Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 15
Morphological and Elemental Evaluation of Investigative Mouthwashes to Repair Acid-Eroded Tooth Surface
Authors Amaechi BT , Mohseni S, Dillow AM, Cvelich MH, Stevanovic A, Abah AI, Movaghari Pour F , Farah R , Kataoka Y, Restrepo MC, Zhang D, Leigh LE, Basilan J
Received 23 September 2022
Accepted for publication 14 December 2022
Published 5 January 2023 Volume 2023:15 Pages 1—11
DOI https://doi.org/10.2147/CCIDE.S390240
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Christopher E. Okunseri
Bennett Tochukwu Amaechi,1 Sahar Mohseni,1 Andrew M Dillow,1 Mackenzie H Cvelich,2 Ana Stevanovic,3 Alphonsus Igoche Abah,1 Fatemeh Movaghari Pour,1 Rayane Farah,1 Yuko Kataoka,1 Maria Camila Restrepo,1 Dennis Zhang,4 Leonora Ethleen Leigh,4 Joel Basilan4
1Department of Comprehensive Dentistry, University of Texas Health San Antonio, San Antonio, TX, USA; 2Department of Developmental Dentistry, University of Texas Health San Antonio, San Antonio, TX, USA; 3Kleberg Advanced Microscopy Center, University of Texas at San Antonio, San Antonio, TX, USA; 4Research & Development – Oral Care, BASF Corporation, Tarrytown, NY, USA
Correspondence: Bennett Tochukwu Amaechi, Department of Comprehensive Dentistry, School of Dentistry, University of Texas Health San Antonio, 7703 Floyd Curl Drive, San Antonio, TX, 78229-3900, USA, Tel +1 210 567 3185, Email [email protected]
Purpose: Erosive tooth wear (ETW) is characterized by subsurface demineralization and tooth substance loss with crater formation. Remineralization of subsurface demineralization has previously been demonstrated; however, repair of the eroded surface is still under investigation. This study investigated the effectiveness of mouthwashes containing hydrolyzed wheat protein (HWP) in repairing ETW through promotion of organized crystal growth.
Methods: Enamel Erosion was created on 210 enamel blocks by 10-minute demineralization in 1% Citric Acid (pH 3.5). Then, blocks were randomly assigned to seven groups (30/group); (A) 0.2% HWP, B) 1% HWP, (C) 2% HWP, (D) 1% HWP + 0.05% NaF, (E) Listerine™ mouthwash, (F) 0.02% NaF Crest™ Pro-health mouthwash and (G) artificial saliva (AS) only. Groups were subjected to daily pH-cycling consisting of one 5-minute erosive challenge with citric acid, three 1-minute mouthwash treatment periods, and then storage in AS for the rest of the time for 28 days. Treatment effects were assessed using SEM-EDX. Statistical analysis was by ANOVA and Tukey’s multiple comparison.
Results: In groups exposed to HWP-containing mouthwashes, there was growth of fiber-like crystals that increased in packing density in a dose-dependent manner (0.2%, 1%, 2%) on the eroded enamel surfaces, with increased calcium and phosphate contents on the treated surfaces. The non-HWP-containing groups had the eroded surfaces covered by structureless deposit layer firmly attached to the surface.
Conclusion: Treating eroded enamel surface with HWP-containing mouthwash resulted in repair of the damaged tissue by formation of a protective layer of crystal deposits within and on the eroded enamel tissue.
Keywords: hydrolyzed wheat protein, Enameguard, erosive tooth wear, acid erosion, erosion repair, remineralization
Introduction
Dental erosion, otherwise known as erosive tooth wear (ETW), is the progressive, irreversible loss of tooth structure due to exposure to acids of non-bacteria origin.1 The loss of tooth structure due to ETW may result in loss of tooth morphology, dentin hypersensitivity, poor esthetics, and in severe cases, a decrease of the vertical dimension of occlusion.2 The erosive process renders the tooth surface hypomineralized, making it more susceptible to abrasion and attrition, exacerbating tooth structure loss.1 Although there is great variability in the reported prevalence of dental erosion due to the differences in diagnostic systems used in various studies, there is evidence that the incidence is increasing.3 The reported prevalence of ETW in dentin in deciduous teeth varied between 0% and 82% and in permanent teeth ranged from 0% to 54%.4,5
ETW can be caused by frequent exposure of the teeth to gastric acid through vomiting or reflux, commonly seen in patients with gastroesophageal reflux disease, bulimia, and anorexia.6–8 Also, an individual’s dietary habits, medications, environmental conditions, and lifestyle can predispose the person to ETW.9–18 Foods with relatively high acidity, such as acidic fruits and fruit juices, soft drinks, sports drinks, energy drinks, other carbonated beverages, and sour candies, contribute to the erosion of the dentition.10–12 Acidic medications like vitamin C and Aspirin have also been linked to ETW,13 while users of drugs, such as ecstasy and methamphetamine, have shown increases in ETW, possibly due to the associated dehydration leading to increased soft-drink consumption.14,15
The above discussion on ETW causative factors implies that the affected individuals are predisposed to this condition by their medical conditions or addiction to the causative agent. Thus, individualized counseling, which requires the patient’s compliance, is hardly successful, while restorative and esthetic treatments to repair the damaged tooth structure are very expensive. Effective and simple, non-invasive interventions are needed to improve the efficacy and efficiency of ETW control. Unlike most tissues in the body, tooth enamel cannot regenerate once a crater has formed. Among the interventions available to arrest the further progression of ETW on affected tooth surfaces is the remineralization of the eroded enamel lesion to form a new mineral layer to resist subsequent acid attacks.19–21 Remineralization of eroded lesions has been performed with agents such as highly concentrated fluoride (F), hydroxyapatite (HAP), Casein Phosphopeptide Amorphous Calcium Phosphate (CPP-ACP) or self-assembling peptides, applied either as toothpaste, mouthwash, brush-on liquid, varnish, or gel. Highly concentrated fluoride agents have been used to prevent ETW and inhibit the progression of ETW. It is thought to work by forming a calcium fluoride-like byproduct on the tooth surface, which serves as a reservoir of fluoride and calcium (Ca) ions to prevent tooth demineralization during the erosive challenge.21,22 However, the calcium-fluoride materials do not last long in the presence of low-pH acids.23 Stannous fluoride dentifrice was found to inhibit ETW significantly better than sodium fluoride (NaF).24 When fluoride is combined with titanium or stannous ions, it promotes a tooth surface more resistant to acid attack.25 Mouthwash with fluoride and calcium saturates the oral environment with minerals that inhibit demineralization and promote remineralization during acid attacks. In a recent study, rinsing the mouth with fluoride mouthwash or fluoride and stannous-chloride-containing mouthwash before an acid exposure reduced softening of the enamel surface.26 CPP-ACP with fluoride (CPP-ACP-F) was found to be more effective at remineralizing eroded tooth surfaces than CPP-ACP without fluoride.21,27 Another study comparing CPP-ACP-F paste and 0.2% sodium fluoride mouthwash found that both the mouthwash and the paste were effective; however, the 0.2% sodium fluoride mouthwash was more effective at preventing dental erosion.28 Several studies have demonstrated the potential of synthetic HAP to remineralize eroded tooth surfaces by forming a protective layer that acts as an expendable shield that protects the tooth material from further acidic attacks.29–32 During the further acidic challenge, the protective HAP layer dissolves, releasing calcium and phosphate ions that act as a buffer system and cause a shift of the equilibrium from dissolution to homeostasis. Besides forming a protective mineral layer, HAP also directly remineralizes the underlying demineralized tissue, where it forms solid interfaces between HAP crystallites from the particles and the enamel.33 Clinical trials and in vitro studies have proved that self-assembling peptides applied as a brush-on liquid are effective in remineralizing demineralized tooth tissue.34,35
In the present era of tissue engineering and biomimetics, reconstructing enamel-like structures on the tooth surface is increasingly being studied in the material sciences and dentistry. Various types of proteins have been used to form scaffolds that modulate the mineralization of organized calcium phosphate crystallites.36–45 One of these proteins being studied is hydrolyzed wheat protein, Enameguard™ (BASF Corporation, Florham Park, USA), a chelating agent that works by guiding and catalyzing the regeneration of lost enamel in an eroded tooth surface. In this report, we investigated the effectiveness of new mouthwashes containing hydrolyzed wheat protein (HWP) in repairing eroded enamel lesions through the promotion of organized crystal growth on an acid-eroded enamel surface. We demonstrated an in vitro biomimetic synthesis of fiber-like apatite crystals under physiological conditions simulated by the pH-cycling method to rebuild the enamel structure on an eroded enamel surface. The new mouthwashes were compared with a standard fluoride mouthwash containing 0.05% NaF (Crest ProHealth; Procter & Gamble, Cincinnati, Ohio, USA) and a non-fluoride mouthwash (Listerine cool mint, Johnson & Johnson Consumer Inc., Skillman, New Jersey, USA).
Materials and Methods
Tooth Blocks Preparation
Following the approval (approval #: HSC20080233N) by the Institutional Animal Care and Use Committee (IACUC) of the University of Texas Health Science Center, bovine teeth were obtained from Animal Technologies, Tyler, Texas, USA (an USDA inspected facilities) and used for this study. This study was conducted in accordance with the principles on ethical animal research outlined in the 2010 Basel Declaration and the 1956 ethical guidelines by the International Council for Laboratory Animal Science (ICLAS). The collected bovine teeth were sterilized in accordance with the university procedure. Following sterilization, the teeth were cleaned of soft tissue debris, brushed with pumice slurry using a manual toothbrush, and then examined by transillumination. Two hundred and ten bovine incisor teeth without cracks, hypomineralization, white spot lesions, and other malformations were selected. Using a water-cooled diamond wire saw (Walter Ebner, Switzerland), the roots of each tooth were cut off, and a total of 210 tooth blocks (approximately 3 mm length x 3 mm width x 1.5 mm thick) were produced from the labial surface of the selected teeth. Using adhesive back lapping film of varying grit levels (30µm–1µm) in a MultiPrep™ Precision Polishing machine (Allied High Tech, USA), the enamel surface and the bottom of each block were polished to achieve flat and planoparallel surfaces. Then the samples were sonicated for 2 minutes (power setting 2) in deionized distilled water to remove the smear layers generated during polishing. Next, all surfaces of each block were painted with two coats of acid-resistant nail varnish except a central rectangular area on the enamel surface, measuring 3 mm long by 1 mm wide, which was submitted to the acid erosive challenge.
Eroded Lesion Creation
Each tooth block with a central exposed area of 3 mm x 1 mm was immersed for 10 minutes in 1% Citric acid (pH 3.5). Enamel blocks were exposed to the acidic solutions at room temperature without agitation. A volume of 10 mL of acidic solution was used per block. The acidic solution was produced as follows. 10.0 g of powdered citric acid anhydrous (C1857, Sigma) was weighed and added to a beaker with ~800 mL of De-ionized distilled water (DIW). The pH of the solution was determined using a calibrated pH meter under agitation. The pH of the solution was raised from natural to 3.5 by adding sodium hydroxide solution (Sigma Aldrich), and the volume was adjusted to 1 L with DIW. After 10-minute immersion, the blocks were removed from the solution, rinsed thoroughly with DIW for 10 seconds, and dried carefully with paper towels. The specimens were stored in a moist environment to prevent dehydration and stored at 4°C prior to use.
Biomimetic Repair of Eroded Enamel Surface
The 210 blocks bearing eroded lesions were randomly assigned to the following seven experimental groups (Table 1) with 30 blocks per group; (A) 0.2% HWP, (B) 1% HWP, (C) 2% HWP, (D) 1% HWP + 0.05% NaF, (E) non-fluoridated Listerine™ mouthwash (Listerine cool mint, Johnson & Johnson Consumer Inc., Skillman, New Jersey, USA), (F) 0.02% NaF Crest Pro-health Restore Enamel Mouthwash (Crest ProHealth; Procter & Gamble, Cincinnati, Ohio, USA) and (G) Artificial saliva only. Using heavy-duty dental putty, the 30 blocks in each group were embedded in oblong grooves carved inside a cylindrical acrylic rod attached to the cover of a 250-mL treatment tube. The seven groups were then subjected to remineralization using a pH cycling (demineralization-remineralization) model,46–48 simulating the activities within the oral environment as close as possible. Artificial saliva19 was used as the storage medium in all treatment regimens, while 1% Citric Acid (pH 3.5) prepared as described above was used as the erosive challenge medium (demineralization solution). The cyclic treatment regimen for each day consisted of one 5-minute erosive challenge (citric acid, CA), three 1-minute mouthwash treatment periods, and then storage in artificial saliva (AS) for the rest of the time without agitation (Table 2). For treatment, 200 mL of the treatment medium (CA, AS, or mouthwash) was placed into each 250 mL treatment tube. The AS and mouthwash treatments were magnetically stirred at 350 rpm while the CA was static. The AS remained static during night storage. All treatments were carried out in an incubator at 37°C. The pH of each medium was measured once daily before treatment. After treatment with one medium, the specimens were rinsed with running DIW and dried with a paper towel before immersion into the next medium. The daily regimen was repeated for 28 days. On termination of the experiment, the blocks were harvested from the holders, rinsed with running DIW for 10 seconds, air-dried, kept in a moist environment to prevent dehydration, and stored at 4°C prior to remineralization assessment using a Scanning Electron Microscope (SEM) coupled with Energy-Dispersive X-Ray Spectroscopy (EDX).
 |
Table 1 Compositions of the Experimental Products/Groups |
 |
Table 2 pH Cycling Treatment Sequence |
Structural and Elemental Composition Analysis
SEM coupled with EDX was used to investigate the eroded enamel surface’s structural and quantitative chemical composition and the mineral deposits laid by the different mouthwash formulations. This was performed in representative samples of each product group. Following drying, representative samples from each group were air-dried, mounted on aluminum stubs with carbon tape, and sputter-coated with 5 nm thick gold/palladium alloy (Balzers SCD 050, Balzers, Liechtenstein) for conductivity. Chemical characterization of the samples was performed with a Zeiss Crossbeam 340 cold-field emission SEM, and the image was acquired at a magnification range of x6000–x7500. The Zeiss Crossbeam 340 SEM equipped with the silicon-drift X-Max EDS detector (Oxford Instruments) was used to identify the composition and quantify the elemental abundance of the enamel built-up layer on its surface. The mounted samples were inserted into SEM and imaged by a 15 keV electron beam using a secondary electron and in-lens detectors. The energy and intensity of the characteristic X-rays from all sample constituents were converted into abundance (At%) using the Aztec software (Aztec, Springfield, NJ, USA), and the Calcium and Phosphorus ratio (Ca/P) in each sample was calculated.
Statistical Analysis
The data obtained on atomic weight percentage (At%) of calcium and phosphorus and their Ca/P ratio were subjected to statistical analysis using Stata software version 11.0 (StataCorp, College Station, TX) statistical software was used. The Ca/P ratio of the treated groups, sound enamel surface and the untreated eroded enamel surface were compared using Analysis of Variance (ANOVA), followed by Tukey’s HSD for multiple comparisons. All p-values were 2-sided and considered significant if less than 0.05.
Results
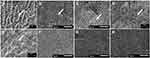
Figure 1A shows the SEM image of the enamel surface following acid erosion. The aprismatic enamel was eroded away, revealing the enamel prisms and the interprismatic spaces. Following the 28-day exposure to the mouthwashes, there was complete crystal growth on the eroded enamel surfaces in the groups that were exposed to the HWP-containing mouthwashes (Figure 1B–E). The crystals had fiber-like structures, and the deposit was porous but increased in packing density in a dose-dependent manner (0.2, 1, 2% HWP). This increase in density with concentration was more obvious following the sonication of the enamel blocks. After sonication, the fiber-like crystals in the group treated with 0.2% HWP concentration were able to come off completely, revealing the original eroded enamel surface prior to treatment (Figure 2B). However, in the groups treated with 1% and 2% HWP concentrations, after sonication, there was obvious evidence of deeper layer of crystal growth (Figure 2C), with the crystals growing out in bundles perpendicular to the enamel surface. The packing density of the crystals increased with an increase in the concentration of the HWP. This difference in density and bundle formation was more obvious with the group exposed to 1% HWP plus 0.05% NaF. In this group, the fiber-like crystals bundled together, forming a coating with a high mineral pack density and branching (Figure 1E). A higher magnification of the SEM image of this group (Figure 2E) showed more clearly the bundle formation of the fiber-like crystals oriented perpendicular to the enamel surface and covered by a web of branched fiber-like crystals, again indicating that the crystals that were formed deeper in the demineralized enamel are selective in direction.
The SEM images (Figure 1F–H) from the groups exposed to Listerine™ mouthwash (F), NaF mouthwash (G), and Artificial saliva only (H) showed that the eroded enamel surfaces were covered by a structureless deposit layer that was firmly attached to the enamel surface (did not come off after sonication (Figure 2D)). These deposits appear to seal the exposed enamel prisms/interprismatic spaces that were visible on the eroded enamel surface (Figure 1A).
Atomic weight percentage of calcium (Ca) and phosphorus (P) was obtained with EDX, and the Ca/P ratio was calculated for sound enamel surface, the eroded enamel surface, and remineralized eroded surface of each group comparison. The mean (±SD) Ca/P ratio of the different groups is shown in Table 3. One-way ANOVA indicated a statistically significant difference (p < 0.01) among the groups, including the sound and eroded enamel. However, there was no statistically significant difference among the treated groups, and between the sound enamel surface and the treated groups (Tukey’s multiple comparison). Statistically significant difference (p < 0.05) was observed between the eroded enamel surface and all treated groups, except Crest Pro-health mouthwash. A representative EDS spectrum of an eroded enamel surface and a remineralized eroded enamel surface with deposits of fiber-like crystals is shown in Figure 3A and B. However, only the groups exposed to 1% HWP + 0.05% NaF and Crest Pro-health Mouthwashes showed fluoride in EDS.
 |
Table 3 Mean, Standard Deviation (SD) and Ratio of Mineral Content of Sound Enamel Surface, the Eroded Enamel Surface, and Eroded Surface with Mineral Deposits in Eroded Surface of Each Group |
 |
Figure 3 EDS spectrum of (A) eroded enamel surface and (B) mineralized eroded enamel surface with deposits of fiber-like crystals. |
Discussion
Erosive tooth wear, a growing public health problem worldwide, may result in crater formation on the tooth surface and subsurface demineralization of the affected tooth tissue. For this reason, the control of ETW involves the remineralization of the demineralized tissue as well as the repair of the eroded tooth surface. Restorative or esthetic treatment to repair the damaged tooth surface is costly; thus, effective and simple, non-invasive interventions are needed to control ETW. Fluoride compounds, HAP, CPP-ACP, CPP-ACP-F, and certain self-assembling peptides applied as toothpaste, mouthwash, brush-on liquid, varnish, or gel have been very effective in remineralizing the demineralized tissue but not the repair of the lost tissue.19–35 Certain proteins have been successfully used to form scaffolds that modulate the mineralization of organized calcium phosphate crystallites and have shown great potential.36–40 Thus, in the present study, we investigated the effectiveness of new mouthwash formulations containing varying concentrations of HWP in repairing eroded enamel tissue through the promotion of organized crystal growth on an acid-eroded enamel surface. This was conducted using a long-established pH cycling model (Table 2), which was developed and accepted as a non-animal alternative to the animal caries reduction test (which is considered the “Gold Standard”) required by the Food & Drug Administration for demonstration of the efficacy of “Anticaries dentifrice product formulations” for over-the-counter human use.46–48 A pH-cycling model serves as a bridge to in vivo caries studies as they mirror clinical conditions, where demineralization and remineralization alternate constantly (ie, pH cycling) and are only interrupted during the very short period of application of investigational products, such as toothpaste or mouthwash.46
In our present study, HWP promoted the growth of fiber-like crystals dose-dependently. These crystals were found at higher magnification and by ultrasonication to be deposited in two layers (Figure 2B and D). The first and a deeper layer was deposited within the spaces in the demineralized enamel tissue, where the crystals were arranged in bundles oriented perpendicular to the enamel surface. This alignment indicates that the crystals’ growth within the demineralized enamel tissue is selective in direction and suggests that the HWP penetrated the demineralized tissue of the eroded enamel and self-assembled into fiber-like bundles oriented perpendicular to the tissue surface. This crystal growth within the enamel tissue occurred only at higher concentrations of the HWP (1%, 2% and 1% + fluoride). It was not dislodged by sonication, indicating a strong bonding and excellent biocompatibility between the newly grown crystal layer and the enamel tissue. The second layer of crystal growth, which was removed by sonication, covered the eroded enamel surface, and was deposited as nondirectional scaffolds increasing in packing density with increasing concentration of the HWP. With a low concentration (0.2%) of the HWP, only one layer of the fiber-like crystals, which covered the eroded enamel surface, were grown and came off completely with sonication, indicating that the deposition was not deep-rooted into the enamel tissue.
Quantitative analysis of the chemical composition of the crystal deposits in representative samples of each group using EDX indicated that the Ca/P ratio also increased with the concentrations of the HWP. This observation, coupled with the finding that with the combination of 1% HWP and 0.05% NaF in the mouthwash, the two deposited crystal layers have higher crystal packing density, strongly suggests that the HWP penetrated the demineralized enamel tissue micropores as well as binds on the surface of the eroded enamel, where it self-assembled into a fiber-like scaffold that templates de novo hydroxyapatite crystallite formation by attracting and integrating calcium, phosphate, and hydroxyl ions from the surrounding solution (artificial saliva) and promotes crystal integrity and growth. This guided biomimetic mineralization process for regenerating damaged enamel simulated the natural process of enamel formation and mineralization, in which amelogenin-rich extracellular organic matrix of enamel is continuously secreted, assembled, processed, and mineralized during enamel development, and indicates the potential of this investigated HWP to repair ETW as well as remineralize demineralized enamel tissue. In the present study, these findings agree with the reports of previous similar studies that used other types of proteins, such as amelogenin-chitosan matrix,36–40 amelogenin,37,38,42 Amelogenin-chitosan Hydrogel,39–41,43 and amelotin-based bio-nano complexes.44 These previous studies reported protein-mediated enamel mineralization.45
The other three investigated mouthwash formulations that do not contain HWP were effective in depositing mineral layers that covered the eroded enamel surface, with strong bonding and biocompatibility between the new layer and the eroded enamel surface, such that the layers were not dislodged by ultrasonication. This finding agrees with the reports of previous studies,19,27 and it is not surprising considering that all samples had a long exposure to remineralizing artificial saliva that contained all the major components of natural saliva (calcium, phosphate, magnesium, chloride, potassium, and hydroxyl ions), and its efficacy in remineralizing eroded lesion has been demonstrated.19
It may be considered a limitation in the present study that the mineral density of the repaired tissue was not quantitatively measured or estimated in any way, such as surface microhardness testing, transverse microradiography, etc. However, the main objective of this study was to demonstrate the ability of the HWP to promote organized crystal growth on an acid-eroded enamel surface through biomimetic synthesis of fiber-like apatite crystals. Our next study involves the SMH measurement to demonstrate remineralization. Furthermore, it may be considered a drawback in this study that the mineralization process was challenged, each day, with an extended 5-minute acid treatment, which may have delayed the consolidation of the deposited crystallites into hydroxyapatite. The acid challenge should have been applied in short periods such as 1 minute over multiple times.
Conclusions
Treatment of eroded enamel surface with a mouthwash containing Enameguard resulted in the repair of the damaged tissue and remineralization of the enamel. With the use of Enameguard, a protective layer of crystal deposits was formed within and on the eroded enamel tissue. The Enameguard-containing mouthwash formulations led to an increase in the Ca and P content of the enamel layer. Therefore, we propose that for repairing an enamel surface worn by acid erosion, using Enameguard in concentrations of 1% and 2%, is a promising approach. Not only did Enameguard remineralize the eroded tissue, but it also replaced the missing tissue.
Within the limitations of this in vitro study, it can be concluded that demineralized and eroded enamel tissue can be remineralized using mouthwashes containing Enameguard as a remineralizing agent. The results of the present in vitro study should be confirmed with a clinical trial to evaluate the effect of oral cavity factors on this remineralization process.
Abbreviations
ETW, erosive tooth wear; F, fluoride; HAP, hydroxyapatite; CPP-ACP, Casein Phosphopeptide Amorphous Calcium Phosphate; Ca, calcium; NaF, sodium fluoride; CPP-ACP-F, CPP-ACP with fluoride; HWP, hydrolyzed wheat protein; DIW, De-ionized distilled water; CA, Citric Acid; AS, artificial saliva; SEM, Scanning Electron Microscope; EDX, Energy-Dispersive X-Ray; EDS, Energy Dispersive Spectroscopy; Ca/P, Ca and P ratio.
Acknowledgments
The authors would like to thank BASF Corporation, 100 Park Ave, Florham Park, NJ, USA for their support in providing the mouthwash formulations for the study.
Disclosure
Mr Dennis Zhang reports grants from BASF, during the conduct of the study. In addition, Mr Dennis Zhang has a patent WO2021113709A1 pending to Colgate Palmolive, BASF Se. The authors report no other conflicts of interest in this work.
Mackenzie Hatfield Cvelich is an officer of the US Navy, however, the views expressed in this article reflects the results of research conducted by the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
References
1. Imfeld T. Dental erosion. Definition, classification and links. Eur J Oral Sci. 1996;104(2 Pt 2):151–155. doi:10.1111/j.1600-0722.1996.tb00063.x
2. Taji S, Seow WK. A literature review of dental erosion in children. Aust Dent J. 2010;55(4):358–475. doi:10.1111/j.1834-7819.2010.01255.x
3. Jaeggi T, Lussi A. Prevalence, incidence and distribution of erosion. Monogr Oral Sci. 2014;25:55–73. doi:10.1159/000360973
4. Kreulen CM, Van ‘t Spijker A, Rodriguez JM, Bronkhorst EM, Creugers NH, Bartlett DW. Systematic review of the prevalence of tooth wear in children and adolescents. Caries Res. 2010;44(2):151–159. doi:10.1159/000308567
5. Salas MM, Nascimento GG, Huysmans MC, Demarco FF. Estimated prevalence of erosive tooth wear in permanent teeth of children and adolescents: an epidemiological systematic review and meta-regression analysis. J Dent. 2015;43(1):42–50. doi:10.1016/j.jdent.2014.10.012
6. Yanushevich OO, Maev IV, Krikheli NI, et al. Prevalence and risk of dental erosion in patients with gastroesophageal reflux disease: a meta-analysis. Dent J. 2022;10(7):126. doi:10.3390/dj10070126
7. Pallier A, Karimova A, Boillot A, et al. Dental and periodontal health in adults with eating disorders: a case-control study. J Dent. 2019;84:55–59. doi:10.1016/j.jdent.2019.03.005
8. Paszynska E, Hernik A, Slopien A, et al. Risk of dental caries and erosive tooth wear in 117 children and adolescents’ anorexia nervosa population-a case-control study. Front Psychiatry. 2022;13:874263. doi:10.3389/fpsyt.2022.874263
9. Amaechi BT, Higham SM. Dental erosion: possible approaches to prevention and control. J Dent. 2005;33(3):243–252. doi:10.1016/j.jdent.2004.10.014
10. Reddy A, Norris DF, Momeni SS, Waldo B, Ruby JD. The pH of beverages in the United States. J Am Dent Assoc. 2016;147(4):255–263. doi:10.1016/j.adaj.2015.10.019
11. Søvik JB, Skudutyte-Rysstad R, Tveit AB, Sandvik L, Mulic A. Sour sweets and acidic beverage consumption are risk indicators for dental erosion. Caries Res. 2015;49(3):243–250. doi:10.1159/000371896
12. Zero DT, Lussi A. Erosion--chemical and biological factors of importance to the dental practitioner. Int Dent J. 2005;55(4 Suppl 1):285–290. doi:10.1111/j.1875-595x.2005.tb00066.x
13. Saads Carvalho T, Lussi A. Chapter 9: acidic beverages and foods associated with dental erosion and erosive tooth wear. Monogr Oral Sci. 2020;28:91–98. doi:10.1159/000455376
14. Milosevic A, Agrawal N, Redfearn P, Mair L. The occurrence of toothwear in users of Ecstasy (3,4-methylenedioxymethamphetamine). Community Dent Oral Epidemiol. 1999;27(4):283–287. doi:10.1111/j.1600-0528.1998.tb02022.x
15. Richards JR, Brofeldt BT. Patterns of tooth wear associated with methamphetamine use. J Periodontol. 2000;71(8):1371–1374. doi:10.1902/jop.2000.71.8.1371
16. Rao KA, Thomas S, Kumar JK, Narayan V. Prevalence of dentinal hypersensitivity and dental erosion among competitive swimmers, Kerala, India. Indian J Community Med. 2019;44(4):390–393. doi:10.4103/ijcm.IJCM_213_19
17. Hara AT, Zero DT. The potential of saliva in protecting against dental erosion. Monogr Oral Sci. 2014;25:197–205. doi:10.1159/000360372
18. Carvalho TS, Colon P, Ganss C, et al. Consensus report of the European Federation of Conservative Dentistry: erosive tooth wear--diagnosis and management. Clin Oral Investig. 2015;19(7):1557–1561. doi:10.1007/s00784-015-1511-7
19. Amaechi BT, Higham SM. In vitro remineralisation of eroded enamel lesions by saliva. J Dent. 2001;29(5):371–376. doi:10.1016/s0300-5712(01)00026-4
20. Attin T, Knöfel S, Buchalla W, Tütüncü R. In situ evaluation of different remineralization periods to decrease brushing abrasion of demineralized enamel. Caries Res. 2001;35(3):216–222. doi:10.1159/000047459
21. Somani R, Jaidka S, Singh DJ, Arora V. Remineralizing potential of various agents on dental erosion. J Oral Biol Craniofac Res. 2014;4(2):104–108. doi:10.1016/j.jobcr.2014.05.001
22. Passos VF, de Vasconcellos AA, Pequeno JH, Rodrigues LK, Santiago SL. Effect of commercial fluoride dentifrices against hydrochloric acid in an erosion-abrasion model. Clin Oral Investig. 2015;19(1):71–76. doi:10.1007/s00784-014-1213-6
23. Attin T, Deifuss H, Hellwig E. Influence of acidified fluoride gel on abrasion resistance of eroded enamel. Caries Res. 1999;33(2):135–139. doi:10.1159/000016507
24. Lussi A, Carvalho TS. The future of fluorides and other protective agents in erosion prevention. Caries Res. 2015;49(Suppl 1):18–29. doi:10.1159/000380886
25. Bellamy PG, Harris R, Date RF, et al. In situ clinical evaluation of a stabilised, stannous fluoride dentifrice. Int Dent J. 2014;64(Suppl 1):43–50. doi:10.1111/idj.12102
26. Lussi A, Buzalaf MAR, Duangthip D, et al. The use of fluoride for the prevention of dental erosion and erosive tooth wear in children and adolescents. Eur Arch Paediatr Dent. 2019;20(6):517–527. doi:10.1007/s40368-019-00420-0
27. Chawhuaveang DD, Yu OY, Yin IX, Lam WYH, Chu CH. Topical agents for nonrestorative management of dental erosion: a narrative review. Healthcare. 2022;10(8):1413. doi:10.3390/healthcare10081413
28. Körner P, Nguyen TP, Hamza B, Attin T, Wegehaupt FJ. Enamel softening can be reduced by rinsing with a fluoride mouthwash before dental erosion but not with a calcium solution. Oral Health Prev Dent. 2021;19(1):587–594. doi:10.3290/j.ohpd.b2259087
29. Lelli M, Putignano A, Marchetti M, et al. Remineralization and repair of enamel surface by biomimetic Zn-carbonate hydroxyapatite containing toothpaste: a comparative in vivo study. Front Physiol. 2014;5:333. doi:10.3389/fphys.2014.00333
30. Colombo M, Beltrami R, Rattalino D, Mirando M, Chiesa M, Poggio C. Protective effects of a zinc-hydroxyapatite toothpaste on enamel erosion: SEM study. Ann Stomatol. 2017;7(3):38–45. doi:10.11138/ads/2016.7.3.038
31. Poggio C, Gulino C, Mirando M, Colombo M, Pietrocola G. Protective effect of zinc-hydroxyapatite toothpastes on enamel erosion: an in vitro study. J Clin Exp Dent. 2017;9(1):e118–e122. doi:10.4317/jced.53068
32. Hornby K, Evans M, Long M, Joiner A, Laucello M, Salvaderi A. Enamel benefits of a new hydroxyapatite containing fluoride toothpaste. Int Dent J. 2009;59(6S1):325–331. doi:10.1002/idj.2009.59.6s1.325
33. Fabritius-Vilpoux K, Enax J, Herbig M, Raabe D, Fabritius HO. Quantitative affinity parameters of synthetic hydroxyapatite and enamel surfaces in vitro. Bioinspired Biomimet Nanobiomater. 2019;8(2):141–153. doi:10.1680/bibn.18.00035
34. Oberdoli D, Bommer C, Begzati A, Haliti F, Heinzel-Gutenbrunner M, Juric H. Randomized clinical trial investigating self-assembling peptide P11-4 for treatment of early occlusal caries. Sci Rep. 2020;10(1):4195. doi:10.1038/s41598-020-60815-8
35. Kind L, Stevanovic S, Wuttig S, et al. Biomimetic remineralization of carious lesions by self-assembling peptide. J Dent Res. 2017;96(7):790–797. doi:10.1177/0022034517698419
36. Ruan Q, Zhang Y, Yang X, Nutt S, Moradian-Oldak J. An amelogenin-chitosan matrix promotes assembly of an enamel-like layer with a dense interface. Acta Biomater. 2013;9(7):7289–7297. doi:10.1016/j.actbio.2013.04.004
37. Wang J, Liu Z, Ren B, et al. Biomimetic mineralisation systems for in situ enamel restoration inspired by amelogenesis. J Mater Sci Mater Med. 2021;32(9):115. doi:10.1007/s10856-021-06583-x
38. Ruan Q, Moradian-Oldak J. Amelogenin and enamel biomimetics. J Mater Chem B. 2015;3:3112–3129. doi:10.1039/C5TB00163C
39. Ruan Q, Moradian-Oldak J. Development of amelogenin-chitosan hydrogel for in vitro enamel regrowth with a dense interface. J Vis Exp. 2014;(89):51606. doi:10.3791/51606
40. Ruan Q, Siddiqah N, Li X, Nutt S, Moradian-Oldak J. Amelogenin-chitosan matrix for human enamel regrowth: effects of viscosity and supersaturation degree. Connect Tissue Res. 2014;55(Suppl1):150–154. doi:10.3109/03008207.2014.923856
41. Ruan Q, Liberman D, Bapat R, Chandrababu KB, Phark JH, Moradian-Oldak J. Efficacy of amelogenin-chitosan hydrogel in biomimetic repair of human enamel in pH-cycling systems. J Biomed Eng Inform. 2016;2(1):119–128. doi:10.5430/jbei.v2n1p119
42. Fan Y, Sun Z, Moradian-Oldak J. Controlled remineralization of enamel in the presence of amelogenin and fluoride. Biomaterials. 2009;30(4):478–483. doi:10.1016/j.biomaterials.2008.10.019
43. Mukherjee K, Ruan Q, Liberman D, White SN, Moradian-Oldak J. Repairing human tooth enamel with leucine-rich amelogenin peptide–chitosan hydrogel. J Mater Res. 2016;31(5):556–563. doi:10.1557/jmr.2016.64
44. Neshatian M, Holcroft J, Kishen A, De Souza G, Ganss B. Promoting mineralization at biological interfaces Ex vivo with novel amelotin-based bio-nano complexes. Mater Today Bio. 2022;14:100255. doi:10.1016/j.mtbio.2022.100255
45. Moradian-Oldak J. Protein-mediated enamel mineralization. Front Biosci. 2012;17(6):1996–2023. doi:10.2741/4034
46. Amaechi BT. Protocols to study dental caries in vitro: pH cycling models. Methods Mol Biol. 2019;1922:379–392. doi:10.1007/978-1-4939-9012-2_34
47. Featherstone JD, Stookey GK, Kaminski MA, Faller RV. Recommendation for a non-animal alternative to rat caries testing. Am J Dent. 2011;24(5):289–294.
48. Stookey GK, Featherstone JD, Rapozo-Hilo M, et al. The Featherstone laboratory pH cycling model: a prospective, multi-site validation exercise. Am J Dent. 2011;24(5):322–328.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.