Back to Journals » OncoTargets and Therapy » Volume 12
Monoclonal antibody AC10364 inhibits cell proliferation in 5-fluorouracil resistant hepatocellular carcinoma via apoptotic pathways
Authors Wu J, Qu J, Cao H, Jing C, Wang Z, Xu H, Ma R
Received 23 February 2019
Accepted for publication 16 May 2019
Published 28 June 2019 Volume 2019:12 Pages 5053—5067
DOI https://doi.org/10.2147/OTT.S206517
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Takuya Aoki
Jianzhong Wu,1,* Junwei Qu,1,* Haixia Cao,1 Changwen Jing,1 Zhuo Wang,1 Heng Xu,2 Rong Ma1
1Research Center for Clinical Oncology, Jiangsu Cancer Hospital, Jiangsu Institute of Cancer Research, Nanjing Medical University Affiliated Cancer Hospital, Nanjing, Jiangsu 210000, People’s Republic of China; 2Laboratory of Pharmaceutical Chemistry, Jiangsu Province Institute of Materia Medica, Nanjing Tech University, Nanjing, Jiangsu 211816, People’s Republic of China
*These authors contributed equally to this work
Background: This study was designed to investigate the antitumor activity of the mAb (AC10364) in vitro and elucidate the related mechanisms of inhibition to cell growth using bel/fu cells treated with AC10364.
Methods: The inhibitory effects of AC10364 on the proliferation of Bel/fu cells were examined using a cytotoxicity assay. Apoptosis of Bel/fu cells was detected using FITC annexin V and PI staining following treatment with AC10364 for 24 h. The factors regulating apoptosis were identified by Western blot using lysates of Bel/fu cells treated with AC10364 for 0, 12, 24, or 36 h. Genes associated with tumorigenesis or growth were analyzed by reverse transcription–quantitative polymerase chain reaction using Bel/fu cells treated for 12, 24, or 36 h with AC10364.
Results: The early apoptotic ratios of Bel/fu cells treated with AC10364 increased in a dose-dependent manner. The levels of caspases, including cleaved caspase-3, caspase-3 and caspase-9, were significantly high in Bel/fu cells treated with AC10364 (P<0.001). Compared with untreated cells, those exposed to AC10364 had showed significant downregulation of the expression of binding protein gene (G protein subunit α 15, GNA15) and other protein-coding genes, including fms-related tyrosine kinase 1(FLT1), nicotinamide phosphoribosyltransferase (NAMPT), netrin 4 (NTN4), platelet-derived growth factor subunit A (PDGFA), S100 calcium binding protein A11 (S100A11), tubulin β 3 class III (TUBB3), aldo-keto reductase family 1 member C3 (AKR1C3), endothelial PAS domain protein 1 (EPAS1), and interferon α-inducible protein 27 (IFI27) (P<0.001). Two other genes, AXL receptor tyrosine kinase (AXL) and carboxypeptidase A4 (CPA4), were significantly upregulated (P<0.001).
Conclusion: AC10364 inhibited cell viability and proliferation through aberrant expression of multiple genes associated with tumorigenesis or growth, which suggests that these genes may be promising therapeutic candidates for cancer therapy.
Keywords: monoclonal antibody, inhibitory, apoptosis, genes
Introduction
While many cancer patients benefit from postoperative chemotherapy which could improve the survival rate,1 development of tumor resistance to chemotherapy (chemoresistance) remains a major obstacle in cancer therapy. Multidrug resistance, which is the principal mechanism by which numerous cancer types develop resistance to chemotherapy drugs, is a major factor contributing to the failure of various forms of chemotherapy.2 Therefore, the development of novel therapeutic methods or strategies become the keys to cancer therapy.
Targeted immunotherapy using mAbs has become a key approach for ensuring the successful treatment of numerous cancer types and improving survival.3,4 Such as, Bevacizumab (Avastin), cetuximab (Erbitux), rituximab (Rituxan), and trastuzumab (Herceptin) are now widely used to treat non-small cell lung cancer, colorectal cancer, lymphoma, breast cancer.5,6 However, the majority of mAbs do not directly induce cell death but rather rely on immune effector mechanisms. Meanwhile, most tumor types could be prevented from immune attacks by different methods.5 The studies concentrated on the ability of mAbs to inhibit growth and induce apoptosis could not clarify inhibition mechanisms of cancer cell death.7,8 The development of mAbs which inhibited the growth of cancer cells relies on combine new targets and novel mechanisms of action.
This study focused on a novel mAb (AC10364) that was developed and screened through indirect immunofluorescence assay and flow-activated cell sorter analysis (FACS) from an anticancer antibody library generated by immunization of mice with human carcinoma cells.9 We have confirmed that Bel/fu, KATO III cells appeared to be more sensitive to mAb AC10364 in cytotoxicity assay, KATO III, Bel/fu, MCF 7 had strong reactivity to mAb AC10364 with two bands (about 54, 56-kDa), and AGS, Hela, NCI-N87 had weak reactivity to AC10364 with one band (about 54-kDa) in Western blotting using cells lysis. In this study, we examined the inhibitory effect of AC10364 on Bel/fu cells, and investigated cell cycle distribution after AC10364 treatment to elucidate the related inhibition mechanisms using molecular techniques.
Materials and methods
Cell lines
The Hep3B and L-02 cell lines, which were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in accordance with ATCC protocols, were used as contrasts for the cytotoxicity assay. Bel/fu, a 5-fluorouracil (5-FU)-resistant line of liver cancer, was presented from Dr. Wan-Zhou Zhao (Sino European Innovation Center, Nanjing, China), and has been authenticated by STR profile (Genetic Testing Biotechnology, Suzhou, China). BEL-7402 cells were domesticated through continuous 5-FU stimulation, after which a drug-resistant cell strain was formatted and named Bel/fu. The use of Bel/fu cell line has been approved by the human subject ethics committee of the Jiangsu Cancer Hospital. All cells were grown in RPMI 1640 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 10% fetal bovine serum (Hyclone, CA, USA) at 37 °C under 5% CO2.
Cytotoxicity assay
The effects of AC10364 (developed in our laboratory) on the viability and proliferation of cancer cells were assessed. Cell viability and proliferation were determined using Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan). Briefly, Bel/fu, Hep3B, and L-02 cells were seeded into 96-well plates at a density of 3×103 (50 µL) per well and incubated for 24 h at 37 °C. Following incubation, AC10364 (10 mg/mL) was serially diluted with RPMI 1640 medium to achieve concentrations of 4, 12, 37, 111, 333 and 1,000 μg/mL. Then, 50 μL of the mAb was mixed with an equal volume of the cells. After incubation for 72 h at 37 °C, the cells were incubated with 3 µL of CCK-8 for 4 h at 37 °C, and their absorbance was measured at 450 nm by using a microplate reader (Thermo Fisher Scientific, Inc.). Wells containing untreated or L-02 cells served as the controls. Growth inhibition curves were plotted as percentages of untreated control cells with respect to the standard curves, and half-maximal inhibitory concentrations (IC50) were calculated. Morphological changes in Bel/fu cells upon incubation with the mAb (250 μg/mL) were observed under a phase-contrast microscope (Nikon, Japan) at 100× magnification.
Cell cycle analysis
Cell cycle analysis was performed as previously described.10 Bel/fu cells were cultured in three different concentrations of AC10364 (50, 150, or 250 μg/mL) for 24 h at 37 °C under 5% CO2. The same concentrations of non-specific mouse immunoglobulin G (IgG) (Invitrogen, CA, USA) were used as isotype controls. The Bel/fu cells were harvested, fixed with 70% ethanol at 4 °C overnight, and then stained with 50 µg/mL of propidium iodide (PI) containing 0.25 mg/mL of RNase A at room temperature for 30 min. Analysis was performed using a FACS-CaliburTM instrument equipped with CELLQuestTM software (Becton Dickinson, NJ, USA).
Cell death analysis
Bel/fu cells were treated with AC10364 for 24 h at 37 °C under 5% CO2. The same concentrations (250 µg/mL) of non-specific mouse IgG were used as isotype controls. Then, the cells were washed twice with ice-cold, phosphate-buffered saline and incubated in 5 µL of annexin V fluorescein isothiocyanate (FITC) and 5 µL of PI at room temperature in the dark for 15 min. Analysis was performed using a FACS-CaliburTM instrument equipped with CELLQuestTM software (Becton Dickinson, NJ, USA).
Western blot analysis
A total of 1×106 Bel/fu cells were treated with AC10364 (150 µg/mL) and collected 0, 12, 24, or 36 h after mAb incubation. Following centrifugation at 1,200×g for 5 min at 4 °C, the pellet was lysed using P-MERTM mammalian protein extraction reagent (1 mL; Thermo Fisher Scientific, Inc.) containing 1% Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific, Inc.) for 30 min at 4 °C. The supernatant was collected after centrifugation at 1,200×g for 10 min at 4 °C. The protein concentration was measured using a spectrophotometer and modulated to a concentration of 5 µg/µL. The sample was denatured using sample loading buffer (Invitrogen, CA, USA) for 10 min at 95 °C and stored at 4 °C for future use. Exactly 10 µL of cell lysate protein was loaded per lane on an SDS-PAGE setup with 12% gel (approximately 50 µg/lane), transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA), immune-blotted with AC10364 at 4 °C overnight, and probed with specific Abs, including anti-β-actin, anti-tumor protein p53 (p53) (Santa Cruz Biotechnology, Inc., Dallas, TX, USA), anti-caspase-3, anti-cleaved caspase-3, anti-caspase-9, anti-B-cell lymphoma 2 (Bcl-2), anti-Bad, anti-Bcl-xl, anti-Bcl-2-associated X protein (Bax) (Cell Signaling Technology, Inc., Danvers, MA, USA), anti-Fas (Abcam, USA). All antibodies were diluted in blocking buffer (1% BSA-TBST). The PVDF membranes were washed five times for 5 min each time, treated with the appropriate horseradish-conjugated secondary antibody (Santa Cruz Biotechnology, USA), and then incubated at a dilution of 1:5,000, in accordance with the manufacturer’s protocol, in blocking buffer (1% BSA-TBST) for 1 h at room temperature. Thereafter, the PVDF membranes were washed five times for 5 min each time and visualized using ECL chemiluminescence reagent (Thermo Scientific, USA) by Imager (Bio-Rad, USA).
Reverse transcription–quantitative polymerase chain reaction analysis (RT–qPCR)
A total of 1×106 Bel/fu cells were treated with AC10364 (250 µg/mL) at 37 °C under 5% CO2 and collected at 0, 12, 24, or 36 h after incubation. Total RNA was extracted from the Bel/fu cells by using a mirVana microRNA isolation kit in accordance with the manufacturer’s protocol (Thermo Fisher Scientific, Inc.) and eluted in 100 µL of heated elution solution (Applied Biosystems, USA) in accordance with the manufacturer’s protocol. Cellular RNA was obtained using Trizol extraction reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and eluted in 30 µL of ddH2O. Complementary cDNA was synthesized from total RNA (2 µg per sample) using a PrimeScript RT Reagent Kit (Takara Biotechnology Co., Ltd., Dalian, China) in accordance with the manufacturer’s protocol. RT–qPCR analysis was performed using a StepOnePlus™ Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.) with SYBR Premix Ex Taq II (Takara Biotechnology Co., Ltd.) to determine the expression of each mRNA.11 The forward and reverse primers are listed in the Table S1. Thermocycling conditions were as follows: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. GAPDH was used as the reference gene. Relative levels of gene expression are presented as ΔCq = Cq gene – Cq reference; the fold change (FC) of gene expression was calculated. Relative gene expression data were analyzed by real-time qPCR and the 2−ΔΔCt method proposed by Livak and Schmittgen in 2001.12 All experiments were conducted in duplicate, and all gene sequences were extracted from NCBI. All primers were designed by Primer 3.0 and obtained from Thermo Fisher Scientific, Inc.
Statistical analysis
All data were analyzed by ANOVA (Version 19, IBM SPSS Statistics). P-values <0.05 were considered statistically significant. Data from the cytotoxicity assay and Western blot analysis are presented as mean ± standard deviation, and data from our gene analysis indicate mean values with 95% confidence intervals. Differences between two groups were examined using an unpaired t-test or Mann-Whitney test. Comparisons among multiple groups were performed using one-way ANOVA and a post hoc test under the assumption that least-significant differences were equal to the variances to realize multiple comparisons of the inhibitory rates of cancer cells and expressions of p53, caspase-3, caspase-9, Bcl-2, and Bax proteins after mAb treatment. A hierarchical clustering analysis with gene tree was used in the heat map. Cluster analysis was performed using HCL cluster software (MeV4.9.0) to group differentially expressed genes exhibiting similar expression patterns. Data were logarithmically transformed prior to all statistical analyses (log2). Absolute values were used for comparing FC among expressed genes. Relative expression levels of genes are shown in log 2 scale. FC for each time point (12 h, 24 h and 48 h) was compared with untreated controls (0 h). The network analysis was performed to construct molecular interaction networks using online STRING database (https://string-db.org/cgi/input.pl). The network mapping was imported using Cytoscape software (version 3.6.1). Gene Ontology term analysis of the main gene expression was dot-plotted through clusterProfiler packages in R language.
Results
AC10364 inhibits cancer cell viability and proliferation
The inhibitory effects of AC10364 on the viability and proliferation of Bel/fu cells were examined using a cytotoxicity assay. Figure 1A presents the morphological changes in Bel/fu cells treated with AC10364 (250 μg/mL) as observed under a phase-contrast microscope. Cell growth was halted at 24 h after mAb treatment. The inhibition curves in Figure 1B reveal that the Bel/fu cell line is sensitive to AC10364 (IC50, 78.7 μg/mL); by comparison, Hep3B cells exhibited weaker sensitivity to AC10364 (IC50, 566.6 μg/mL). Compared with those of L-02 cell viability, the inhibitory rates of Bel/fu cancer cell viability significantly increased along with an increase in AC10364 concentration (P<0.001; Figure 1B). When Bel/fu cells were treated with 250 μg/mL AC10364 for 24, 48, and 72 h, the inhibitory rates of Bel/fu cell proliferation increased with longer treatment time and were significantly higher than those of L-02 cell inhibition at the same time points (P<0.01; Figure 1C). These results demonstrate that AC10364 inhibits the viability and proliferation of Bel/fu cells in a dose- and time-dependent manner.
AC10364 affects the cell cycle distribution
The cell cycle distribution of Bel/fu cells was analyzed following treatment with 50, 150, or 250 μg/mL of AC10364 for 24 h. As shown in Figure 2A and B, the percentage of the cell population in the G1 phase decreased, whereas the proportion in the S phase increased, compared with the corresponding percentages in the control cells; that suggests cell cycle is arrested in the S phase after AC10364 treatment. No substantial change was observed in the G2/M phase of mAb- or IgG-treated Bel/fu cells. In addition, the cell population in the G1 and S phases remained unchanged with increasing concentration of AC10364 treatment (Figure 2B).
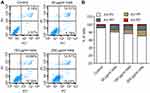
AC10364 mAb induces Bel/fu cell apoptosis
Apoptosis of cancer cells was detected using FITC annexin V and PI staining following treatment with AC10364 for 24 h. Here, annexin V-positive cells, including both early (AV+/PI-) and late (AV+/PI+) apoptotic cells, were considered apoptotic cells. Figure 3A and B demonstrate that the early apoptotic ratios of Bel/fu cells treated with 50, 150, or 250 μg/mL of mAb substantially increased in a dose-dependent manner by 5.78%, 10.39%, and 15.03%, respectively, relative to the corresponding ratios in the control cells. By contrast, minimal differences were observed in the late apoptotic ratios of Bel/fu cells treated with various concentrations of mAb relative to the corresponding ratios in the control cells. AC10364 treatment increased the late apoptotic ratios of Bel/fu cells, but not in a dose-dependent manner (Figure 3B).
In Western blot analysis, factors regulating apoptosis were identified using lysates of Bel/fu cells treated with mAb for 0, 12, 24, or 36 h (Figure 4). The levels of cleaved caspase-3 was gradually increasing with longer treatment time (P<0.001). Expression of caspase-3 was significantly higher in Bel/fu cells treated with AC10364 than in the control cells, which suggests that apoptosis induced by the mAb is irreversible (P<0.001). Similarly, expression of caspase-9 was significantly higher in Bel/fu cells treated with AC10364, which suggests that treatment activates the mitochondrial apoptosis pathway (P<0.001). Expression of p53 in Bel/fu cells gradually increased from 0 h to 36 h after mAb treatment (P<0.001). Although expression of Bcl-2 in Bel/fu cells gradually decreased from 0 to 36 h after mAb treatment, there was no significant difference in Bcl-2 expression compared with that in the control (P>0.05), while the expression of Bcl-xL was remarkably decreased (P<0.001). The expressions of Bax and Bad in treated cells significantly increased relative to that in the control cells only at 36 h (P<0.01, P<0.001 respectively). In addition, the level of Fas, as an apoptotic initiator, was steadily increased from 0 to 36 h after mAb treatment (P<0.001). The results of the Western blot analysis are consistent with those of FITC annexin V and PI staining and confirm that AC10364 induces apoptosis in Bel/fu cells.
AC10364 mAb regulates the expression of multiple genes
Given that the objective of this study was to elucidate the molecular mechanisms underlying the effect of AC10364 on cancer growth, the validity of the expression data was assessed by analyzing a subset of candidate genes (53 tumorigenesis- or growth-associated genes) through RT–qPCR. Different sets of RNA samples were obtained at different time points (12, 24, and 36 h after treatment), and the cluster analysis results of these genes are presented in Figure 5. Distinct clusters of genes were identified. The cluster genes AXL receptor tyrosine kinase (AXL), BCL2-like 1, BRCA1, DNA repair-associated, Bloom syndrome RecQ-like helicase, interferon regulatory factor 8, suppression of tumorigenicity 5, erb-b2 receptor tyrosine kinase 2, V-set immunoregulatory receptor, signal transducer and activator of transcription (STAT3), and STAT5B were initially upregulated; expression of these genes peaked at 12 h, after which it was subsequently downregulated. The clustered genes DNA topoisomerase II α, ribonucleotide reductase catalytic subunit M1, thymidylate synthetase, RAS protein activator-like 2, insulin-like growth factor 1 receptor, WW and C2 domain-containing 1, stathmin 1, and zinc finger protein 112 were slightly upregulated at 12, 24, and 36 h. Other cluster genes, including G protein subunit α 15 (GNA15), aldo-keto reductase family 1 member C3 (AKR1C3), endothelial PAS domain protein 1 (EPAS1), and interferon α-inducible protein 27 (IFI27), were downregulated continuously from 12 h to 36 h. Gene Ontology enrichment analysis of the main gene expression were summarized in Figure 6. The functional relationships among these genes were depicted in a gene regulatory network (Figure 7). EGFR gene has the highest connectivity among all detected genes in the network. Subsequently, the STAT3 gene and ERBB2 gene also have higher connectivity than others.
 |
Figure 6 Gene ontology enrichment analysis of the main gene expression. |
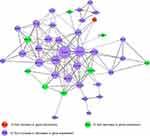 |
Figure 7 A network illustrating the connectedness of the genes associated with tumorigenesis or growth. |
A total of 12 regulated genes were selected, and the expression of these genes is presented in Figure 8. GNA15 and other protein-coding genes, including fms-related tyrosine kinase 1 (FLT1), nicotinamide phosphoriosyltransferase (NAMPT), netrin 4 (NTN4), platelet-derived growth factor subunit A (PDGFA), S100 calcium-binding protein A11 (S100A11), tubulin β class III (TUBB3), AKR1C3, EPAS1, and IFI27, were significantly downregulated by AC10364 treatment (P<0.001); however, two genes, AXL and carboxypeptidase A4 (CPA4), were significantly upregulated (P<0.01; Figure 8). Most genes with a three- to fourfold difference in expression were significantly downregulated, whereas CPA4, which showed more than a fourfold difference in expression, was significantly upregulated by AC10364 treatment. The expression levels of most genes were similar at 24 and 36 h following after AC10364 treatment. By contrast, expression of GNA15 and CPA4 was further down- or upregulated at 36 h compared with corresponding levels at 24 h. AXL expression was upregulated more significantly at 12 h than at 24 or 36 h, and NTN4 expression was downregulated more considerably at 12 h than at 24 or 36 h. AKR1C3 expression was downregulated at 12 h and then even further at 24 and 36 h (Figure 6). These results demonstrate that AC10364 treatment changes the expression of multiple genes associated with tumorigenesis or cell growth in Bel/fu cells.
Discussion
In cancer immunotherapy, effective therapeutic Abs typically require cytostatic and cytotoxic abilities.9 In the present study, AC10364 inhibited the proliferation and viability of Bel/fu cells with an IC50 of 78.7 μg/mL. Morphological changes were detected in Bel/fu cells upon incubation with mAb (250 μg/mL), which suggests that viability and proliferation were inhibited (Figure 1A). Furthermore, in the previous study, we have reported the reactivity with cells lysis of mAb AC10364 in Western blot, which showed there were different reactivities in binging strength and molecular weight of reactive protein among different cancer cells. Indeed, the sensitivity to AC10364 on cell inhibition in Bel/fu cells was higher than that of Hep3B and LO-2 cells. The possible reason is the reactivity of mAb AC10364 to Bel/fu cells was stronger than Hep3B and LO-2 cells, suggesting that it is necessary to clarify the AC10364’s binding molecule on the surface of Bel/fu cells. Marked S-phase arrest and apoptosis with growth inhibition were observed in AC10364-treated Bel/fu cells (Figures 2 and 3). Treatment with the mAb decreased the percentage of cells undergoing the G1 phase and increased the proportion of cells in the S phase; this suggests that DNA damage caused by a certain phase block may be the result of accumulation of damage in each phase or the result of DNA sensitivity to damage in that phase. Western blot analysis also revealed significantly increased expression of p53, a tumor-suppressor protein, following AC10364 treatment compared with that in control cells (P<0.001; Figure 4D). This protein mediates G1 phase arrest in response to various stress stimuli.13 Nevertheless, in the present study, the G1 phase was not blocked despite an AC10364-mediated increase in the expression of p53. It is possible that incomplete DNA replication and repair of DNA damage may cause the majority of cells to fail at the cell-division phase and undergo arrest at the S phase.
Caspases are proteolytic enzymes that serve critical roles in apoptosis. Activation of caspases results in irreversible biochemical and morphological changes in cells. Caspase-3, for example, is the first of all effector caspases activated to amplify downstream apoptotic processes,14 and activation of the enzyme occurs rapidly during cell death.15 In this study, we used Western blot analysis to reveal the apoptotic pathways induced by AC10364 treatment. Specifically, treatment activated the mitochondrial apoptosis pathway, as indicated by activation of the initiators of this pathway, including caspase-3 and caspase-9. Consistent with previous findings on caspase-3/7 staining in indirect immunofluorescence assay (IFA),9 apoptosis was detected by annexin V staining of Bel/fu cells 24 h after mAb treatment (Figure 3).
A series of oncogenes and proto-oncogenes in cancer cells are activated and overexpressed, which resulted in out-of-control growth of the malignant cancer cells. It is generally believed that the mechanism of cell apoptosis is inhibited, leading to the failure of clearance of death cells. One of the oncogenes belongs to the family of growth factors and the other belongs to the family of growth factor receptors, which these oncogenes and their expression products are also important regulators of apoptosis. After many kinds of oncogenes are expressed, they block the apoptosis process of cancer cells and increase the number of cancer cells.16,17 Therefore, a method for cancer therapy could be designed by the reconstruction of apoptosis signal transmission system through apoptosis mechanism of cancer cells. EGFR is the expression product of proto-oncogene c-erbB1 and is one of the members of epidermal growth factor receptor (HER) family which plays an important regulatory role in cell physiological process.18,19 In the present study, EGFR gene has the highest connectivity and was a regulatory hub in the network (Figure 7), suggesting that EGFR, as a hub gene, plays an important role in cell growth and proliferation. However, the expression of EGFR was slightly regulated in mAb-treated Bel/fu cells. Meanwhile, these genes which >2 fold decreased or increased in gene expression (P<0.001), such as AXL, FLT1, PDGFA, PDGFRB, has inferior connectivity in network (Figure 7). The possible reason maybe that the effect of mAb AC10364 on inhibiting the growth of Bel/fu cells has not fully exerted at concentration of 250 μg/mL.
We assessed the validity of the expression by analyzing a subset of genes using real-time RT-PCR in mAb-treated Bel/fu cells (Figure 8). The expression levels of these genes (AXL, FLT1, GNA15, NAMPT, NTN4, PDGFA, S100A11, TUBB3, AKR1C3, CPA4, EPAS1, and IFI27) were significant increased or decreased in Bel/fu cells after AC10364 treatment (P<0.001). AXL belongs to the receptor tyrosine kinase family. When combined with its ligand, growth arrest specific 6, AXL may activate the Ptdlns 3-kinase and downstream signaling pathways.20 AXL participates in cell adhesion, proliferation, and anti-apoptotic activities.21 Aberrant expression of AXL is associated with the pathogenesis and development of various tumor types and contributes to inhibition of tumor apoptosis, tumor angiogenesis, and invasion.22,23 In the present study, aberrant expression of AXL was observed at 12, 24, and 36 h after mAb treatment, suggesting that AXL plays an important role in inhibiting Bel/fu cell proliferation. The expression levels of all tested genes, except those of AXL and CPA4, significantly decreased following mAb treatment. CPA4, an enzyme in humans encoded by the CPA4 gene, is secreted from cells in a soluble form and, once activated, has a neutral pH optimum. That suggests that its function is related to the extracellular environment rather than the secretory pathway.24 Kayashima et al reported that CPA4 is preferentially expressed in benign prostatic hyperplasia and may be a strong candidate gene for prostate cancer aggressiveness.25 The observed increase in CPA4 expression in the present study indicates that the gene may be associated with the anti-apoptotic response of cells treated with AC10364. Angiogenesis is an important hallmark of cancer.26 Vascular endothelial growth factor (VEGF), which exerts its functions mainly by binding to VEGF receptor 1 (FLT1/VEGFR1) and VEGF receptor 2, is the primary regulator of angiogenesis. Downregulation of FLT1 is associated with inhibition of cell growth by mAb treatment. Therefore, FLT1 functions as a decoy VEGF receptor enabling regulation of VEGF in the vascular endothelium.27 The findings suggested that these inhibited or activated genes associated with cell growth, such as AXL, FLT1, are closely related to the process of apoptosis after Bel/fu cells were treated with mAb AC10364. When apoptosis of Bel/fu cells treated with AC10364 was induced, the expression of hub gene (such as EGFR) has also activated by these interaction genes down- or upregulated. Therefore, the disorder of expression of the hug gene inhibited the growth of Bel/fu cells treated with AC10364, accompanied by the process of cell apoptosis. In conclusion, AC10364 significantly inhibited cell viability and proliferation and induced apoptosis in Bel/fu cells. The mAb increased or decreased expression of multiple genes associated with tumorigenesis or growth compared with expression in untreated cells, suggesting that these genes may be promising therapeutic targets for cancer therapy.
Acknowledgments
The mAb named AC10364 was developed by Heng Xu. The hybridoma of AC10364 was preserved in the China Center for Type Culture Collection (CCTCC No.: C201440).
The present study was supported by the Key Projects of Jiangsu Science and Technology Department (Grant No. BE2016795), the Jiangsu Provincial Science Foundation (Grant No. BK20141019), and the Projects of Jiangsu Cancer Hospital (Grant No. ZK201608).
Disclosure
Dr Heng Xu reports the patent Monoclonal Antibody against Bel/fu cells and Hybridoma Cell Line licensed to ZL201410194267.9. The authors declare no other conflicts of interests in this work.
References
1. Dhanasekaran R, Limaye A, Cabrera R. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis, and therapeutics. Hepat Med. 2012;4:19–37. doi:10.2147/HMER.S16316
2. Higgins CF. Multiple molecular mechanisms for multidrug resistance transporters. Nature. 2007;446(7137):749–757. doi:10.1038/nature05630
3. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2016;12(4):252–264. doi:10.1038/nrc3239
4. Sadozai H, Gruber T, Hunger RE, Schenk M. Recent successes and future directions in immunotherapy of cutaneous melanoma. Front Immunol. 2017;8:1617. doi:10.3389/fimmu.2017.01617
5. Grillo-Lopez AJ, Hedrick E, Rashford M, Benyunes M. Rituximab: ongoing and future clinical development. Semin Oncol. 2002;29(1S2):105–112. doi:10.1053/sonc.2002.30145
6. Yip YL, Ward RL. Anti-ErbB-2 monoclonal antibodies and ErbB-2-directed vaccines. Cancer Immunol Immunother. 2002;50(11):569–587. doi:10.1007/s002620100226
7. de Weers M, Tai YT, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840–1848. doi:10.4049/jimmunol.1003032
8. Crescence L, Beraud E, Sbarra V, Bernard JP, Lombardo D, Mas E. Targeting a novel onco-glycoprotein antigen at tumoral pancreatic cell surface by mAb16D10 induces cell death. J Immunol. 2012;189(7):3386–3396. doi:10.4049/jimmunol.1102647
9. Xu H, Tian YN, Dun BY, et al. A novel monoclonal antibody induces cancer cell apoptosis and enhances the activity of chemotherapeutic drugs. Asian Pac J Cancer Prev. 2014;15(11):4423–4428.
10. Shen X, Zhu HF, He FR, et al. An anti-transferrin receptor antibody enhanced the growth inhibitory effects of chemotherapeutic drugs on human non-hematopoietic tumor cells. Int Immunopharmacol. 2008;8(13–14):1813–1820. doi:10.1016/j.intimp.2008.08.022
11. Li SC, Ma R, Wu JZ, et al. Delineation of gastric cancer subtypes by co-regulated expression of receptor tyrosine kinases and chemosensitivity genes. Am J Transl Res. 2015;7(8):1429–1439.
12. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
13. Sun XX, Dai MS, Lu H. Mycophenolic acid activation of p53 requires ribosomal proteins L5 and L11. J Biol Chem. 2008;283(18):12387–12392. doi:10.1074/jbc.M801387200
14. Salvesen GS, Riedl SJ. Caspase mechanisms. Adv Exp Med Biol. 2008;615:13–23. doi:10.1007/978-1-4020-6554-5_2
15. Tyas L, Brophy VA, Pope A, Rivett AJ, Tavare JM. Rapid caspase-3 activation during apoptosis revealed using fluorescence-resonance energy transfer. EMBO Rep. 2000;1(3):266–270. doi:10.1093/embo-reports/kvd050
16. Horvitz HR. Nobel lecture. worms, life and death. Biosci Rep. 2003;23(5–6):239–303.
17. Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2014;2014:150845. doi:10.1155/2014/150845
18. Franklin WA, Veve R, Hirsch FR, Helfrich BA, Bunn PA
19. Serrano MJ, Alvarez-Cubero MJ, De Miguel Pérez D, et al. Significance of EGFR expression in circulating tumor cells. Adv Exp Med Biol. 2017;994:285–296. doi:10.1007/978-3-319-55947-6_16
20. O’Bryan JP, Frye RA, Cogswell PC, et al. Axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11(10):5016–5031. doi:10.1128/mcb.11.10.5016
21. Nagata K, Ohashi K, Nakano T, et al. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem. 1996;271(47):30022–30027. doi:10.1074/jbc.271.47.30022
22. Wu YM, Robinson DR, Kung HJ. Signal pathways in up-regulation of chemokines by tyrosine kinase MER/NYK in prostate cancer cells. Cancer Res. 2004;64(20):7311–7320. doi:10.1158/0008-5472.CAN-04-0972
23. Li Y, Ye X, Tan C, et al. Axl as a potential therapeutic target in cancer: role of Axl in tumor growth, metastasis and angiogenesis. Oncogene. 2009;28(39):3442–3455. doi:10.1038/onc.2009.212
24. Tanco S, Zhang X, Morano C, Avilés FX, Lorenzo J, Fricker LD. Characterization of the substrate specificity of human carboxypeptidase A4 and implications for a role in extracellular peptide processing. J Biol Chem. 2010;285(24):18385–18396. doi:10.1074/jbc.M109.060350
25. Kayashima T, Yamasaki K, Yamada T, et al. The novel imprinted carboxypeptidase A4 gene (CPA4) in the 7q32 imprinting domain. Hum Genet. 2003;112(3):220–226. doi:10.1007/s00439-002-0891-3
26. Xu WW, Li B, Lam AK, et al. Targeting VEGFR1- and VEGFR2-expressing non-tumor cells is essential for esophageal cancer therapy. Oncotarget. 2015;6(3):1790–1805. doi:10.18632/oncotarget.2781
27. Claussa M. Functions of the VEGF receptor-1 (FLT-1) in the vasculature. Trends Cardiovasc Med. 1998;8(6):241–245. doi:10.1016/S1050-1738(98)00015-2
Supplementary material
 |
Table S1 The forward and reverse primers of all genes |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.