Back to Journals » Infection and Drug Resistance » Volume 15
Mortality-Related Risk Factors and Novel Antimicrobial Regimens for Carbapenem-Resistant Enterobacteriaceae Infections: A Systematic Review
Authors Hu Q, Chen J, Sun S , Deng S
Received 27 September 2022
Accepted for publication 9 November 2022
Published 28 November 2022 Volume 2022:15 Pages 6907—6926
DOI https://doi.org/10.2147/IDR.S390635
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Qin Hu,1– 4,* Jinglan Chen,1,2,5,* Shusen Sun,1,6 Sheng Deng1,2,5
1Department of Pharmacy, Xiangya Hospital, Central South University, Changsha, People’s Republic of China; 2Institute for Rational and Safe Medication Practices, National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, People’s Republic of China; 3Hospital Institute Administration, Central South University, Changsha, People’s Republic of China; 4Xiangya Health Development Research Center, Changsha, People’s Republic of China; 5The Hunan Institute of Pharmacy Practice and Clinical Research, Changsha, People’s Republic of China; 6Department of Pharmacy Practice, College of Pharmacy and Health Sciences, Western New England University, Springfeld, MA, USA
*These authors contributed equally to this work
Correspondence: Sheng Deng, Email [email protected]
Objective: Carbapenem-resistant Enterobacteriaceae (CRE) has become a significant public health problem in the last decade. We aimed to explore the risk factors of mortality in patients with CRE infections and to focus on the current evidence on antimicrobial regimens for CRE infections, particularly from the perspective of mortality.
Methods: A systematic literature review was performed by searching the databases of EMBASE, PubMed, and the Cochrane Library to identify studies that evaluated mortality-related risk factors and antimicrobial regimens for CRE infections published from 2012 to 2022.
Results: In total, 33 and 28 studies were included to analyze risk factors and antibiotic treatment, respectively. The risk factors most frequently reported as significantly associated with CRE mortality were antibiotic use (92.9%; 26/28 studies), comorbidities (88.7%; 23/26 studies), and hospital-related factors (82.8%; 24/29 studies). In 10 studies that did not contain ceftazidime/avibactam (CAZ-AVI) therapy, seven demonstrated significantly lower mortality in combination therapy than in monotherapy. However, 5 of 6 studies identified no substantial difference between CAZ-AVI monotherapy and CAZ-AVI combination therapy. Six studies reported substantially lower mortality in CAZ-AVI regimens than in other regimens.
Conclusion: Several risk factors, particularly antibiotic use and patients’ comorbidities, are strong risk factors for CRE mortality. The optimal regimen for CRE infections remains controversial. Combination therapy should be considered when carbapenems, colistin, tigecycline, or aminoglycosides are administered. CAZ-AVI appears to be a promising antibiotic for CRE infections. Most importantly, treatment should be individualized according to the source and severity of the disease or other highly related risk factors.
Keywords: carbapenem resistant Enterobacteriaceae, CRE, mortality, risk factors, antimicrobial, treatment
Introduction
The global emergence of antimicrobial resistance poses a threat to human health.1 Carbapenem-resistant Enterobacteriaceae (CRE) or carbapenemase-producing Enterobacteriaceae (CPE) are Gram-negative bacteria that are resistant to the carbapenem drug class.2 The major resistance mechanisms of CRE are: enzyme production, efflux pumps and porin mutations.3 Of these, the production of carbapenemase including KPC, NDM, OXA-48, IMP, and VIM is the main resistance mechanism among CRE.4 The KPC enzyme accounts for a high proportion and has the ability to hydrolyze not just carbapenems but also several other antibiotics, leading to high mortality rate.5 CRE has become a major public health problem in the last decade due to the gradual increase in carbapenem resistance and the lack of effective antibiotics.6,7
The infection types of CRE are mainly bloodstream, pneumonia, respiratory, and urinary tract infections (UTIs). CRE infection is associated with increased mortality.2,6,8 In particular, CRE-caused bloodstream infections (CRE-BSIs) are associated with extremely high mortality, 30%-80%.9,10 A recent meta-analysis that included 62 studies showed a mortality rate of 54.3% for BSIs and 13.5% for UTIs associated with carbapenem-resistant K. pneumoniae (CRKP).10
Several studies have evaluated risk factors for CRE mortality but results were inconsistent. These risk factors included Pitt bacteremia score, immunocompromised status, previous exposure to carbapenems, lack of infection source control, and inappropriate antibiotic treatment, etc.11–14 Nevertheless, many studies have considered antibiotic use as significant risk factor for CRE infection and death.15,16 The main treatment options for CRE infections are regimens utilizing carbapenem, tigecycline, colistin, aminoglycoside, or ceftazidime/avibactam (CAZ-AVI). The Infectious Diseases Society of America (IDSA) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) have provided recommendations for treating CRE infections.1,17 However, the optimal antimicrobial regimen for CRE infections is unknown as there are controversies regarding which is the safest and most effective antibiotic regimen among the available choices. More importantly, an increasing body of evidence suggests that therapy should be individualized according to the source and severity of the infection or other related factors.18 Thus, it is difficult to establish the “gold standard” for treating CRE infections.
Our systematic review aimed to explore mortality risk factors in patients with CRE infections and to focus on current evidence on antimicrobial regimens for CRE infections. The results may provide clinical insight into reducing mortality in CRE patients and develop appropriate antibiotic regimens that offer a better prognosis for patients.
Methods
Mortality-Related Risk Factors
Search Strategy
Two authors independently searched the PubMed, Embase, and Cochrane Library databases to identify relevant studies from January 2012 to January 2022. The search strategy contained five core components, which were linked using the AND operator: (1) carbapenem (eg, carbapenem antibiotics), (2) resistance, (3) Enterobacteriaceae (eg, Klebsiella pneumoniae, Escherichia coli), (4) mortality (eg, death rate, case fatality rate), and (5) risk factors (eg, health correlates, the population at risk). Subject headings and free texts (ie, Medical Subject Headings [MeSH] terms) were identified for the five core components. In addition, relevant articles were selected by searching the references identified by this strategy. The complete search strategies are provided in the Supplementary Material (Part 1).
Selection Criteria
The CDC defines CRE as members of the Enterobacterales order resistant to at least 1 carbapenem antibiotic (meropenem, imipenem or ertapenem) or producing a carbapenemase enzyme.1 Studies were eligible for inclusion if they 1) were hospitalized patients with CRE infections, 2) reported mortality-related risk factors, and 3) were prospective/retrospective observational cohort, case-control studies or randomized controlled trials (RCTs). Exclusion criteria were 1) studies not published in English, 2) reviews, case reports, or experimental studies, 3) studies conducted in patients ≤14 years, 4) studies that did not differentiate between infection and colonization, 5) studies that did not differentiate CRE and other bacteria, 6) studies that had unclear definition and ineligible analysis, and 7) studies that did not provide adequate information.
Quality Assessment
The quality of cohort or case-control studies was assessed based on the Newcastle-Ottawa Scale (NOS) score. Studies with a NOS score ≥ 5 were further analyzed. The scoring details are shown in the Supplementary Material (Part 1).
Data Extraction
Two authors independently extracted relevant data and information from included studies. The following information was collected: first author, publication year, country, study period, study design, pathogen, infection type, the definition of resistance, mortality day, sample size, the numbers of non-survivors, and characteristics of the study population. Data and information on mortality-related risk factors were also extracted.
Data Synthesis
The risk factors were divided into ten groups according to clinical characteristics: demographics, comorbidities, clinical severity assessment scores, hospital-related factors, invasive procedures, type of infection, antibiotic use, clinical index, CRE strain-related factors, and other factors (such as antibiotic resistance and dialysis).
The significance of the association between risk factors and CRE mortality was investigated by examining the statistical data reported in the study. All risk factors with a significant association in the univariate or multivariate analysis were included in the statistical analysis, and odds ratios (OR) for the associations were recorded. Subsequently, we calculated the proportions of studies that reported significance for each risk factor. We also calculated the sample size of each study.
Antimicrobial Regimens
A literature search was performed using the PubMed database from January 2012 to January 2022 to identify studies investigating the treatments of CRE infections. The search strategy contained four core components, which were linked using the AND operator: (1) carbapenem-resistant Enterobacteriaceae, (2) antibacterial agents, (3) treatments and (4) infections. Subject headings and free texts (MeSH terms) were identified for each core component. The search strategy is provided in the Supplementary Material (Part 2).
Studies were eligible for inclusion if they: 1) were hospitalized patients with CRE infections, 2) studied antimicrobial regimens of patients, 3) had reported clinical outcomes of patients treated for CRE infections, 4) were prospective/retrospective observational cohort, case-control studies or randomized controlled trials (RCTs). Exclusion criteria were 1) studies not published in English, 2) reviews, case reports, or experimental studies, 3) studies conducted in patients ≤ 14 years, 4) studies did not differentiate between infection and colonization, 5) studies did not differentiate CRE and other bacteria, 6) studies had unclear definition and ineligible analysis, 7) studies did not provide adequate information, and 8) studies did not included more than 30 cases. The primary outcome of the systematic review was 30-day mortality. When 30-day mortality was unavailable, 14-day mortality, 28-day mortality and in-hospital mortality were extracted.
Results
Mortality-Related Risk Factors
Results of Included Studies
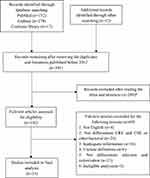
In total, 448 articles were identified through database searching, and 12 additional articles were identified from reference lists. After removing duplicates and literature published before 2012, 391 articles were screened for eligibility, and 289 were excluded after reading the abstract and title. The remaining 102 full-text articles were assessed for eligibility, and 33 studies were included in the analysis.11–14,19–47 The flow of the study selection is shown in Figure 1.
Study Characteristics
Table 1 shows the characteristics of the 33 studies from nine countries or regions. All were observational studies, 28 of which were retrospective and 5 of which were prospective, including 6 case-control, 27 cohort studies. Among the 33 studies, 9 were multicenter studies, and 24 were single-center studies. The sample size ranged from 39 to 661. The most frequently investigated pathogen was CRKP, followed by CRE (including K. pneumonia, Escherichia coli and other CRE pathogens), and CPE. The primary infections were BSIs (15 studies), followed by any infections (mainly pneumonia and UTIs, 14 studies).
 |
Table 1 Characteristics of the Eligible Studies (Mortality-Related Risk Factors) |
CRE Mortality-Related Risk Factors
Binary logistic regression analysis model was used to analysis the mortality-related risk factors in all the included studies. The proportion of studies demonstrating an association between chosen risk factors and the mortality of CRE in univariate analysis is shown in Table 2. In particular, only those factors examined in at least two eligible studies were presented. Table 3 shows the significant risk factors ranked according to the proportion of reports. The most reported significant risk factors were antibiotic use (92.9% of studies; 26/28) followed by comorbidities (88.7%; 23/26), hospital-related factors (82.8%; 24/29), and clinical severity assessment scores (82.1%; 23/28) base on univariate analysis.
 |
Table 2 Proportion of Studies Demonstrating an Association Between Chosen Risk Factors and the Mortality of CRE in Univariate Analysis |
 |
Table 3 The Proportion of Risk Factors Groups in Univariate Analysis and Multivariate |
Furthermore, in univariate analysis, the individual significant risk factors associated with CRE mortality were the Sequential Organ Failure Assessment (SOFA) Score (100% of studies; 5/5), inappropriate definitive therapy (100%; 2/2), the Pitt bacteremia score (85.7%; 6/7), hematologic malignancy (72.7%; 8/11), septic shock (76.2%; 16/21), and bloodstream infections (75%; 6/8). Additionally, in Table 2, no risk factors with an OR value < 1 are shown. The most reported risk factors were microbiological eradication (100% of studies; 4/4), colistin-based combination (80%; 4/5), appropriate antibiotic therapy (55%; 11/20), and combination therapy (50%; 8/16).
The summary of studies that reported a significant association with CRE mortality in multivariate analysis is shown in Table 3. Antibiotic use accounted for the highest proportion of studies (81.0%; 17/21), followed by type of infections (77.8%; 14/18), and clinical severity assessment scores (73.7%; 14/19).
Antimicrobial Regimens
Characteristics of Included Studies
In total, 28 eligible studies were included.11,13,21,26,31,32,36,38–41,43,48–63 The characteristics of the studies are presented in Table 4. Only studies that reported mortality as a treatment outcome were presented. Of the 28 studies, 15 were multicenter, and 13 were single-center studies. All were observational studies (24 retrospective; 4 prospective, 5 case-control, and 23 cohort studies). The sample size of the included studies ranged from 36 to 595.
 |  |  |  |
Table 4 Mortality of Infections Caused by CRE Among Different Antibiotic Treatment Regimens |
Ten studies focused on CPE, 12 CRKP, and 5 CRE. The primary infections were BSIs (17 studies), followed by pneumonia, respiratory, and urinary tract infections. In 22 studies, mortality at 28 or 30 days was provided. Three studies reported 14-day mortality, and 3 reported in-hospital mortality.
Antimicrobial Therapy and Outcome
All studies reported antibiotic treatment regimens and their associated mortalities. Mortalities ranged from 10% to 59% in 12 studies in patients who received combination therapies. However, mortalities ranged from 8.3% to 64.7% in 11 studies in patients who received monotherapies. Except for the 9 studies focused on CAZ-AVI therapy, 8 studies demonstrated significantly lower mortalities using combination therapies, 4 studies reported lower mortalities using monotherapies, and the remaining 7 studies reported no difference. However, in CAZ-AVI studies, 5 studies identified no substantial differences between CAZ-AVI monotherapy and CAZ-AVI combination therapies. Only one study reported significantly lower mortality in CAZ-AVI combination therapies.
Colistin, tigecycline, aminoglycosides, carbapenems, and CAZ-AVI were the most commonly used monotherapy antibiotics (Table 4). The mortality rates of monotherapies were attributed to use of: polymyxins, 40% to 66.7% in seven studies; tigecycline, 18.2% to 68.4% in six studies; aminoglycosides, 5.8–41% in four studies; carbapenems, 20% to 56.4% in eight studies; and CAZ-AVI, 22% to 47.6% in four studies (excluded 0% and 100%). In contrast, the corresponding mortalities of combination therapies were 22.6%-68.3%, 35%-69%, 32%-50%, 19.9%-53.8%, and 27%-41.5%, respectively. Carbapenem-containing therapies were associated with lower mortality than carbapenem-sparing therapies in three studies.48,55,57 Two studies reported no significant differences between these two types of therapies.38,39 In addition, CAZ-AVI- based therapies had substantially lower mortality than other regimens in six studies.
The “Old” Antibiotics
The older antibiotics for treating CRE infections are polymyxins, tigecycline, aminoglycosides, fosfomycin, and aztreonam. Polymyxins and tigecycline have been used as first-line agents to treat CRE infections. However, these monotherapies were often unsatisfactory, and the efficacy was uncertain even when combined with other antibiotics.64 Aminoglycosides are limited by nephrotoxicity and are second-line agents due to the availability of newer β-lactams and β-lactamase inhibitor combinations.65 The combination of polymyxin and tigecycline showed a good synergistic effect in vitro evaluation.66 In vitro synergy was also observed when polymyxins were combined with aminoglycosides or carbapenems.67,68 However, the clinical effect of synergy has not been identified.
In a single-center retrospective study, the outcome of 89 CRKP-caused BSI cases showed polymyxin-based therapy improved the survival rate compared to tigecycline-based treatment.13 Conversely, another nationwide multicenter study (64 patients) analyzed BSIs caused by CRKP (n = 50) and E. coli (n = 14), showing that tigecycline monotherapy was a choice if the strains exhibited the minimum inhibitory concentration (MIC) ≤ 0.5 mg/L, and colistin monotherapy was not suitable.41 Additionally, another study evaluated the treatment outcomes of a cohort of 36 patients with BSI due to OXA-48-like CPE, found that colistin-based dual combinations and preferably triple combinations were associated with significantly better outcomes when compared to non-colistin-based regimens (P < 0.001).43 Similarly, combination therapy, mostly polymyxin B plus amikacin, showed a survival benefit compared with other regimens in patients with Klebsiella pneumoniae carbapenemase-producing K. pneumoniae (KPC-KP) BSIs.54
Despite renal toxicity and second-line status, aminoglycosides still have potential roles in treating CRE infections, especially when combined with newer agents. A small sample size study demonstrated that aminoglycosides had effectively treated CRKP-BSIs if the pathogen was susceptible to aminoglycoside, showing a 75% clinical cure rate.65 Two observational studies revealed that aminoglycosides had better clinical outcomes compared to polymyxins or tigecycline in patients with CRE bacteriuria.69,70 A prospective cohort study in patients with CRKP-UTIs observed that patients treated with aminoglycosides (adjusted hazard ratio HR 0.34, 95% confidence interval CI 0.15–0.73, P=0.0049) were less likely to fail compared to patients treated with tigecycline (adjusted HR 2.92, 95% CI 1.03–5.13, P=0.0425).70 Similarly, the clinical success of aminoglycosides was 78.9% compared to other antibiotics (37.0%, P=0.007) in kidney transplant recipients with polymyxin-resistant CRE infections.69 Data are limited regarding tigecycline, fosfomycin, and aztreonam treatments for CRE infections.
Carbapenems
Due to the increasing resistance to CRE, carbapenems are no longer reserved as a last-resort therapy for high-risk CRE infections. However, carbapenems in treating CRE infections are still widely debated.6 Our analysis showed that carbapenem-containing treatment had lower mortality than other regimens. Dosing strategies of carbapenems for CRE infections include using high doses with prolonged infusion, double carbapenems, or combination with other antibiotics.
Two comparative studies on the efficacy of double carbapenems versus other antibiotics for CRE treatment showed similar results.55,71 A case-control (1:2) observational two-center study that involved critically ill adults demonstrated significantly lower mortality in patients treated with double carbapenems than standard treatment (ie, colistin, tigecycline, or gentamicin) (47.9% vs 29.2%, P = 0.04).55 Likewise, a single-center retrospective study observed that the double carbapenem regimen was also effective compared with the best available regimens in patients infected with CRE, including those with severe clinical conditions, and even in extremely high meropenem MICs.71
A large sample study suggested that high-dose carbapenem-based combination therapy was a protective factor (HR 0.69, 95% CI 0.47–1.00, P=0.05) for CRKP-caused BSIs, even in high-level carbapenem resistance.57 In a small sample study, 19 critically-ill patients with BSIs caused by KPC-KP (MICs ≥16 mg/L) were given combination therapy including meropenem, tigecycline, plus colistin or gentamicin. Meropenem was administered as an extended 3-hour infusion (2 g every 8 hours). High-dose meropenem failed to reach pharmacokinetics/pharmacodynamics targets.72 However, another cohort study revealed that high-dose continuous-infusion meropenem optimized using real-time TDM (Therapeutic Drug Monitoring) improved clinical outcomes in the patients infected with KPC-KP (meropenem MIC ≤ 64 mg/L).73 Real-time TDM-guided meropenem may represent a valuable adjunct for optimized care.74 Tigecycline and colistin were the two antimicrobials most commonly combined with meropenem,73 but their clinical effects of synergy are not entirely clear.
Ceftazidime-Avibactam
The CAZ-AVI was approved in 2015 to treat complicated intra-abdominal, urinary tract infections and hospital-acquired pneumonia.75 Before introducing CAZ-AVI, combination therapy was associated with lower mortality than monotherapy for CRE infections. However, it seems inconsistent when CAZ-AVI was administered to CRE patients. A relatively large multicenter cohort of 138 patients with KPC-KP bacteremia infections revealed significantly lower mortality when treated with CAZ-AVI-containing regimens as salvage therapy after first-line treatment (36.5% vs 55.8%, P =0.005). The results indicated no significant difference in mortality between CAZ-AVI monotherapy and combination therapy.59 Subsequently, the largest study published to date confirmed that combination therapies, including CAZ-AVI, were not associated with any significant change compared to CAZ-AVI monotherapy in mortality (26.1% vs 25.0%, P = 0.79),51 which was also supported by three other observational studies.11,58,61 On the contrary, lower mortality was observed in 41 critically ill patients treated with CAZ-AVI combined with antibiotics against CRKP infections (24.4% vs 47.6%, P = 0.028) suggesting that tigecycline, carbapenems, and fosfomycin could be optional concomitant antimicrobials.49
Several studies analyzed the efficacy of CAZ-AVI regimens compared to other antibacterial regimens on mortality in patients with CRE infections. Two multicenter observational studies compared the effectiveness of CAZ-AVI versus polymyxins for CRE, demonstrating the superiority of CAV-AVI over polymyxins in treating infections caused by KPC-KP or CRKP.21,53 Interestingly, a potential survival benefit was found in a large cohort study comprising 577 adults with KPC-KP infections treated with prolonged CAZ-AVI infusions (over three hours).51 Together, preliminary evidence suggests that CAZ-AVI appears to be a promising antibiotic for treating CRE infections. However, this option requires further evaluation.
Discussion
Most of the research on CRE infections was observational studies with a moderate to high risk of bias. It is challenging to perform RCTs on CRE infections due to the different susceptibility of CRE strains and many confounding factors.56 The lack of RCTs has hindered the development of guidelines for managing CRE infections.76
Several systematic reviews and meta-analyses have been conducted on specific pathogens, such as CRKP and carbapenem-resistant Acinetobacter baumannii (CRAB), focusing on mortality and predictors.10,16,77 Additionally, two meta-analyses analyzed the association between CRE and mortality.8,10 Another systematic review analyzed mortality risk factors with carbapenem-resistant Gram-negative bacterial (CR-GNB) infections.15 No systematic studies or meta-analyses have evaluated mortality-related risk factors for all CRE pathogens.
The duration of antibiotic treatment is controversial. Some studies reported that patients who received a short course of antimicrobial therapy had a poorer prognosis.43,44 Other studies revealed that the short duration of antibiotic treatment was a protective factor.78 The difference may be due to frequent changes in clinical conditions in critically ill patients with CRE infections, and antibiotic regimens are often modified during treatment. Therefore, it is difficult to evaluate the effect of the duration of antibiotic treatment on clinical outcomes.43 The IDSA does not provide recommendations on the duration of therapy. Instead, IDSA advises clinicians that prolonged treatment is unnecessary against infections by resistant pathogens compared to infections caused by the same bacterial species with more susceptible phenotypes.1
The protective factors with an OR value less than 1 are mainly regarding antibiotic therapy, such as appropriate antibiotic treatment and combination therapies with a carbapenem, suggesting that the proper use of antibiotics may reduce the risk of CRE mortality. Proper antibiotic use has become an essential measure to prevent and treat CRE infections.79
Few monotherapy studies, except for CAZ-AVI, reported lower mortality outcomes, partly because patients who received monotherapies had less severe symptoms or a quickly controllable source of infection.48 The ESCMID guidelines recommended that “old” antibiotics, including polymyxin, tigecycline, and aminoglycosides, be considered in patients with non-severe CRE infections. Newer antibiotics (meropenem-vaborbactam or ceftazidime-avibactam) are used in critically ill patients.17 In clinical practice, combination therapies are commonly administered to patients with severe infections. However, studies have shown that the efficacy of combination therapies is uncertain. The in vitro synergy of specific antibiotics may not always translate into clinical effects. Dosages and duration of antibiotics and the susceptibility profiles of CRE pathogens may affect the treatment effectiveness.17,80
The effectiveness of colistin monotherapy was not satisfactory principally because the suboptimal dosing could not reach appropriate plasma concentrations. Still, it would increase the risk of death, particularly in severely ill patients with renal dysfunction.81 In our analysis, colistin monotherapy was a mortality-related risk factor for CRE infections. Furthermore, a study supported a survival benefit in colistin-based dual combinations, preferably in triple combinations.43 Therefore, combination with other in vitro active antibiotics might be the optimal option when treating CRE patients with colistin. In addition, aminoglycosides were more effective than polymyxins for treating CRE bacteriuria based on the ESCMID guidelines.
Carbapenem-containing regimens for CRE infections have been a long-standing topic of debate. The ESCMID guidelines suggested that clinicians should avoid carbapenem-containing combination therapies for CRE infections unless the MIC of meropenem is 8 mg/L.17 However, IDSA guidelines recommend that meropenem should be avoided if isolates are carbapenemase producers, despite susceptibility to meropenem.1 Our review obtained favorable outcomes when carbapenem-containing regimens were administered to CRE patients, and three studies supported a better outcome in patients treated with carbapenem-containing therapies than other treatments.48,55,57 In contrast, two studies reported no differences.38,39 Furthermore, high-dose continuous-infusion meropenem optimized by real-time TDM improved clinical outcomes even when there were extremely high meropenem MICs,73,74 which can be an option for clinicians.
In addition to CAZ-AVI, other novel antibiotics against CRE infections have been approved or in advanced clinical development, including ceftolozane-tazobactam, meropenem-vaborbactam, and imipenem-relebactam.82,83 We cannot accurately evaluate their effectiveness and safety due to the lack of data available for these new antibiotics against CRE. Preliminary evidence revealed a potential role of CAZ-AVI in patients with CRKP infections. Our review and two other meta-analyses demonstrate no substantial difference between CAZ-AVI monotherapy and CAZ-AVI combination therapy.84,85 More post-marketing data from real-world studies and RCTs are needed to evaluate the effectiveness and safety of CAZ-AVI in treating CRE infections. However, the drug resistance of CAZ-AVI has gradually increased in recent years, and their effectiveness has been decreased due to β-lactamase production, efflux pumps and target modifications.86
Our systematic review has the following limitations: 1) No RCTs were included. 2) The size of included studies was small. 3) Owing to the high heterogeneity of the included studies in terms of study design, patient populations and CRE pathogens, and so comparative statistical analysis or meta-analysis of the results was not possible. and 4) In addition to antibiotic use, risk factors such as complications and septic shock also accounted for a high proportion of the dead patients. We could not control for these variables when analyzing the efficacy of antimicrobial regimens due to the limited data. It needs further investigation whether the patient’s antibiotic regimen was different in the sepsis/non-sepsis group, organ dysfunctions/non- organ dysfunctions, or mild/critical patients’ group.
Conclusions
Our systematic review has explored mortality-related risk factors and antimicrobial regimens of CRE infection. According to our review, antibiotics use, patients’ comorbidities, and hospital-related factors are the most important mortality risk factors in patients with CRE infections. Combination therapies may offer a comparative advantage over monotherapy except for CAZ-AVI. When treating CRE infections, colistin monotherapy should be avoided. Aminoglycosides can be used for CRE bacteriuria. High-dose continuous-infusion meropenem and double carbapenems regimens could be considered. CAZ-AVI appears to be a promising drug for treating CRE infections, especially those involving bacteremia. Clinicians must consider mortality-related risk factors, and treatment should be individualized based on the source and severity of the disease.
Funding
This study was supported by grants from Xiangya Hospital Management Research Fund of Central South University (2019GL08), Natural Science Foundation of Hunan Province, China (2022JJ30922), and the Program of Natural Science Foundation of Hunan Province, China (2022JJ80045).
Disclosure
We declare that we have no conflicts of interest.
References
1. Tamma PD, Aitken SL, Bonomo RA, et al. Infectious Diseases Society of America guidance on the treatment of Extended-spectrum beta-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin Infect Dis. 2021;72(7):e169–e183. doi:10.1093/cid/ciaa1478
2. Centers for Disease Control and Prevention Facility. Guidance for control of carbapenem resistant Enterobacteriaceae (CRE)ō; 2015. Available from: https://www.cdc.gov/hai/organisms/cre/.
3. Suay-García B, Pérez-Gracia MT. Present and future of carbapenem-resistant Enterobacteriaceae (CRE) infections. Antibiotics. 2019;8(3):122. doi:10.3390/antibiotics8030122
4. Han R, Shi Q, Wu S, et al. Dissemination of carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) among carbapenem-resistant Enterobacteriaceae isolated from adult and children patients in China. Front Cell Infect Microbiol. 2020;10:314. doi:10.3389/fcimb.2020.00314
5. Campos AC, Albiero J, Ecker AB, et al. Outbreak of Klebsiella pneumoniae carbapenemase-producing K pneumoniae: a systematic review. Am J Infect Control. 2016;44(11):1374–1380. doi:10.1016/j.ajic.2016.03.022
6. Trecarichi EM, Tumbarello M. Therapeutic options for carbapenem-resistant Enterobacteriaceae infections. Virulence. 2017;8(4):470–484. doi:10.1080/21505594.2017.1292196
7. Durante-Mangoni E, Andini R, Zampino R. Management of carbapenem-resistant Enterobacteriaceae infections. Clin Microbiol Infect. 2019;25(8):943–950. doi:10.1016/j.cmi.2019.04.013
8. Soontaros S, Leelakanok N. Association between carbapenem-resistant Enterobacteriaceae and death: a systematic review and meta-analysis. Am J Infect Control. 2019;47(10):1200–1212. doi:10.1016/j.ajic.2019.03.020
9. Falcone M, Tiseo G, Antonelli A, et al. Clinical features and outcomes of bloodstream infections caused by New Delhi metallo-β-lactamase-producing Enterobacterales during a regional outbreak. Open Forum Infect Dis. 2020;7(2):ofaa011. doi:10.1093/ofid/ofaa011
10. Xu L, Sun X, Ma X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob. 2017;16(1):18. doi:10.1186/s12941-017-0191-3
11. Chen L, Han X, Li Y, et al. Assessment of mortality-related risk factors and effective antimicrobial regimens for treatment of bloodstream infections caused by carbapenem-resistant Enterobacterales. Antimicrob Agents Chemother. 2021;65(9):e0069821. doi:10.1128/AAC.00698-21
12. Zuo Y, Zhao D, Song G, et al. Risk factors, molecular epidemiology, and outcomes of carbapenem-resistant Klebsiella pneumoniae infection for hospital-acquired pneumonia: a matched case-control study in Eastern China during 2015–2017. Microb Drug Resist. 2021;27(2):204–211. doi:10.1089/mdr.2020.0162
13. Shen L, Lian C, Zhu B, et al. Bloodstream infections due to carbapenem-resistant Klebsiella pneumoniae: a single-center retrospective study on risk factors and therapy options. Microb Drug Resist. 2021;27(2):227–233. doi:10.1089/mdr.2019.0455
14. Seo H, Lee SC, Chung H, et al. Clinical and microbiological analysis of risk factors for mortality in patients with carbapenem-resistant Enterobacteriaceae bacteremia. Int J Antimicrob Agents. 2020;56(4):106126. doi:10.1016/j.ijantimicag.2020.106126
15. Palacios-Baena ZR, Giannella M, Manissero D, et al. Risk factors for carbapenem-resistant Gram-negative bacterial infections: a systematic review. Clin Microbiol Infect. 2021;27(2):228–235. doi:10.1016/j.cmi.2020.10.016
16. Qian Y, Bi Y, Liu S, et al. Predictors of mortality in patients with carbapenem-resistant Klebsiella pneumoniae infection: a meta-analysis and a systematic review. Ann Palliat Med. 2021;10(7):7340–7350. doi:10.21037/apm-21-338
17. Paul M, Carrara E, Retamar P, et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin Microbiol Infect. 2022;28(4):521–547. doi:10.1016/j.cmi.2021.11.025
18. Rodríguez-Baño J, Gutiérrez-Gutiérrez B, Machuca I, et al. Treatment of infections caused by Extended-spectrum-beta-lactamase-, ampc-, and carbapenemase-producing Enterobacteriaceae. Clin Microbiol Rev. 2018;31(2). doi:10.1128/CMR.00079-17
19. Palacios-Baena ZR, Oteo J, Conejo C, et al. Comprehensive clinical and epidemiological assessment of colonisation and infection due to carbapenemase-producing Enterobacteriaceae in Spain. J Infect. 2016;72(2):152–160. doi:10.1016/j.jinf.2015.10.008
20. Lin YT, Chuang C, Su CF, et al. Efficacy of appropriate antimicrobial therapy on the survival of patients with carbapenem nonsusceptible Klebsiella pneumoniae infection: a multicenter study in Taiwan. Medicine. 2015;94(33):e1405. doi:10.1097/MD.0000000000001405
21. Fang J, Li H, Zhang M, et al. Efficacy of ceftazidime-avibactam versus polymyxin B and risk factors affecting clinical outcomes in patients with carbapenem-resistant Klebsiella pneumoniae infections a retrospective study. Front Pharmacol. 2021;12:780940. doi:10.3389/fphar.2021.780940
22. Andrey DO, Pereira Dantas P, Martins WBS, et al. An emerging clone, Klebsiella pneumoniae carbapenemase 2-producing K. pneumoniae sequence type 16, associated with high mortality rates in a CC258-endemic setting. Clin Infect Dis. 2020;71(7):e141–e150. doi:10.1093/cid/ciz1095
23. Chotiprasitsakul D, Srichatrapimuk S, Kirdlarp S, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae: a 5-year experience at a tertiary care hospital. Infect Drug Resist. 2019;12:461–468. doi:10.2147/IDR.S192540
24. Cristina ML, Alicino C, Sartini M, et al. Epidemiology, management, and outcome of carbapenem-resistant Klebsiella pneumoniae bloodstream infections in hospitals within the same endemic metropolitan area. J Infect Public Health. 2018;11(2):171–177. doi:10.1016/j.jiph.2017.06.003
25. Li Y, Li J, Hu T, et al. Five-year change of prevalence and risk factors for infection and mortality of carbapenem-resistant Klebsiella pneumoniae bloodstream infection in a tertiary hospital in North China. Antimicrob Resist Infect Control. 2020;9(1):79. doi:10.1186/s13756-020-00728-3
26. Capone A, Giannella M, Fortini D, et al. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin Microbiol Infect. 2013;19(1):E23–E30. doi:10.1111/1469-0691.12070
27. Geng TT, Xu X, Huang M. High-dose tigecycline for the treatment of nosocomial carbapenem-resistant Klebsiella pneumoniae bloodstream infections: a retrospective cohort study. Medicine. 2018;97(8):e9961. doi:10.1097/MD.0000000000009961
28. Rivera-Espinar F, Machuca I, Tejero R, et al. Impact of KPC production and high-level meropenem resistance on all-cause mortality of ventilator-associated pneumonia in association with Klebsiella pneumoniae. Antimicrob Agents Chemother. 2020;64(6):e02164. doi:10.1128/AAC.02164-19
29. Tumbarello M, Trecarichi EM, De Rosa FG, et al. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother. 2015;70(7):2133–2143. doi:10.1093/jac/dkv086
30. Mora-Guzman I, Rubio-Perez I, Domingo-Garcia D, et al. Intra-abdominal infections by carbapenemase-producing Enterobacteriaceae in a surgical unit: counting mortality, stay, and costs. Surg Infect. 2021;22(3):266–273. doi:10.1089/sur.2020.137
31. Falcone M, Russo A, Iacovelli A, et al. Predictors of outcome in ICU patients with septic shock caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Microbiol Infect. 2016;22(5):444–450. doi:10.1016/j.cmi.2016.01.016
32. Wang X, Wang Q, Cao B, et al. Retrospective observational study from a Chinese network of the impact of combination therapy versus monotherapy on mortality from carbapenem-resistant Enterobacteriaceae bacteremia. Antimicrob Agents Chemother. 2019;63(1):e01511. doi:10.1128/AAC.01511-18
33. Zhang H, Guo Z, Chai Y, et al. Risk factors for and clinical outcomes of carbapenem-resistant Klebsiella pneumoniae nosocomial infections: a retrospective study in a tertiary hospital in Beijing, China. Infect Drug Resist. 2021;14:1393–1401. doi:10.2147/IDR.S298530
34. Bar-Yoseph H, Cohen N, Korytny A, et al. Risk factors for mortality among carbapenem-resistant Enterobacteriaceae carriers with focus on immunosuppression. J Infect. 2019;78(2):101–105. doi:10.1016/j.jinf.2018.10.003
35. Tuon FF, Graf ME, Merlini A, et al. Risk factors for mortality in patients with ventilator-associated pneumonia caused by carbapenem-resistant Enterobacteriaceae. Braz J Infect Dis. 2017;21(1):1–6. doi:10.1016/j.bjid.2016.09.008
36. Liu KS, Tong YS, Lee MT, et al. Risk factors of 30-day all-cause mortality in patients with carbapenem-resistant Klebsiella pneumoniae bloodstream infection. J Pers Med. 2021;11(7):616. doi:10.3390/jpm11070616
37. Lim FK, Liew YX, Cai Y, et al. Treatment and outcomes of infections caused by diverse carbapenemase-producing carbapenem-resistant Enterobacterales. Front Cell Infect Microbiol. 2020;10:579462. doi:10.3389/fcimb.2020.579462
38. Li C, Li Y, Zhao Z, et al. Treatment options and clinical outcomes for carbapenem-resistant Enterobacteriaceae bloodstream infection in a Chinese university hospital. J Infect Public Health. 2019;12(1):26–31. doi:10.1016/j.jiph.2018.08.002
39. Lee NY, Tsai CS, Syue LS, et al. Treatment outcome of bacteremia due to non-carbapenemase-producing carbapenem-resistant Klebsiella pneumoniae bacteremia: role of carbapenem combination therapy. Clin Ther. 2020;42(3):e33–e44. doi:10.1016/j.clinthera.2020.01.004
40. Su CF, Chuang C, Lin YT, et al. Treatment outcome of non-carbapenemase-producing carbapenem-resistant Klebsiella pneumoniae infections: a multicenter study in Taiwan. Eur J Clin Microbiol Infect Dis. 2018;37(4):651–659. doi:10.1007/s10096-017-3156-8
41. Lin YT, Su CF, Chuang C, et al. Appropriate treatment for bloodstream infections due to carbapenem-resistant Klebsiella pneumoniae and Escherichia coli: a nationwide multicenter study in Taiwan. Open Forum Infect Dis. 2019;6(2):ofy336. doi:10.1093/ofid/ofy336
42. Di Domenico EG, Cavallo I, Sivori F, et al. Biofilm production by carbapenem-resistant Klebsiella pneumoniae significantly increases the risk of death in oncological patients. Front Cell Infect Microbiol. 2020;10:561741. doi:10.3389/fcimb.2020.561741
43. Balkan II, Aygun G, Aydin S, et al. Blood stream infections due to OXA-48-like carbapenemase-producing Enterobacteriaceae: treatment and survival. Int J Infect Dis. 2014;26:51–56. doi:10.1016/j.ijid.2014.05.012
44. Zhou C, Jin L, Wang Q, et al. Bloodstream infections caused by carbapenem-resistant Enterobacterales: risk factors for mortality, antimicrobial therapy and treatment outcomes from a prospective multicenter study. Infect Drug Resist. 2021;14:731–742. doi:10.2147/IDR.S294282
45. Tian X, Huang C, Ye X, et al. Carbapenem-resistant Enterobacter cloacae causing nosocomial infections in southwestern China: molecular epidemiology, risk factors, and predictors of mortality. Infect Drug Resist. 2020;13:129–137. doi:10.2147/IDR.S234678
46. Brescini L, Morroni G, Valeriani C, et al. Clinical and epidemiological characteristics of KPC-producing Klebsiella pneumoniae from bloodstream infections in a tertiary referral center in Italy. BMC Infect Dis. 2019;19(1):611. doi:10.1186/s12879-019-4268-9
47. Chang YY, Chuang YC, Siu LK, et al. Clinical features of patients with carbapenem nonsusceptible Klebsiella pneumoniae and Escherichia coli in intensive care units: a nationwide multicenter study in Taiwan. J Microbiol Immunol Infect. 2015;48(2):219–225. doi:10.1016/j.jmii.2014.05.010
48. Navarro-san francisco C, Mora-Rillo M, Romero-Gómez MP, et al. Bacteraemia due to OXA-48-carbapenemase-producing Enterobacteriaceae: a major clinical challenge. Clin Microbiol Infect. 2013;19(2):E72–E79. doi:10.1111/1469-0691.12091
49. Zheng G, Zhang J, Wang B, et al. Ceftazidime-avibactam in combination with in vitro non-susceptible antimicrobials versus ceftazidime-avibactam in monotherapy in critically ill patients with carbapenem-resistant Klebsiella pneumoniae infection: a retrospective cohort study. Infect Dis Ther. 2021;10(3):1699–1713. doi:10.1007/s40121-021-00479-7
50. Gu J, Xu J, Zuo TT, et al. Ceftazidime-avibactam in the treatment of infections from carbapenem-resistant Klebsiella pneumoniae: ceftazidime-avibactam against CR-KP infections. J Glob Antimicrob Resist. 2021;26:20–25. doi:10.1016/j.jgar.2021.04.022
51. Tumbarello M, Raffaelli F, Giannella M, et al. Ceftazidime-avibactam use for Klebsiella pneumoniae carbapenemase-producing K. Pneumoniae infections: a retrospective observational multicenter study. Clin Infect Dis. 2021;73(9):1664–1676. doi:10.1093/cid/ciab176
52. Villegas MV, Pallares CJ, Escandon-Vargas K, et al. Characterization and clinical impact of bloodstream infection caused by carbapenemase-producing Enterobacteriaceae in seven Latin American countries. PLoS One. 2016;11(4):e0154092. doi:10.1371/journal.pone.0154092
53. Van Duin D, Lok JJ, Earley M, et al. Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis. 2018;66(2):163–171. doi:10.1093/cid/cix783
54. Medeiros GS, Rigatto MH, Falci DR, et al. Combination therapy with polymyxin B for carbapenemase-producing Klebsiella pneumoniae bloodstream infection. Int J Antimicrob Agents. 2019;53(2):152–157. doi:10.1016/j.ijantimicag.2018.10.010
55. De Pascale G, Martucci G, Montini L, et al. Double carbapenem as a rescue strategy for the treatment of severe carbapenemase-producing Klebsiella pneumoniae infections: a two-center, matched case-control study. Crit Care. 2017;21(1):173. doi:10.1186/s13054-017-1769-z
56. Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M, et al. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis. 2017;17(7):726–734. doi:10.1016/S1473-3099(17)30228-1
57. Giannella M, Trecarichi EM, Giacobbe DR, et al. Effect of combination therapy containing a high-dose carbapenem on mortality in patients with carbapenem-resistant Klebsiella pneumoniae bloodstream infection. Int J Antimicrob Agents. 2018;51(2):244–248. doi:10.1016/j.ijantimicag.2017.08.019
58. Sousa A, Perez-Rodriguez MT, Soto A, et al. Effectiveness of ceftazidime/avibactam as salvage therapy for treatment of infections due to OXA-48 carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother. 2018;73(11):3170–3175. doi:10.1093/jac/dky295
59. Tumbarello M, Trecarichi EM, Corona A, et al. Efficacy of ceftazidime-avibactam salvage therapy in patients with infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Infect Dis. 2019;68(3):355–364. doi:10.1093/cid/ciy492
60. de Oliveira MS, de Assis DB, Freire MP, et al. Treatment of KPC-producing Enterobacteriaceae: suboptimal efficacy of polymyxins. Clin Microbiol Infect. 2015;21(2):e1–e7. doi:10.1016/j.cmi.2014.07.010
61. King M, Heil E, Kuriakose S, et al. Multicenter study of outcomes with ceftazidime-avibactam in patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother. 2017;61(7):e00449. doi:10.1128/AAC.00449-17
62. Satlin MJ, Chen L, Gomez-Simmonds A, et al. Impact of a rapid molecular test for Klebsiella pneumoniae carbapenemase and ceftazidime-avibactam use on outcomes after bacteremia caused by carbapenem-resistant Enterobacterales. Clin Infect Dis. 2022;ciac354. doi:10.1093/cid/ciac354
63. Chen J, Yang Y, Yao H, et al. Prediction of prognosis in adult patients with carbapenem-resistant Klebsiella pneumoniae infection. Front Cell Infect Microbiol. 2021;11:818308. doi:10.3389/fcimb.2021.818308
64. Fritzenwanker M, Imirzalioglu C, Herold S, et al. Treatment options for carbapenem- resistant Gram-negative infections. Dtsch Arztebl Int. 2018;115(20–21):345–352. doi:10.3238/arztebl.2018.0345
65. Shields RK, Clancy CJ, Press EG, et al. Aminoglycosides for treatment of bacteremia due to carbapenem-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2016;60(5):3187–3192. doi:10.1128/AAC.02638-15
66. Dubrovskaya Y, Chen TY, Scipione MR, et al. Risk factors for treatment failure of polymyxin B monotherapy for carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother. 2013;57(11):5394–5397. doi:10.1128/AAC.00510-13
67. Yu L, Zhang J, Fu Y, et al. Synergetic effects of combined treatment of colistin with meropenem or amikacin on carbapenem-resistant Klebsiella pneumoniae in vitro. Front Cell Infect Microbiol. 2019;9:422. doi:10.3389/fcimb.2019.00422
68. Ni W, Yang D, Guan J, et al. In vitro and in vivo synergistic effects of tigecycline combined with aminoglycosides on carbapenem-resistant Klebsiella pneumoniae. J Antimicrob Chemother. 2021;76(8):2097–2105. doi:10.1093/jac/dkab122
69. Freire MP, de Oliveira Garcia D, Cury AP, et al. The role of therapy with aminoglycoside in the outcomes of kidney transplant recipients infected with polymyxin- and carbapenem-resistant Enterobacteriaceae. Eur J Clin Microbiol Infect Dis. 2019;38(4):755–765. doi:10.1007/s10096-019-03468-4
70. van Duin D, Cober E, Richter SS, et al. Impact of therapy and strain type on outcomes in urinary tract infections caused by carbapenem-resistant Klebsiella pneumoniae. J Antimicrob Chemother. 2015;70(4):1203–1211. doi:10.1093/jac/dku495
71. Cancelli F, Oliva A, De Angelis M, et al. Role of double-carbapenem regimen in the treatment of infections due to carbapenemase producing carbapenem-resistant Enterobacteriaceae: a single-center, observational study. Biomed Res Int. 2018;2018:2785696. doi:10.1155/2018/2785696
72. Del Bono V, Giacobbe DR, Marchese A, et al. Meropenem for treating kpc-producing Klebsiella pneumoniae bloodstream infections: should we get to the pk/pd root of the paradox? Virulence. 2017;8(1):66–73. doi:10.1080/21505594.2016.1213476
73. Pea F, Della Siega P, Cojutti P, et al. Might real-time pharmacokinetic/pharmacodynamic optimisation of high-dose continuous-infusion meropenem improve clinical cure in infections caused by KPC-producing Klebsiella pneumoniae? Int J Antimicrob Agents. 2017;49(2):255–258. doi:10.1016/j.ijantimicag.2016.10.018
74. Cojutti P, Sartor A, Righi E, et al. Population pharmacokinetics of high-dose continuous-infusion meropenem and considerations for use in the treatment of infections due to KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2017;61(10):e00794. doi:10.1128/AAC.00794-17
75. Dietl B, Martínez LM, Calbo E, et al. Update on the role of ceftazidime-avibactam in the management of carbapenemase-producing Enterobacterales. Future Microbiol. 2020;15:473–484. doi:10.2217/fmb-2020-0012
76. Agyeman AA, Bergen PJ, Rao GG, et al. A systematic review and meta-analysis of treatment outcomes following antibiotic therapy among patients with carbapenem-resistant Klebsiella pneumoniae infections. Int J Antimicrob Agents. 2020;55(1):105833. doi:10.1016/j.ijantimicag.2019.10.014
77. Du X, Xu X, Yao J, et al. Predictors of mortality in patients infected with carbapenem-resistant Acinetobacter baumannii: a systematic review and meta-analysis. Am J Infect Control. 2019;47(9):1140–1145. doi:10.1016/j.ajic.2019.03.003
78. Cienfuegos-Gallet AV, Ocampo de Los Ríos AM, Sierra VP, et al. Risk factors and survival of patients infected with carbapenem-resistant Klebsiella pneumoniae in a KPC endemic setting: a case-control and cohort study. BMC Infect Dis. 2019;19(1):830. doi:10.1186/s12879-019-4461-x
79. Wilson AP, Livermore DM, Otter JA, et al. Prevention and control of multi-drug-resistant Gram-negative bacteria: recommendations from a Joint Working Party. J Hosp Infect. 2016;92(Suppl 1):S1–S44. doi:10.1016/j.jhin.2015.08.007
80. Falagas ME, Lourida P, Poulikakos P, et al. Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: systematic evaluation of the available evidence. Antimicrob Agents Chemother. 2014;58(2):654–663. doi:10.1128/AAC.01222-13
81. Jacobs M, Grégoire N, Mégarbane B, et al. Population pharmacokinetics of colistin methanesulfonate and colistin in critically ill patients with acute renal failure requiring intermittent hemodialysis. Antimicrob Agents Chemother. 2016;60(3):1788–1793. doi:10.1128/AAC.01868-15
82. Doi Y. Treatment options for carbapenem-resistant Gram-negative bacterial infections. Clin Infect Dis. 2019;69(Suppl 7):S565–S575. doi:10.1093/cid/ciz830
83. Ackley R, Roshdy D, Meredith J, et al. Meropenem-vaborbactam versus ceftazidime-avibactam for treatment of carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother. 2020;64(5):e02313. doi:10.1128/AAC.02313-19
84. Fiore M, Alfieri A, Di Franco S, et al. Ceftazidime-avibactam combination therapy compared to ceftazidime-avibactam monotherapy for the treatment of severe infections due to carbapenem-resistant pathogens: a systematic review and network meta-analysis. Antibiotics. 2020;9(7):338. doi:10.3390/antibiotics9070388
85. Onorato L, Di Caprio G, Signoriello S, et al. Efficacy of ceftazidime/avibactam in monotherapy or combination therapy against carbapenem-resistant Gram-negative bacteria: a meta-analysis. Int J Antimicrob Agents. 2019;54(6):735–740. doi:10.1016/j.ijantimicag.2019.08.025
86. Wang Y, Wang J, Wang R, et al. Resistance to ceftazidime-avibactam and underlying mechanisms. J Glob Antimicrob Resist. 2020;22:18–27. doi:10.1016/j.jgar.2019.12.009
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.