr. 2020;217:59–65.e1. doi:10.1016/j.jpeds.2019.08.023
29. Beck C, Gallagher K, Taylor LA, Goldstein JA, Mithal LB, Gernand AD. Chorioamnionitis and risk for maternal and neonatal sepsis: a systematic review and meta-analysis. Obstet Gynecol. 2021;137(6):1007–1022. doi:10.1097/AOG.0000000000004377
30. Štimac M, Vukelić V, Peruško Matasić N, Juretić E, Kos M, Babić DE. Effect of chorioamnionitis on mortality, early onset neonatal sepsis and bronchopulmonary dysplasia in preterm neonates with birth weight of ≤1500 grams. Coll Antropol. 2014;38(1):167–171.
31. Fjalstad JW, Stensvold HJ, Bergseng H, et al. Early-onset sepsis and antibiotic exposure in term infants: a nationwide population-based study in Norway. Pediatr Infect Dis J. 2016;35(1):1–6. doi:10.1097/INF.0000000000000906
32. Kellogg JA, Ferrentino FL, Goodstein MH, Liss J, Shapiro SL, Bankert DA. Frequency of low level bacteremia in infants from birth to two months of age. Pediatr Infect Dis J. 1997;16(4):381–385. doi:10.1097/00006454-199704000-00009
33. Piantino JH, Schreiber MD, Alexander K, Hageman J. Culture negative sepsis and systemic inflammatory response syndrome in neonates. NeoReviews. 2013;14(6):e294–305. doi:10.1542/neo.14-6-e294
34. Jatsho J, Nishizawa Y, Pelzom D, Sharma R. Clinical and bacteriological profile of neonatal sepsis: a prospective hospital-based study. Int J Pediatr. 2020;2020:e1835945. doi:10.1155/2020/1835945
35. Song WS, Park HW, Oh MY, et al. Neonatal sepsis-causing bacterial pathogens and outcome of trends of their antimicrobial susceptibility a 20-year period at a neonatal intensive care unit. Clin Exp Pediatr. 2021;65:350–357. doi:10.3345/cep.2021.00668
36. Mariani M, Parodi A, Minghetti D, et al. Early and late onset neonatal sepsis: epidemiology and effectiveness of empirical antibacterial therapy in a III level neonatal intensive care unit. Antibiotics. 2022;11(2):284. doi:10.3390/antibiotics11020284
37. Kaliyeva SS, Lavrinenko AV, Tishkambayev Y, et al. Microbial landscape and antibiotic susceptibility dynamics of skin and soft tissue infections in Kazakhstan 2018–2020. Antibiotics. 2022;11(5):659. doi:10.3390/antibiotics11050659
38. Cantey JB, Anderson KR, Kalagiri RR, Mallett LH. Morbidity and mortality of coagulase-negative staphylococcal sepsis in very-low-birth-weight infants. World J Pediatr. 2018;14(3):269–273. doi:10.1007/s12519-018-0145-7
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

