Back to Journals » Journal of Inflammation Research » Volume 15
Monitoring and Management of the Patient with Immune Checkpoint Inhibitor-Induced Inflammatory Arthritis: Current Perspectives
Received 20 January 2022
Accepted for publication 18 March 2022
Published 25 May 2022 Volume 2022:15 Pages 3105—3118
DOI https://doi.org/10.2147/JIR.S282600
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Karmela K Chan, Anne R Bass
Department of Medicine, Hospital for Special Surgery/Weill Cornell Medicine, New York, NY, USA
Correspondence: Anne R Bass, Email [email protected]
Abstract: In this review, we draw from observational studies, treatment guidelines and our own clinical experience to describe approaches to monitoring and management of immune checkpoint inhibitor (ICI)-induced inflammatory arthritis, including polymyalgia rheumatica. This condition occurs in about 4% of ICI-treated cancer patients and can persist for a year or longer. Mild arthritis can generally be managed with non-steroidal anti-inflammatory drugs, intraarticular steroids injections and/or low dose corticosteroids. Higher grade arthritis should be brought under control with corticosteroids, but early introduction of a steroid-sparing agent is recommended to minimize steroid toxicity. In order to assess the effectiveness of any arthritis treatment, tender and swollen joint counts and patient reported measures of physical function, such as the health assessment questionnaire, should be obtained at each visit. Referral to a rheumatologist is recommended for patients with high grade arthritis to help guide the use of disease-modifying antirheumatic drugs.
Keywords: arthritis, checkpoint inhibitor, immunotherapy, adverse event, cancer, treatment
Introduction
Since the approval of ipilimumab in 2011 for metastatic melanoma, a raft of immune checkpoint inhibitors (ICI) have come into use for a wide variety of metastatic cancers,1–4 for adjuvant therapy of locally advanced tumors,5,6 and for microsatellite instability (MSI)-high tumors agnostic of tumor origin.7,8
The inhibitory “checkpoint molecules” targeted by these agents include cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed cell death-1 (PD-1) which are expressed on the surface of T cells, as well as the PD-1 ligand (PD-L1) expressed by tumor macrophages and some cancer cells (Table 1). When engaged, checkpoint molecules turn off T cell activation and prevent runaway autoreactivity and cytotoxicity. However, engagement of checkpoint molecules also prevents effective clearance of cancer cells by the immune system. Blockade of CTLA-4, PD-1 or PD-LI by ICI prevents inhibitory signaling and allows ongoing anti-tumor immunity.9
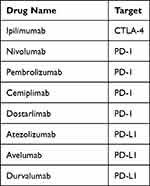 |
Table 1 Currently Available Immune Checkpoint Inhibitors |
While ICI can effectively treat many cancers, they can also result in immune-related adverse events (irAE) as a consequence of off-target activation of T-lymphocytes. These irAE can involve a variety of organ systems, and the joints are not exempt.10 ICI-induced inflammatory arthritis (“ICI-arthritis”) often goes unrecognized by oncologists who, like most non-rheumatologists, are often unaccustomed to taking a musculoskeletal history or performing a musculoskeletal examination. Left untreated, ICI-induced arthritis can render a patient unable to perform the most basic of tasks such as walking, washing, or dressing, and joint damage from ICI-induced arthritis can, in severe cases, lead to the need for joint replacement.11 Unlike other irAE, ICI-arthritis tends to persist.12,13 In our ICI-arthritis registry, 58% of patients continued to have symptoms and require treatment at 12 months of follow-up.12 Both the persistence of ICI-arthritis and its potential for joint damage justify early intervention to control disease. In this review, we discuss evaluation, treatment and monitoring of ICI-arthritis by drawing on literature review, treatment guidelines and our own clinical experience.
Incidence and Risk Factors
The incidence of arthralgia in ICI clinical trials ranges from 1% to 43%, and of arthritis 1–7% according to a 2017 systematic literature review.14 The frequency of arthralgia in clinical trials varies based on the ICI used, and is reported to be 11%, 8% or 5% after treatment with combination anti-PD-1/CTLA-4, anti-PD-(L)1 or anti-CTLA-4, respectively.15 The incidence of true inflammatory arthritis (with joint swelling) after ICI was 3.8% in one prospectively followed cancer cohort.16 The frequency of ICI-induced polymyalgia in ICI treated patients has not been established. In one retrospective study, ICI-arthritis was found to be more common in patients with melanoma and genitourinary cancers than in patients with lung cancer, more common with combination ICI than with anti-PD1 monotherapy, and more common in patients with a history of a non-rheumatic autoimmune condition.17 Patients with ICI-arthritis are more likely than the general population to carry at least one RA-associated HLA allele (referred to as the “shared epitope”) though they are less likely to be homozygous for the allele than RA patients.18
Clinical Presentation
ICI-arthritis can present in a variety of ways.19 Most commonly it presents as polyarthritis, similar to rheumatoid arthritis (RA). ICI-arthritis can also present as a mono- or oligoarthritis with or without enthesitis akin to a spondyloarthropathy. Another ICI-arthritis phenotype is a polymyalgia rheumatica (PMR)-like syndrome, in which there is bilateral shoulder and hip girdle pain and prolonged morning stiffness. Many patients with ICI-induced PMR-like symptoms have concomitant peripheral synovitis.20,21 Because peripheral synovitis is so common in the ICI-associated PMR phenotype, because many ICI-arthritis cohorts in the literature include patients with ICI-PMR, and because it has not been established that ICI-associated PMR is in fact an entity distinct from ICI-arthritis, we have included it here under the ICI-arthritis umbrella. Finally, there is also an entity, termed “activated osteoarthritis”, in which patients experience an exacerbation of joint pain in an area of previously identified osteoarthritis, but without morning stiffness or inflammatory synovial fluid.22 It is unclear whether this represents a true irAE or the chance occurrence of an osteoarthritis flare after ICI treatment. Median time of onset of ICI-arthritis is 2.8 months after ICI initiation, though the range is wide, and some patients can present after immunotherapy has been discontinued.12
Patient Assessment
Although oncologists rely on the Common Terminology Criteria for Adverse Events (CTCAE) assessment of irAE grade (Table 2),23 a more refined assessment of arthritis extent and severity allows clinicians to better judge the effectiveness of therapy. This includes documentation of the specific joints affected, and of the patient’s level of pain and physical function. Many electronic medical records have a homunculus to document tender and/or swollen joints.
 |
Table 2 Common Terminology Criteria for Adverse Events (CTCAE) V5.0 Grading of Arthritis |
The Clinical Disease Activity Index (CDAI) is a simple measure of arthritis disease activity that adds the total tender and swollen joint count (0–28 each) plus the patient’s and the physician’s global arthritis activity scores (0–10 each, in 0.5 increments).24 A CDAI ≤ 2.8 represents remission, 2.8–10 represents low disease activity, >10–22 moderate disease activity and >22 high disease activity. The Health Assessment Questionnaire (HAQ) can be used to assess function and is graded on a scale of 0–3.0.25 The duration of morning stiffness can be used as another metric of ICI-arthritis disease activity. These measures are validated in RA and, while not validated in ICI-arthritis, do offer clinical utility.
Laboratory Testing
Measuring an erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP) can provide additional information about the degree of inflammation and corroborate clinical findings, however inflammatory markers are not always elevated. In our ICI-arthritis registry, median [IQR] CRP at presentation was 1.5 mg/dl [0.7, 3.1] in patients with small joint involvement (“RA-like”), and 1.1 mg/dl [0.2, 12.3] in those with a PMR-like presentation.12
Serologic studies may be positive in ICI-arthritis, but rarely help with diagnosis or management. For example, approximately 22% of patients with ICI-arthritis will have a positive ANA, but ICI-induced lupus is rare and other lupus related autoantibodies are uncommon (eg, 9% are SSA/SSB positive).26 Nine percent of patients with ICI-arthritis have a positive CCP and/or rheumatoid factor, but many seronegative ICI-arthritis patients have an RA-like presentation19 and seropositive patients can present with any ICI-arthritis phenotype, including PMR.12 Whether seropositive ICI-arthritis patients were seropositive prior to treatment is usually not known. In our study of 60 melanoma patients treated with combination ICI (anti-CTLA4/PD1) in the context of a clinical trial, we found that the frequency of pre-ICI RF and CCP was low (6.7%), and that seropositivity was not associated with post-ICI arthralgia.96 DeMoel et al made a similar observation in a cohort of 133 ipilimumab (anti-CTLA4)-treated melanoma patients.27 Patients with ICI-arthritis who are seronegative at presentation have been shown not to seroconvert over time.28 It is unknown whether the natural history of ICI-arthritis differs in those who are seropositive vs seronegative either in terms of disease severity or disease duration, and the absence of antibody positivity should not stand in the way of ICI-arthritis treatment.
Imaging
Plain radiographs may demonstrate underlying osteoarthritis in patients with ICI-arthritis, but this does not rule out a superimposed inflammatory process. Radiographs can also be used to rule out bone metastases. Erosions are rarely seen on radiography at presentation, however in one small study erosions were demonstrated on magnetic resonance imaging (MRI) as early as 4 weeks in 3/8 imaged patients.29 In another study, musculoskeletal ultrasound demonstrated erosions in 1/4 ICI-arthritis patients imaged, and an effusion and proliferative synovitis in one other.30 In a study of 6 patients with ICI associated PMR, ultrasound was able to demonstrate biceps tenosynovitis in 5 and subacromial/subdeltoid bursitis in 3 of them.31 In that same study, FDG-PET/CT scans performed prior to corticosteroid initiation demonstrated uptake at the shoulders, hip joints, greater trochanters and ischial tuberosities in all patients.
Joint Aspiration
When joint effusions are present, joint aspiration and synovial fluid analysis allows objective assessment of the degree of inflammation. A synovial fluid white cell count >2000/mm3 is indicative of an inflammatory process, while a count <200/mm3 rules it out. Microscopy can also help rule out crystal-induced joint inflammation. Finally, joint aspiration also provides an opportunity to inject the joint with corticosteroids if indicated.
Biology of ICI Arthritis and Implications for Treatment
Our understanding of the biology of ICI-arthritis is still in its infancy. In one case report, synovial pathology from a patient with ICI-arthritis was strikingly reminiscent of RA with B cell aggregates, CD4+ and CD8+ T cell infiltration and scattered histiocytes.32 In contrast, in another published case of ICI-arthritis due to nivolumab (anti-PD1), B cells were absent, but there was extensive infiltration of memory T cells and histiocytes. In this second case, there was significantly more tumor necrosis factor (TNF) but less interleukin (IL)-6 staining compared to RA controls.33 Most notable about this case was a complete lack of staining for PD-1 in the synovial tissue, suggesting continued occupancy of PD-1 by nivolumab even though the tissue was obtained ~200 days after nivolumab discontinuation. The long duration of ICI binding in the synovial tissue could explain the long duration of ICI-arthritis, even in patients who discontinue ICI treatment.
More recently, our group has used mass cytometry, single cell RNA sequencing and assessment of T cell clonality to better describe the pathology of ICI-arthritis.34 These studies have identified a unique CD38hi CD127− CD8+ T cell population that displays cytotoxic, effector, and interferon (IFN) response signatures, and is markedly expanded in the joints of patients with ICI-arthritis as compared to RA or psoriatic arthritis controls. Examination of synovial tissue from one patient who underwent bilateral knee arthroplasty also demonstrated considerable sharing of T cell receptor clonotypes in the CD38hi CD8 T cell fraction from both knees, despite the two specimens having very different histologic features on light microscopy (one knee with acute inflammation and the other with lymphoid aggregates reminiscent of RA). Although this study suggests that targeting IFN to treat ICI-arthritis would be therapeutic, IFN signaling is critical to effective cancer responses to anti-PD-1 therapy.35 There is one case report, however, describing pre- and post-treatment synovial tissue from an ICI-arthritis patient successfully treated with tofacitinib, a Janus kinase (JAK) inhibitor, showing resolution of extensive T cell infiltrates on light microscopy and a reduction in the number of IFNγ-, IL17A- and IL22-producing CD4+ T cells.36
ICI-Arthritis Management
Review of the Literature
We reviewed the literature through March 2022 to identify published approaches to ICI-arthritis management (See Supplement for search strategy and Supplemental Tables 1 and 2). We identified 15 case series (“case series”) with >3 patients that included patient level data20,29,30,37–48 and 11 observational cohort studies (“cohort studies”) without individual patient level data12,13,17,21,49–55 encompassing 595 ICI-arthritis and 95 ICI-PMR patients. Only 3 of the cohort studies were prospective,12,13,52 and there were no randomized controlled trials, emphasizing the low quality of the evidence. Most of the studies suffer from referral and/or publication bias. In the case series, 106/138 (76.8%) ICI-arthritis and 39/44 (88.6%) ICI-PMR patients were treated with systemic steroids. There were similar rates of steroid use in the cohort studies (61–100% of ICI-arthritis and 75–100% of ICI-PMR patients), and mean maximum steroid dose was 30 to 60 mg/day. Disease modifying antirheumatic drug (DMARD)-use varied widely. In the case series, 62/138 (44.9%) ICI-arthritis patients were treated with a conventional synthetic DMARD (csDMARD), and 10/138 (7.2%) with a biologic DMARD (bDMARD), primarily TNF inhibitors (TNFi). Among the cohort studies, csDMARDs were used in up to 40% of patients, with equal numbers receiving hydroxychloroquine or methotrexate. Smaller numbers of patients were treated with sulfasalazine, leflunomide or azathioprine. Where reported, bDMARD use in the cohort studies varied widely (between 4.8% and 47.6%) with 75% (42/56) receiving a TNFi and 19.6% (11/56) receiving tocilizumab, an IL-6 receptor inhibitor (IL6Ri). In the case series, ICI was either held or discontinued in 34.3% (34/99) of ICI-arthritis patients. Although these studies provide some insight into treatment practices in the rheumatology community, they do not allow us to determine the relative safety or efficacy of any given approach.
Treatment Guidelines
Several medical oncology societies, including the European Society for Medical Oncology (ESMO), the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) have developed management guidelines for irAE, including ICI-arthritis (Table 3).56–59 It must be recognized that, given the weakness of the underlying evidence, these guidelines are necessarily “eminence-based”. They are, however, a useful starting point for physicians confronting ICI-arthritis for the first time. In general, they recommend NSAIDs for grade 1 arthritis, low dose prednisone (10–20mg daily) for grade 2 and high dose (0.5–1 mg/kg/day) for grade 3 arthritis. DMARDS are recommended as steroid sparing agents for steroid refractory cases and to enable tapering of steroids. They recommend consideration of holding ICI for grade 2 arthritis and holding for grade 3. ASCO recommends referral to a rheumatologist for ICI-arthritis that is grade 2 or higher.29 This is important since oncologists may not be familiar with conventional or biologic DMARDS. Most guidelines provide little information about the specific choice of DMARD in steroid refractory patients or for monitoring over time. Other organizations with more abbreviated guidance documents include the Society for immunotherapy of Cancer (SITC) and the European League Against Rheumatism (EULAR).59,60 SITC suggests that the specific choice of DMARD be individualized based on arthritis severity, comorbidities, and anticipated time to efficacy. EULAR emphasizes shared decision-making between patients, oncologists, and rheumatologists. EULAR recommends conventional DMARDs for patients who have an insufficient response to corticosteroids, and biologic DMARDs (specifically a TNF or IL6R inhibitor) for patients with severe irAE or with an inadequate response to a conventional DMARD.
 |
Table 3 Arthritis Management Guidelines56–58 |
Safety of Anti-Rheumatic Drugs in ICI-Treated Cancer Patients
There is virtually no data on the safety of DMARDS, either conventional or biologic, in the treatment of ICI-arthritis; however, lessons can be learned from preclinical studies, and from the literature on other irAE. Table 4 lists dosing regimens, mechanism of action, and common side effects of DMARDS used for ICI-arthritis.61–64
 |
Table 4 Commonly Used Disease-Modifying Anti-Rheumatic Drugs (DMARDs) |
Corticosteroids remain the foundation of treatment for most irAE because of their effectiveness and rapid onset of action. However, a retrospective study of patients with ICI-induced hypophysitis demonstrated that treatment with high dose corticosteroids was associated with a dramatic reduction in overall survival compared to low dose corticosteroids.65 The negative association between corticosteroid treatment and survival is greatest when they are given within 2 months of ICI initiation.66 This could, however, be because experiencing an early irAE necessitates early holding of the ICI, or because higher corticosteroid doses are needed for early irAE (eg, colitis) than for later ones (eg, endocrine). A long duration of corticosteroid use is associated with an increased infection risk67 and with osteoporosis.
Hydroxychloroquine is a commonly used DMARD for low grade ICI-arthritis40 and is thought to be immunomodulatory rather than immunosuppressive (ie, it is not associated with an increased risk of infection). A recent preclinical study suggested that hydroxychloroquine may impair anti-PD-1 associated reductions in tumor growth68 but the hydroxychloroquine dosages used in that study (the equivalent of 600 mg in humans) was higher than is used in rheumatology practice (typically 200–400 mg). Sulfasalazine is another non-immunosuppressive DMARD that can be used for low grade ICI-arthritis in patients who are not sulfa allergic. Both hydroxychloroquine and sulfasalazine are slow acting agents, however, and take 2–3 months to work.
Methotrexate is a folic acid antagonist which is commonly used to treat RA and psoriatic arthritis. The medication can cause hematologic and hepatic toxicity, as well as gastrointestinal intolerance. Methotrexate is inexpensive, widely available, and used for many rheumatic conditions. However, as an antimetabolite its immunosuppressive effects are broad ranging rather than targeted, raising the possibility that it could negatively impact cancer survival when used over the long term for ICI-arthritis. It is also slow acting, taking 2–3 months to have its effect.
Indirect evidence from preclinical and clinical studies suggests that TNF inhibition is likely to be safe in ICI-treated patients.69 One study comparing steroid treatment alone with steroid and infliximab together in patients with ICI-induced colitis failed to show a difference in survival.70 However, in one large prospective melanoma registry, patients treated with TNFi for high grade irAE had shorter survival than those who did not require immunosuppression (eg, endocrine irAE), and patients treated with TNFi ± corticosteroids did worse than those treated with corticosteroids alone.71 The vast majority of TNFi-treated patients in this study had colitis due to anti-CTLA-4 and were treated early in the cohort, so there may have been unmeasured confounders that influenced their results, but it does suggest that potent immune suppression may interfere with the efficacy of ICI. Along these lines, a retrospective study of 184 patients with ICI-colitis that compared patients treated with infliximab, a TNFi, to vedolizumab, which targets integrin α4β7 and prevents gut but not systemic inflammation, showed infliximab to be associated with a higher rate of cancer progression.72
Although less commonly used than TNFi, IL6Ri are also effective for irAE management including ICI-arthritis and PMR.73–75 Elevated levels of IL-6 and CRP (which closely correlates with IL-6) are associated with reduced survival in ICI-treated patients.76–78 Investigators have demonstrated synergy between IL6Ri and anti-PD(L)1 when used concomitantly in a preclinical model.79 Among patients with RA, there is a risk of colon perforation with the IL6Ri tocilizumab,80 although it has been used in a trial setting for patients with Crohn’s disease.81 As such, this agent should be used with caution in patients who have ICI-colitis.
The last several years have seen the introduction of several oral JAK inhibitors for the treatment of RA, including tofacitinib, baricitinib and upadacitinib.82 These agents block signaling through the JAK STAT pathway and have important inhibitory effects on gamma IFN, which signals through JAK1 and JAK2. Of note, intact IFN signaling has been shown to be necessary for cancer responses to anti-PD-1.35
Abatacept, which is a CTLA4 agonist, has the direct opposite effect of ipilimumab (anti-CTLA4), and it has been used to treat life-threatening irAE such as myocarditis.83 There is limited and conflicting evidence as to the role of IL17 in irAE development.84,85 Although there are a number of case reports in which anti-IL17 was used to treat ICI-induced psoriasis, in one instance the tumor response was subsequently lost.86 Anti-IL12/IL23 (ustekinumab) was reported to be effective in two cases of refractory ICI-colitis but has not been used for ICI-arthritis.87 With regard to rituximab, a systematic literature review identified no published cases of rituximab treated patients with ICI arthritis but did note that the drug was effective in 6/9 patients with neurologic irAE.88
ICI Rechallenge After ICI-Arthritis Development
Studies suggest that about half of patients who are rechallenged with ICI after experiencing an irAE will have a recurrence of the same or a different irAE.89 In a study using the World Health Organization database VigiBase, 28.8% of patients rechallenged with the same ICI had a recurrence of the same irAE, and recurrence rates were highest for ICI-arthritis (45%, 95% CI 28–62%).90
Our approach:
For patients with ICI-arthritis, the major therapeutic questions are:
- Can this patient be managed without systemic steroids?
- If steroids are used, what dose is appropriate?
- If steroids cannot be tapered, what steroid-sparing agent should be used?
- Should the patient’s ICI be held and, if so, when/whether should ICI be resumed?
We aim to taper prednisone to ≤10 mg daily while maintaining an ICI-arthritis grade ≤1. This contrasts with our approach to treating RA, where our goal is remission. We accept a low level of ICI-arthritis disease activity in order to avoid intensive immunosuppression that might abrogate cancer responses.
As recommended in most treatment guidelines recommendation, we use NSAIDS, acetaminophen, intraarticular steroid injections and/or, at times, low doses of prednisone for grade 1 arthritis, and moderate to high steroid dosages for grade 2 and 3 arthritis. We generally aim to taper prednisone to 10 mg over 2 to 4 weeks. The ease with which this can be accomplished without a flare of symptoms provides additional information that can help determine whether a steroid sparing agent is needed, and if so which one to use. Hydroxychloroquine and sulfasalazine are most appropriate for patients with mild arthritis who can easily taper prednisone to ≤10 mg. Patients who fail to respond to hydroxychloroquine or sulfasalazine, or who have higher grade arthritis, can be treated with methotrexate or a biologic DMARD. For patients with high grade arthritis who cannot quickly taper prednisone to ≤10 mg/day, we prefer biologic DMARDS over methotrexate in order to avoid prolonged high dose corticosteroid use. We generally turn to TNFi rather than IL6Ri due to their tolerability, their effectiveness for de novo rheumatoid arthritis95 and the extensive experience using TNFi for ICI-colitis.70 In our experience, patients with the PMR phenotype of ICI arthritis also response to TNFi. In patients with refractory arthritis, methotrexate can be added to a biologic DMARD for better arthritis control; alternatively, the TNFi can be switched to an IL6Ri.
We generally hold the ICI until the patient’s arthritis is grade ≤1 and they are on prednisone ≤10 mg daily. The decision to resume ICI should be made in consultation with the patient’s oncologist and will depend on the original severity of the arthritis, the number of ICI doses the patient has already received, the status of the cancer, and the availability of other treatment options. For example, in a patient with melanoma who has been on ICI for a year, whose cancer remitted and who presented with grade 3 arthritis, the oncologist may not feel any need to resume ICI. In contrast, in the case of a patient with lung cancer who presents with grade 2 arthritis after only two months of ICI, and whose cancer has stabilized on imaging, the oncologist may be anxious to resume ICI. Patients with high grade ICI arthritis who would like to resume ICI can sometimes be successfully managed with concomitant TNFi.
Reassessment of arthritis severity (grade and CDAI) and response to therapy is needed during follow-up, regardless of whether the patient continues ICI therapy. Although measurement of ESR and CRP can provide adjunctive information, treatment can be guided largely by the patient’s joint symptoms, physical exam findings, and functional status. Most patients achieve full benefit from DMARDs, whether conventional or biologic, within three months. Failure to respond, or failure to taper corticosteroids to an acceptably low dose, is an indication to adjust therapy, or to add an additional agent in cases of a partial response.
ICI-arthritis can resolve, however, and when the patient’s symptoms have completely dissipated, tapering of medications is indicated. If prednisone has already been tapered off, then the interval between doses of TNFi or IL6Ri can be increased. In patients on methotrexate, the dose can be cut in half and later discontinued, keeping in mind that the effect of medication discontinuation may lag by as much as three months.
Patients whose cancer has progressed on ICI therapy may transition to other cancer treatments, including chemotherapy. Combining immunosuppressive DMARDS, systemic corticosteroids, and chemotherapy will significantly increase the risk of infection; it may be prudent to hold DMARDS in that setting.
Patient with Preexisting Autoimmune Diseases
The overall risk of toxicity from ICI is similar in patients with or without pre-existing autoimmune conditions.91 About 50% of patients with an underlying autoimmune condition will experience a flare of their disease after ICI initiation, although disease flares appear to be more common with anti-PD-(L)1 than with anti-CTLA-4.92 One question that arises in these patients is whether to hold their immunosuppressive medications at the time of ICI initiation. A retrospective study of ICI-treated non-small cell lung cancer patients (without autoimmune disease) demonstrated that patients on prednisone >10 mg daily at the time of ICI initiation had worse overall survival after ICI treatment.93 Similarly, a retrospective study of anti-PD1 treated melanoma patients demonstrated lower cancer response rates in patients who continued immunosuppression at the time of anti-PD1 initiation (15% vs 44% (p = 0.033)).94 In contrast, a prospective study of 415 patients with autoimmune diseases enrolled in a melanoma registry did not demonstrate a significant reduction in overall survival in ICI-treated patients on versus off immunosuppression after adjusting for known prognostic factors, although there was a trend in that direction.91
Given these contradictory results and the overall low level of evidence, the decision whether to hold immunosuppression at the time of ICI initiation should be made jointly with the patient, taking into account the severity of their autoimmune condition and the risk associated with disease flare. In general, our approach is to continue antirheumatic drugs in patients with active life- or organ-threatening autoimmune diseases (eg, vasculitis and severe lupus). We generally continue medications such as hydroxychloroquine and sulfasalazine at the time of ICI initiation since they are immunomodulatory and not immunosuppressive. Sometimes, we choose an alternative treatment for a patient’s preexisting autoimmune condition at the time of ICI initiation. An example of this would be switching a patient with RA being treated with a JAK inhibitor to a TNFi or and IL6Ri, since JAK inhibitors may abrogate responses to anti-PD-1.35
Conclusion
Early recognition and management of ICI-induced arthritis is important in order to minimize its impact on patients’ physical function, quality of life and its potential to cause joint damage. Treatment must take into account the impact of immunosuppression on cancer ICI responses, especially because ICI-induced arthritis has a tendency to persist, even after ICI discontinuation. There are now a number of published prospective ICI-induced arthritis cohorts characterizing ICI-induced arthritis.12,13 However, translational studies are needed to identify pathways that are important to ICI-induce arthritis pathogenesis but not critical to cancer control. This in turn could inform future randomized controlled trials comparing the safety and efficacy of different targeted treatment approaches.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi:10.1056/NEJMoa1200694
2. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–1356. doi:10.1056/NEJMoa1709684
3. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi:10.1056/NEJMoa1712126
4. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381:2020–2031. doi:10.1056/nejmoa1910231
5. Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19):1789–1801. doi:10.1056/NEJMOA1802357
6. Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396(10265):1817–1828. doi:10.1016/S0140-6736(20)32531-9
7. Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019;25(13):3753–3758. doi:10.1158/1078-0432.CCR-18-4070
8. André T, Shiu -K-K, Kim TW, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383(23):2207–2218. doi:10.1056/NEJMOA2017699
9. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–249. doi:10.1146/ANNUREV-PATHOL-042020-042741
10. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. doi:10.1056/NEJMra1703481
11. Wang R, Singaraju A, Marks KE, et al. Clonally expanded CD38. 2021.
12. Chan KK, Tirpack A, Vitone G, et al. Higher checkpoint inhibitor arthritis disease activity may be associated with cancer progression: results from an observational registry. ACR Open Rheumatol. 2020;2(10):595–604. doi:10.1002/ACR2.11181
13. Braaten TJ, Brahmer JR, Forde PM, et al. Immune checkpoint inhibitor-induced inflammatory arthritis persists after immunotherapy cessation. Ann Rheum Dis. 2019;79:332–338. doi:10.1136/annrheumdis-2019-216109
14. Cappelli LC, Gutierrez AK, Bingham CO, Shah AA. Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: a systematic review of the literature. Arthritis Care Res. 2017;69(11):1751–1763. doi:10.1002/ACR.23177
15. Arnaud-Coffin P, Maillet D, Gan HK, et al. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int J Cancer. 2019;145(3):639–648. doi:10.1002/IJC.32132
16. Richter MD, Crowson C, Kottschade LA, et al. Rheumatic immune-related adverse events secondary to anti–programmed death-1 antibodies and preliminary analysis on the impact of corticosteroids on anti-tumour response: a case series. Ann Rheum Dis. 2019;17(3):1–10. doi:10.1007/s00262-017-2069-9
17. Cunningham-Bussel A, Wang J, Prisco LC, et al. Predictors of rheumatic immune-related adverse events and de novo inflammatory arthritis after immune checkpoint inhibitor treatment for cancer. Arthritis Rheumatol. 2022;74(3):527–540. doi:10.1002/ART.41949/ABSTRACT
18. Cappelli LC, Dorak MT, Bettinotti MP, Bingham CO, Shah AA. Association of HLA-DRB1 shared epitope alleles and immune checkpoint inhibitor-induced inflammatory arthritis. Rheumatology. 2019;58(3):476–478. doi:10.1093/RHEUMATOLOGY/KEY358
19. Ghosh N, Tiongson MD, Stewart C, et al. Checkpoint inhibitor-associated arthritis: a systematic review of case reports and case series. J Clin Rheumatol. 2021;27(8):E317–E322. doi:10.1097/RHU.0000000000001370
20. Calabrese C, Cappelli LC, Kostine M, Kirchner E, Braaten T, Calabrese L. Polymyalgia rheumatica-like syndrome from checkpoint inhibitor therapy: case series and systematic review of the literature. RMD Open. 2019;5(1):e000906. doi:10.1136/rmdopen-2019-000906
21. Martin De Fremont G, Belkhir R, Henry J, et al. Features of polymyalgia rheumatica-like syndrome after immune checkpoint inhibitor therapy. Ann Rheum Dis. 2022;81(3):E52. doi:10.1136/annrheumdis-2020-217225
22. Reid P, Liew DFL, Akruwala R, Bass AR, Chan KK. Activated osteoarthritis following immune checkpoint inhibitor treatment: an observational study. J Immunother Cancer. 2021;9(9). doi:10.1136/JITC-2021-003260
23. National Cancer Institute. Common terminology criteria for adverse events (CTCAE) common terminology criteria for adverse events (CTCAE) v5.0; 2017. Available from: https://www.meddra.org/.
24. Anderson J, Caplan L, Yazdany J, et al. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res. 2012;64(5):640–647. doi:10.1002/ACR.21649
25. Bruce B, Fries JF. The Stanford health assessment questionnaire: dimensions and practical applications. Health Qual Life Outcomes. 2003;1:20. doi:10.1186/1477-7525-1-20
26. Ghosh N, Chan KK, Jivanelli B, Bass AR, Ayaz NA. Autoantibodies in patients with immune-related adverse events from checkpoint inhibitors. J Clin Rheumatol. 2021;27:1–8. doi:10.1097/rhu.0000000000001777
27. De Moel EC, Rozeman EA, Kapiteijn EH, et al. Autoantibody development under treatment with immune-checkpoint inhibitors. Cancer Immunol Res. 2019;7(1):6–11. doi:10.1158/2326-6066.CIR-18-0245
28. Cappelli LC, Darrah E, Shah AA, Bingham CO. Patients with checkpoint inhibitor‐induced inflammatory arthritis do not become seropositive for anti‐cyclic citrullinated peptide when followed over time. ACR Open Rheumatol. 2022;4(1):83–84. doi:10.1002/acr2.11363
29. Subedi A, Williams SG, Yao L, et al. Use of magnetic resonance imaging to identify immune checkpoint inhibitor-induced inflammatory arthritis. JAMA Netw open. 2020;3(2):e200032. doi:10.1001/JAMANETWORKOPEN.2020.0032
30. Cappelli LC, Gutierrez AK, Baer AN, et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis. 2017;76(1):43–50. doi:10.1136/annrheumdis-2016-209595
31. Van Der Geest KSM, Sandovici M, Rutgers A, et al. Imaging in immune checkpoint inhibitor-induced polymyalgia rheumatica. Ann Rheum Dis. 2020;1–2. doi:10.1136/annrheumdis-2020-217381
32. Medina H, Eickhoff J, Edison J. Thinking inside the box. J Clin Rheumatol. 2019. doi:10.1097/RHU.0000000000001088
33. Murray-Brown W, Wilsdon TD, Weedon H, et al. Nivolumab-induced synovitis is characterized by florid T cell infiltration and rapid resolution with synovial biopsy-guided therapy. J Immunother Cancer. 2020;8(1):1–6. doi:10.1136/jitc-2019-000281
34. Wang R, Singaraju A, Marks KE, et al. Clonally expanded CD38hi cytotoxic CD8 T cells define the T cell infiltrate in checkpoint inhibitor-associated arthritis. bioRxiv. 2021. doi:10.1101/2021.10.19.464961
35. Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375(9):819–829. doi:10.1056/NEJMOA1604958/SUPPL_FILE/NEJMOA1604958_DISCLOSURES.PDF
36. Murray K, Floudas A, Murray C, et al. First use of tofacitinib to treat an immune checkpoint inhibitor-induced arthritis. BMJ Case Rep CP. 2021;14(2):e238851. doi:10.1136/BCR-2020-238851
37. Belkhir R, Le Burel S, Dunogeant L, et al. Rheumatoid arthritis and polymyalgia rheumatica occurring after immune checkpoint inhibitor treatment. Ann Rheum Dis. 2017;76(10):1747–1750. doi:10.1136/annrheumdis-2017-211216
38. Calabrese C, Kirchner E, Kontzias K, Velcheti V, Calabrese LH. Rheumatic immune-related adverse events of checkpoint therapy for cancer: case series of a new nosological entity. RMD Open. 2017;3(1):e000412. doi:10.1136/rmdopen-2016-000412
39. Leipe J, Christ LA, Arnoldi AP, et al. Characteristics and treatment of new-onset arthritis after checkpoint inhibitor therapy. RMD Open. 2018;4(2):1–7. doi:10.1136/rmdopen-2018-000714
40. Roberts J, Smylie M, Walker J, et al. Hydroxychloroquine is a safe and effective steroid-sparing agent for immune checkpoint inhibitor–induced inflammatory arthritis. Clin Rheumatol. 2019;38(5):1513–1519. doi:10.1007/s10067-019-04451-2
41. Smith MH, Bass AR. Arthritis after cancer immunotherapy: symptom duration and treatment response. Arthritis Care Res. 2017. doi:10.1002/acr.23467
42. Lee J, Graham A, Sion A. Evaluation of arthralgias in adult oncology patients receiving immune checkpoint inhibitors. J Oncol Pharm Pract. 2019;25(8):1867–1872. doi:10.1177/1078155218822707
43. Lidar M, Giat E, Garelick D, et al. Rheumatic manifestations among cancer patients treated with immune checkpoint inhibitors. Autoimmun Rev. 2018;17(3):284–289. doi:10.1016/j.autrev.2018.01.003
44. Mooradian MJ, Nasrallah M, Gainor JF, et al. Musculoskeletal rheumatic complications of immune checkpoint inhibitor therapy: a single center experience. Semin Arthritis Rheum. 2019;48(6):1127–1132. doi:10.1016/j.semarthrit.2018.10.012
45. Verspohl SH, Holderried T, Behning C, Brossart P, Schäfer VS. Prevalence, therapy and tumour response in patients with rheumatic immune-related adverse events following immune checkpoint inhibitor therapy: a single-centre analysis. Ther Adv Musculoskelet Dis. 2021;13:1–14. doi:10.1177/1759720X211006963
46. Mitchell EL, Lau PKH, Khoo C, et al. Rheumatic immune-related adverse events secondary to anti–programmed death-1 antibodies and preliminary analysis on the impact of corticosteroids on anti-tumour response: a case series. Eur J Cancer. 2018;105(2018):88–102. doi:10.1016/j.ejca.2018.09.027
47. Van Der Geest KSM, Sandovici M, Rutgers A, et al. Management of immune checkpoint inhibitor-induced polymyalgia rheumatica. Ann Rheum Dis. 2020;1–2. doi:10.1136/annrheumdis-2020-218276
48. Ceccarelli F, Botticelli A, Gelibter AJ, et al. Correspondence on “Immune checkpoint inhibitor-induced inflammatory arthritis persists after immunotherapy cessation” by Braaten et al. Ann Rheum Dis. 2022;81(1):1–2. doi:10.1136/annrheumdis-2019-216867
49. Buder-Bakhaya K, Benesova K, Schulz C, et al. Characterization of arthralgia induced by PD-1 antibody treatment in patients with metastasized cutaneous malignancies. Cancer Immunol Immunother. 2018;67(2):175–182. doi:10.1007/s00262-017-2069-9
50. Cappelli LC, Brahmer JR, Forde PM, et al. Clinical presentation of immune checkpoint inhibitor-induced inflammatory arthritis differs by immunotherapy regimen. Semin Arthritis Rheum. 2018;48:1–5. doi:10.1016/j.semarthrit.2018.02.011
51. Roberts J, Ennis D, Hudson M, et al. Rheumatic immune-related adverse events associated with cancer immunotherapy: a nationwide multi-center cohort. Autoimmun Rev. 2020;19(8):102595. doi:10.1016/j.autrev.2020.102595
52. Kostine M, Rouxel L, Barnetche T, et al. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer - Clinical aspects and relationship with tumour response: a single-centre prospective cohort study. Ann Rheum Dis. 2018;77(3):393–398. doi:10.1136/annrheumdis-2017-212257
53. Liu Y, Jaquith JM, Mccarthy-Fruin K, et al. Immune checkpoint inhibitor-induced inflammatory arthritis: a novel clinical entity with striking similarities to seronegative rheumatoid arthritis. Clin Rheumatol. 2020;39(12):3631–3637. doi:10.1007/s10067-020-05162-9
54. Ornstein MC, Calabrese C, Wood LS, et al. Myalgia and arthralgia immune-related adverse events (irAEs) in patients with genitourinary malignancies treated with immune checkpoint inhibitors. Clin Genitourin Cancer. 2019;17(3):177–182. doi:10.1016/j.clgc.2019.01.021
55. Richter MD, Crowson C, Kottschade LA, Finnes HD, Markovic SN, Thanarajasingam U. Rheumatic syndromes associated with immune checkpoint inhibitors: a single-center cohort of sixty-one patients. Arthritis Rheumatol. 2019;71(3):468–475. doi:10.1002/art.40745
56. Haanen JB, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Supplement 4):iv119–iv142. doi:10.1093/annonc/mdx225
57. Schneider BJ, Naidoo J, Santomasso BD, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39:4073–4126. doi:10.1200/JCO.21.01440
58. Thompson JA, Schneider BJ, Brahmer J, et al. NCCN guidelines version 4.2021 management of immunotherapy-related toxicities NCCN guidelines panel disclosures continue NCCN; 2021. Available from: https://www.nccn.org/home/member-.
59. Brahmer JR, Abu-Sbeih H, Ascierto PA, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021;9(6):e002435. doi:10.1136/JITC-2021-002435
60. Kostine M, Finckh A, Bingham CO, et al. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Ann Rheum Dis. 2021;80(1):36–48. doi:10.1136/ANNRHEUMDIS-2020-217139
61. Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16(3):155–166. doi:10.1038/S41584-020-0372-X
62. Choi J, Fenando A. Sulfasalazine. Intraocular Inflamm. 2021;373–377. doi:10.1007/978-3-540-75387-2_30
63. Gerriets V, Bansal P, Goyal A, Khaddour K. Tumor necrosis factor inhibitors. StatPearls; 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482425/.
64. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):16295–16296. doi:10.1101/CSHPERSPECT.A016295
65. Faje AT, Lawrence D, Flaherty K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer. 2018;124(18):3706–3714. doi:10.1002/cncr.31629
66. Maslov DV, Tawagi K, Kc M, et al. Timing of steroid initiation and response rates to immune checkpoint inhibitors in metastatic cancer. J Immunother Cancer. 2021;9(7):e002261. doi:10.1136/JITC-2020-002261
67. Wang Y, Abu-Sbeih H, Mao E, et al. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. J Immunother Cancer. 2018;6(1). doi:10.1186/s40425-018-0346-6
68. Krueger J, Santinon F, Kazanova A, et al. Hydroxychloroquine (HCQ) decreases the benefit of anti-PD-1 immune checkpoint blockade in tumor immunotherapy. PLoS One. 2021;16(6):e0251731. doi:10.1371/JOURNAL.PONE.0251731
69. Chen AY, Wolchok JD, Bass AR. TNF in the era of immune checkpoint inhibitors: friend or foe? Nat Rev Rheumatol. 2021;17(4):213–223. doi:10.1038/s41584-021-00584-4
70. Johnson DH, Zobniw CM, Trinh VA, et al. Infliximab associated with faster symptom resolution compared with corticosteroids alone for the management of immune-related enterocolitis. J Immunother Cancer. 2018;6(1). doi:10.1186/s40425-018-0412-0
71. Verheijden RJ, May AM, Blank CU, et al. Association of anti-TNF with decreased survival in steroid refractory ipilimumab and anti-PD1 treated patients in the Dutch Melanoma Treatment Registry. Clin Cancer Res. 2020;26:2268–2274. doi:10.1158/1078-0432.ccr-19-3322
72. Zou F, Faleck D, Thomas A, et al. Original research: efficacy and safety of vedolizumab and infliximab treatment for immune-mediated diarrhea and colitis in patients with cancer: a two-center observational study. J Immunother Cancer. 2021;9(11):e003277. doi:10.1136/JITC-2021-003277
73. Stroud CRG, Hegde A, Cherry C, et al. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract. 2019;25(3):551–557. doi:10.1177/1078155217745144
74. Dimitriou F, Hogan S, Menzies AM, Dummer R, Long GV. Interleukin-6 blockade for prophylaxis and management of immune-related adverse events in cancer immunotherapy. Eur J Cancer. 2021;157:214–224. doi:10.1016/j.ejca.2021.08.031
75. Fa’ak F, Zobniw CM, Buni M, et al. Selective immune suppression using interleukin-6 blockade in immune related adverse events. J Immunother Cancer. 2021;9(Suppl 2):A853–A853. doi:10.1136/JITC-2021-SITC2021.816
76. Damuzzo V, Solito S, Pinton L, et al. Clinical implication of tumor-associated and immunological parameters in melanoma patients treated with ipilimumab. Oncoimmunology. 2016;5(12):e1249559. doi:10.1080/2162402X.2016.1249559
77. Tachibana H, Nemoto Y, Ishihara H, et al. Predictive impact of early changes in serum C-reactive protein levels in nivolumab plus ipilimumab therapy for metastatic renal cell carcinoma. Clin Genitourin Cancer. 2021;20:e81–e88. doi:10.1016/J.CLGC.2021.10.005
78. Shi Y, Liu X, Du J, et al. Circulating cytokines associated with clinical outcomes in advanced non-small cell lung cancer patients who received chemoimmunotherapy. Thorac Cancer. 2022;13(2):219–227. doi:10.1111/1759-7714.14248
79. Tsukamoto H, Fujieda K, Miyashita A, et al. Combined blockade of IL6 and PD-1/PD-L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment. Cancer Res. 2018;78(17):5011–5022. doi:10.1158/0008-5472.CAN-18-0118
80. Strangfeld A, Richter A, Siegmund B, et al. Risk for lower intestinal perforations in patients with rheumatoid arthritis treated with tocilizumab in comparison to treatment with other biologic or conventional synthetic DMARDs. Ann Rheum Dis. 2017;76(3):504–510. doi:10.1136/ANNRHEUMDIS-2016-209773
81. Danese S, Vermeire S, Hellstern P, et al. Randomised trial and open-label extension study of an anti-interleukin-6 antibody in Crohn’s disease (ANDANTE I and II). Gut. 2019;68(1):40–48. doi:10.1136/gutjnl-2017-314562
82. Harrington R, Al Nokhatha SA, Conway R. JAK inhibitors in rheumatoid arthritis: an evidence-based review on the emerging clinical data. J Inflamm Res. 2020;13:519. doi:10.2147/JIR.S219586
83. Salem J-E, Allenbach Y, Vozy A, et al. Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N Engl J Med. 2019;380(24):2377–2379. doi:10.1056/NEJMC1901677
84. Tyan K, Baginska J, Brainard M, et al. Cytokine changes during immune-related adverse events and corticosteroid treatment in melanoma patients receiving immune checkpoint inhibitors. Cancer Immunol Immunother. 2021;70(8):2209–2221. doi:10.1007/s00262-021-02855-1
85. Husain B, Kirchberger MC, Erdmann M, et al. Inflammatory markers in autoimmunity induced by checkpoint inhibitors. J Cancer Res Clin Oncol. 2021;147(6):1623–1630. doi:10.1007/S00432-021-03550-5
86. Esfahani K, Miller WH. Reversal of autoimmune toxicity and loss of tumor response by interleukin-17 blockade. N Engl J Med. 2017;376(20):1989–1991. doi:10.1056/NEJMC1703047/SUPPL_FILE/NEJMC1703047_DISCLOSURES.PDF
87. Thomas AS, Ma W, Wang Y. Ustekinumab for refractory colitis associated with immune checkpoint inhibitors. N Engl J Med. 2021;384(6):581–583. doi:10.1056/NEJMC2031717
88. Deftereos SN, Georgonikou D. Effectiveness of rituximab in treating immune-checkpoint-inhibitor-induced immune-related adverse events: results of a systematic review. Ann Oncol. 2021;32(2):282–283. doi:10.1016/J.ANNONC.2020.12.001
89. Simonaggio A, Michot JM, Voisin AL, et al. Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol. 2019;5(9):1310–1317. doi:10.1001/JAMAONCOL.2019.1022
90. Dolladille C, Ederhy S, Sassier M, et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol. 2020;6(6):865–871. doi:10.1001/JAMAONCOL.2020.0726
91. van der Kooij MK, Suijkerbuijk KP, Dekkers OM, Kapiteijn E. Safety and efficacy of checkpoint inhibition in patients with melanoma and preexisting autoimmune disease. Ann Intern Med. 2021;174(9):1345. doi:10.7326/L21-0441
92. Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: a systematic review. Ann Intern Med. 2018;168(2):121–130. doi:10.7326/M17-2073
93. Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non–small-cell lung cancer. J Clin Oncol. 2018;36(28):2872–2878. doi:10.1200/JCO.2018.79.0006
94. Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2017;28(2):368–376. doi:10.1093/annonc/mdw443
95. Scott DL, Wolfe F, Huizinga TWJ. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–1108. doi:10.1016/S0140-6736(10)60826-4
96. Ghosh N, Postow M, Zhu C, et al. Lower baseline autoantibody levels are associated with immune-related adverse events from immune checkpoint inhibition. J Immunother Cancer. 2022;10(1):e004008. doi:10.1136/jitc-2021-004008
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
