Back to Journals » Drug Design, Development and Therapy » Volume 16
Molecular Mechanisms of Luteolin Against Atopic Dermatitis Based on Network Pharmacology and in vivo Experimental Validation
Authors Tang L, Gao J, Li X, Cao X, Zhou B
Received 29 August 2022
Accepted for publication 26 November 2022
Published 9 December 2022 Volume 2022:16 Pages 4205—4221
DOI https://doi.org/10.2147/DDDT.S387893
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Liu Tang,1 Jiefang Gao,2 Xiaolei Li,2 Xiaoqin Cao,3 Benhong Zhou1
1Department of Pharmacy, Renmin Hospital of Wuhan University, Wuhan, People’s Republic of China; 2School of Pharmaceutical Sciences, Wuhan University, Wuhan, People’s Republic of China; 3School of Medicine, Jianghan University, Wuhan, People’s Republic of China
Correspondence: Liu Tang; Benhong Zhou, Department of Pharmacy, Renmin Hospital of Wuhan University, Wuhan, People’s Republic of China, Email [email protected]; [email protected]
Purpose: To undercover the underlying mechanisms of luteolin against atopic dermatitis (AD), clinically characterized by recurrent eczematous lesions and intense itching, based on network pharmacology, molecular docking and in vivo experimental validation.
Methods: TCMSP, STITCH and SwissTargetPrediction databases were utilized to screen the corresponding targets of luteolin. Targets related to AD were collected from DisGeNET, GeneCards and TTD databases. PPI network of intersection targets was constructed through STRING 11.0 database and Cytoscape 3.9.0 software. GO and KEGG enrichment analysis were performed to investigate the critical pathways of luteolin against AD. Further, the therapeutic effects and candidate targets/signaling pathways predicted from network pharmacology analysis were experimentally validated in a mouse model of AD induced by 2, 4-dinitrofluorobenzene (DNFB).
Results: A total of 31 intersection targets were obtained by matching 151 targets of luteolin with 553 targets of AD. Among all, 20 core targets were identified by PPI network topology analysis, including IL-6, TNF, IL-10, VEGFA, IL-4, etc., and molecular docking indicated that luteolin binds strongly to these core targets. KEGG pathway enrichment analysis suggested that the intersected targets were significantly enriched in IL-17 signaling pathway, Th17 cell differentiation, Th1 and Th2 cell differentiation, JAK/STAT signaling pathway, etc. The in vivo experiment validated that luteolin could alleviate AD-like skin symptoms, as evidenced by the lower SCORAD score, the reduced infiltration of mast cells and the recovery of skin barrier function. Furthermore, luteolin restored immune balance by regulating the production of Th1/Th2/Th17-mediated cytokines, which were both the predicted core targets. Moreover, luteolin inhibited the phosphorylation of JAK2 and STAT3 in the lesional skin.
Conclusion: Together, the present study systematically clarifies the ameliorative effects and possible molecular mechanisms of luteolin against AD through the combination of network pharmacology and experimental validation, shedding light on the future development and clinical application of luteolin.
Keywords: atopic dermatitis, luteolin, network pharmacology, target prediction, experimental validation
Introduction
Atopic dermatitis (AD), a chronic inflammatory and pruritic skin disorder, is characterized by recurrent eczematous and inflamed skin lesions, compromised barrier function and intense itch.1,2 Itch is burdensome symptom and can negatively influence patients’ sleep quality, social life and emotional well-being. The pathogenesis of AD is multifactorial and complex, most researchers hold the view that immunologic aberrations and epidermal barrier dysfunction constitute critical factors in the development of this disease.3,4 Findings proposed that the Th1/Th2 immune imbalance caused by a predominant Th2 over Th1 immune response has been recognized as a crucial pathogenesis of cutaneous inflammation in AD. Th2-mediated cytokines, such as interleukin (IL)-4, IL-5, IL-13, could activate IgE response to allergens and disrupt skin barrier function. Th17 cells and Th22 cells, another two novel CD4+ T cell subsets, have also been found to be increased in AD lesions and associated with AD severity.5–7
Current treatment options for AD are limited and mainly include topical emollients, corticosteroids, and immunosuppressive agents. However, long-term epicutaneous application of corticosteroid increases the risk of recurrence and results in telangiectasia, petechiae, skin atrophy and thinning.2 Thus, researchers have moved toward the discovery of alternative therapeutic agents for AD from traditional Chinese medicine or natural products with better long-term benefits and less side effects.8
Luteolin, a food‐derived flavonoid, which is present in medicinal plants worldwide, was reported to have many noteworthy pharmacological properties, including anti-inflammatory, anti-oxidant and anti-allergic effects.9–11 One previous pre-clinical study showed that luteolin suppressed dry skin induced spontaneous scratching behavior in mice via inhibiting IgE-mediated mast cell activation.12 However, the role of luteolin on AD remains unknown. With the rapid development of bioinformatics, systems biology and pharmacology, network pharmacology has been considered as a frontier in drug discovery and development. Network pharmacology highlights a paradigm shift from the current “one molecule, one target, one disease” mode a new “network multi-targets” mode.13,14 Therefore, in the present study, we firstly performed network pharmacology approach to predict potential molecular targets and molecular mechanisms of luteolin in treating AD. In silico molecular docking as well as an in vivo experiment was further conducted to confirm the findings. An overview of this study is shown in Figure 1.
 |
Figure 1 Flowchart of the current research study. |
Materials and Methods
Related Databases and Platforms
TCMSP database (http://lsp.nwu.edu.cn/tcmsp.php); SwissTargetPrediction (http://www.swisstargetprediction.ch/); STITCH 4.0 (http://stitch.embl.de/); UniProt database (http://www.uniprot.org); DisGeNET database (http://www.disgenet.org/), GeneCards database (https://www.genecards.org/), Therapeutic Target Database (TTD, https://db.idrblab.org/ttd/); Venny 2.1.0 (http://bioinfogp.cnb.csic.es/tools/venny/index.html); STRING 11.0 (https://string-db.org/), Cytoscape 3.9.0 (http://www.cytoscape.org/); g:profiler database (https://biit.cs.ut.ee/gprofiler/convert); OmicShare cloud platform (https://www.omicshare.com/tools/).
Prediction of the Luteolin Pharmacological Targets
To obtain the potential targets of luteolin, we searched TCMSP, STITCH 4.0 and SwissTargetPrediction databases, followed by filtering with the term “Homo sapiens”. The operation is as follows: Firstly, we directly enter “luteolin” in the TCMSP and STITCH 4.0 databases and then download the corresponding target genes. Secondly, we got the luteolin’s Canonical SMILES from the PubChem database, imported it to the SwissTargetPrediction database and then obtained the predicted targets of luteolin. Through the UniProt database, we analyzed and annotated potential pharmacodynamic targets and the candidate targets were both modified to the official standardized abbreviation, that is, Gene symbol. Duplicated non-human and non-standard target genes were removed.
Prediction of Target Genes Associated with AD
Using “Dermatitis, Atopic” as the key word, the potential targets for the treatment of AD in the DisGeNET, GeneCards and TTD databases were discovered. Among them, GeneCards database was used to screen target genes related to AD with a ‘Relevance score ≥5’ and the DisGeNET database was used to screen target genes related to AD with a ‘Gene-Disease Score ≥0.05’. Combine the search results of the three databases and then remove duplicate targets.
Intersection Targets Between Luteolin and AD
The common potential targets of luteolin and AD, which represented the potential targets of luteolin in the treatment of AD, were further obtained by the online drawing software Venny 2.1.0.
Construction of Protein–Protein Interaction (PPI) Network and Topology Analysis
The intersection targets for luteolin against AD were input to the STRING 11.0 database to construct a PPI network. “Homo sapiens” was chosen and a scoring value >0.7 was selected as the minimum required interaction score protein interactions. Finally, we imported the PPI information into Cytoscape 3.9.0 software to visualize the intersection of PPI diagram. The core targets of luteolin treating AD were mined by Cytohubba and MCODE plugins in Cytoscape 3.9.0 software. Top 20 nodes with the largest “Degree” value generated from the CytoHubba plug-in were selected to establish a Hub gene network. Additionally, the analysis parameters of MCODE were set as follows: degree cutoff = 2, node score cutoff = 0.2, K-score = 2 and Max depth = 100.
GO Functional Enrichment and KEGG Pathway Enrichment Analysis
The common target genes of luteolin and AD were imported into the DAVID database and OmicShare cloud platform for GO function and KEGG pathway analysis. The channels with Q value <0.01 are filtered out and sorted in descending order based on the Q value (adjust P). Top 20 items are selected, and the screening results were visualized by an advanced bubble chart.
Molecular Docking
Firstly, the 3D structure of luteolin was downloaded from the PubChem database. Before docking simulation, the file was imported into PyMOL software to hydrogenate and add charge and then saved in .pdbqt format. Secondly, the X-ray crystal structures of the core targets were retrieved from the PDB database and modified using UCSF Chimera 1.14 software including ligand and water removal and polar hydrogen atoms and Gasteiger charge addition. Finally, luteolin was used as the ligand, and the candidate protein targets were used as receptors for molecular docking using Chimera 1.14.
Experimental Verification
Chemicals
DNFB (purity grade >99%) was obtained from Alfa Aesar, Heysham, UK. Luteolin (purity grade > 99%) was supplied by Aladdin Ltd., Shanghai, China. DEX (dexamethasone, purity grade > 98%) was purchased from Saen Chemical Technology Co. Ltd., Shanghai, China. All other general agents were commercially available.
Experimental Protocol and AD Model
Experimental female KM mice aged 6 weeks were purchased from the Animal Center of Wuhan University (Wuhan, China). All mice were housed in the cages at a 12/12 h day/night cycle and provided a standard diet. The protocol of this experimental study was approved by the Institutional Animal Care and Use Committee (IACUC), Wuhan University Center for Animal Experiment, Wuhan, China), and all the procedures of this study were done in accordance with the Guide for the Care and Use of Laboratory Animals (Eighth Edition, 2011, published by the National Academies Press, Washington, USA). After 1 week of acclimation period, the mice were randomly divided into 4 groups: (i) Normal group, (ii) Model group (DNFB), (iii) 0.5% luteolin (g/mL) group (DNFB +0.5% luteolin), (iv) 0.05% DEX (g/mL) group (DNFB +0.05% DEX).
To experimentally induce AD-like model, we employed cutaneous DNFB sensitization and challenging in mice according to the method of Mandlik et al15 with some modifications. Briefly, 100 μL of 1.0% DNFB (w/v) in vehicle composed of acetone and olive oil (3:1) was applied onto the shaved dorsal skin (2 cm*3 cm) on day 1. Mice were then topically treated with 100 μL of 0.5% DNFB onto the dorsal skin on day 3, 7 and 11. Simultaneously, apart from Normal group and Model group (only treated with the same volume of vehicle), the 0.5% luteolin and 0.05% DEX (100 μL) were dissolved in ethanol in vehicle composed of ethanol and propylene glycol (3:1) and they were topically applied onto the shaved back of mice daily from day 1 to day 13.
Measurements of AD Severity Score, Scratching Behaviors and TEWL
To evaluate the ameliorative effects of luteolin on DNFB-induced AD-like morphology, the dermatitis severity score and scratching behavior episodes within 10 min among different groups were examined in this study. The dermatitis severity score was measured macroscopically at day 13 based on four criteria previously described.16 Briefly, symptom severity was scored as 0 (none), 1 (mild), 2 (moderate), and 3 (severe) for each of the following four different manifestations: erythema/hemorrhage, scaling/dryness, edema/swelling and erosion/excoriation. Immediately after the application of DNFB, scratching behavior was counted for 10 min on day 13. Just scratching of the hind paws to the dorsal skin was recorded as the scratching behavior and the consecutive scratching before putting down the hind paws was counted as once.17
In addition, edema was further evaluated through skin swelling by measuring the difference in the thickness/weight among different groups. After sacrifice, the dorsal skin of each group was separated, respectively, and made into a circle slice using a puncher with the diameter of 8 mm. An analytical balance was used for measuring the skin weight and a vernier calliper was used for measuring the skin thickness.
Transepidermal water loss (TEWL) of the shaved dorsal skin was measured by VAPO SCAN AS-VT100RS (Asch, Japan) and the values were determined from three independent measurements at day 12.
Histological Assay
After sacrifice, the dorsal lesional skin of mice among different groups was harvested carefully. Skin specimens were immersed in 4% paraformaldehyde for 24 h and then embedded into wax blocks. Paraffin-embedded skins were then cut into 4.5-μm thin sections and were stained with hematoxylin-eosin (H&E) to detect epidermal thickness or toluidine blue (TB) to examine mast cells, including the granulated and degranulated forms. Quantitative analysis of epidermal thickness and mast cells was measured using ImageJ software taken at 3 randomly selected fields per section.
Enzyme-Linked Immunosorbent Assay (ELISA)
Whole blood was obtained from the eyeball and then centrifuged at 3500 rpm for 15 min at 4 ℃ to collect serum samples. Skin tissues (100 mg) were homogenized in 9-fold 0.9% saline solution, and the homogenates were centrifuged for 10 min at 4 ℃ at 12,000 rpm to obtain the supernatant. The total protein concentration of supernatants was determined using the bicinchoninic acid (BCA) assay (Kerui Biotechnology Co., Ltd, Wuhan, China). Serum and skin levels of Th1/Th2/Th17-related cytokines were measured using ELISA kits.
Immunohistochemical (IHC) and Immunofluorescence (IF) Analysis
The paraffin sections of mice in each group were subjected to immunohistochemical analysis according to the following steps: deparaffinization and hydration of sections➙3% H2O2 blocking endogenous peroxidase➙EDTA antigen microwave retrieval➙5% BSA serum blocking ➙ Incubation with the primary antibodies: rabbit anti-FLG, anti-p-JAK2 and anti-p-STAT3 (Cell Signaling Technology, Boston, USA), 4℃, overnight➙Incubation with goat anti-rabbit IgG secondary antibody, 37℃, 1h➙DAB or DAPI staining.
Statistical Analysis
One-way ANOVA followed by Dunnett’s post-hoc test using GraphPad Prism 5.0 was used for statistical analysis of data. All the experimental data were presented as the mean ± standard deviation (S.D.) and p-value <0.05 or 0.01was considered significantly.
Results
Identification of the Targets Related to Luteolin Against AD
This study got 56, 10 and 104 candidate targets of luteolin from TCMSP, STITCH and SwissTargetPrediction databases, respectively, and we finally received 151 targets after removing duplicates (Figure 2A). By retrieving the DisGeNET, GeneCards and TTD databases and deleting repeated targets, a total of 553 target genes associated with AD were obtained (Figure 2A). The Venn diagram produced by Venny 2.1.0 showed that 31 intersection targets between luteolin and AD were obtained (Figure 2B).
 |
Figure 2 (A) Potential targets of luteolin and AD-related genes. (B) Intersecting targets between luteolin and AD-related genes determined by Venny 2.1. |
Construction of PPI Network and Topology Analysis
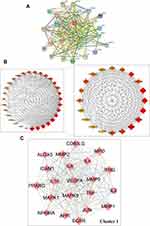
Thirty-one anti-AD targets of luteolin were imported into STRING 11.0 databases to obtain the PPI network (Figure 3A). Cytoscape 3.9.0 was used for network visualization. PPI network stands for a global view of intersection target association, and the PPI network contained 31 nodes and 259 edges. The circles represented the targets of luteolin against AD and the edges represented the interaction between targets. “Degree” value, which is calculated from CytoHubba plug-in, is also an important topological property that can explain the most important nodes in the network. The size and color trend of the nodes varies from the “Degree” value. The first 20 nodes with the highest ‘Degree’ value were extracted to establish a Hub gene network, including MMP9, IL6, TNF, IL10, VEGFA, IL4, ICAM1, MPO, MAPK1, IL2, MAPK8, JUN, EGFR, PPARG, IFNG, MMP1, MMP2, CD40LG, NFKBIA, AHR (Figure 3B). The results of MCODE obtained the most significant cluster with scores of 18.5. Convincingly, cluster 1 comprised 21 nodes and 185 edges, which further confirmed the importance of 20 core genes obtained from CytoHubba analysis (Figure 3C).
Molecular Docking Verification of Luteolin with Core Target Genes
Molecular docking, as one of bioinformatics tools, has been increasingly used in the early stages of drug discovery. The luteolin was molecularly docked with 20 core targets to further verify the interaction between the compound and the core targets. In the docking output results, the more negative docking score is predicted to have a higher binding affinity between the luteolin to protein. In general, the binding energy (affinity) below −5.0 kcal/mol means strong binding activity between the receptor and the ligand.18–20 The bonding interaction data are shown in Table 1, and the binding affinities were found to be ranged from-5.8 to −10.1 kcal/mol.
 |
Table 1 Molecular Docking Results in Terms of the Binding Energy of Luteolin with 20 Anti-AD Core Targets |
GO Function Analysis
As illustrated in Figure 4, the input of the 31 overlapping target genes derived the top 20 biological process (BP) enrichment items including response to cytokines, cellular response to cytokine stimulus, cellular response to chemical stimulus and cytokine-mediated signaling pathway. Cellular component (CC) enrichments involve extracellular region, membrane raft and cell surface. Molecular function (MF), enrichments involve cytokine receptor binding, signaling receptor binding, and MAP kinase activity.
 |
Figure 4 Bubble diagram of GO enrichment analysis (Top 20). (A) Biological process (BP). (B) Cellular component (CC). (C) Molecular function (MF). |
KEGG Pathway Analysis and Luteolin-Target-Pathway Network Construction
The KEGG pathway enrichment analysis revealed that 31 intersection targets were mainly enriched in 71 signaling pathways (adjust P < 0.01). To further uncover the possible mechanisms of luteolin in the prevention of AD, top 20 (based on adjust P value) AD-related KEGG analysis results were visualized by the OmicShare tool. As shown in Figure 5A and Table 2, key mechanisms of luteolin against AD might include IL-17 signaling pathway, T cell receptor signaling pathway, Th17 cell differentiation, TNF signaling pathway, Th1 and Th2 cell differentiation, JAK/STAT signaling pathway, etc. The luteolin-target-pathway network visualized by Cytoscape 3.9.0 software comprised 20 signaling pathways and enriched gene targets is shown in Figure 5B.
 |
Table 2 KEGG Pathway Enrichment Analysis of Luteolin Against AD |
Experimental Verification
Luteolin Alleviates Dorsal Skin Lesions in DNFB-Induced AD Mice
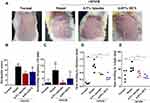
At the end of the experiment, the AD model mice successfully developed severe by repeated application of DNFB (Figure 6A). Compared to the Normal group, the lesional score in the AD model group was significantly increased about 6.75-fold (Figure 6B). In line with the development of skin lesions, repeated treatment with DNFB triggered a significant increase in the scratching episodes within 10 min (Figure 6C). Treatment with 0.5% luteolin markedly decreased DNFB-induced increases in dermatitis score and scratching bouts (Figures 6B and C).
Skin swelling, typically as a result of inflammation or fluid retention in the skin, is an indicator of skin edema.21,22 In this study, skin edema was evaluated by comparing the difference in the thickness/weight among different groups. As shown in Figures 6D and E, the dorsal skin thickness and skin weight were gradually elevated in DNFB-treated AD mice. In contrast, compared to the model group, 0.5% luteolin substantially reduced dorsal skin thickness from (1.19 ± 0.28) to (0.72 ± 0.04) mm (p < 0.01) and alleviated the skin swelling by weight from (43.02 ± 8.71) to (25.38 ± 11.72) mg (p < 0.05).
Luteolin Alleviates Histopathological Changes in DNFB-Induced AD Mice
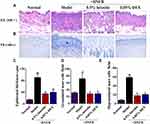
H&E staining was performed to examine the histopathological changes, including the epidermal hyperplasia and inflammatory cell infiltration, which are the major features of AD. In DNFB-induced AD mice, marked increases in epidermal thickness and infiltration of inflammatory cells in the dermis were observed (Figure 7A). TB staining was further carried out to observe the infiltration of granulated and degranulated mast cells into inflamed tissues (Figure 7B). Compared to normal mice, AD mice significantly increased the infiltration of mast cells, including granulated and degranulated forms (p < 0.05 or p < 0.01, Figure 7C–E). However, these above mentioned pathological changes were both remarkedly reversed by topical treatment of 0.5% luteolin (p < 0.05 or p < 0.01, Figures 7C–E).
Luteolin Improves the Skin Barrier Function in DNFB-Induced AD Mice
Clinically, both the affected and unaffected skin of AD patients exhibit epidermal barrier dysfunction, manifested by the enhanced transepidermal water loss (TEWL) and capacity reduced water retention. TEWL is a gold indicator used for evaluating the skin barrier function.23 As shown in Figure 8A, DNFB-sensitized skin showed a significant increase in TEWL value until the end of modeling (p < 0.01, Model [46.22 ± 5.61] g/m2/h vs Normal [8.28 ± 3.95] g/m2/h). 0.5% luteolin significantly reduced the TEWL value from 46.22 ± 5.61 to 28.00 ± 2.17 g/m2/h. The TEWL value in the 0.05% DEX group was 32.65 ± 7.52 g/m2/h, which was significantly higher than that of the 0.5% luteolin group.
The degradation product of filaggrin (FLG) in normal skin is the important source of moisturizing factors (NMF) that are essential for maintaining stratum corneum hydration.21 We further evaluated the expression of FLG protein using immunofluorescence staining (Figure 8B). As expected, the positive expression of FLG (green) was significantly suppressed in the AD model compared with the Normal group, while luteolin reversed the FLG expression.
Luteolin Alleviates Th1, Th2 and Th17-Related Cytokines
Emerging evidences show that elevated Th1/Th2/Th17-associated chemokines could be implicated in AD pathogenesis.24,25 Network pharmacology analysis also revealed that IL-17 signaling pathway, Th17 cell differentiation, TNF signaling pathway, Th1 and Th2 cell differentiation serve as the targets for luteolin against AD. Herein, we estimated Th1/Th2/Th17-associated cytokine levels in the serum and inflamed skin of DNFB-sensitized mice. As illustrated in Figure 9, we observed that topical applied 0.5% luteolin markedly decreased the serum or skin levels of the Th2-related cytokine, IL-4 (p<0.01 or p<0.05, Figure 9A and B) and Th17-related cytokines, such as IL-6 (p< 0.05, Figure 9E and F), TNF-α (p<0.01 or p<0.05, Figure 9G and H) and IL-17 (p<0.05, Figure 9I and J). Th1-related cytokines, such as IFN-γ, are reported to inhibit the differentiation of Th2 cells and the secretion of cytokines, thus restoring the impaired balance of Th1/Th2 immune responses in AD patients.26 In this study, we also found that the production of IFN-γ in the skin was significantly increased in the group of mice treated with luteolin (p < 0.05, Figure 9D) and a similar trend can also be seen in the serum IFN-γ level, while there was no statistically significant difference (p > 0.05, Figure 9C).
Luteolin Inhibits Jak2/Stat3 Signaling Pathway Activation
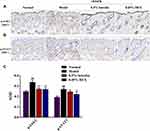
The results of KEGG pathway enrichment in the network pharmacology study demonstrated the mechanism of luteolin in treating AD was significantly associated with the Jak-STAT signaling pathway. Thus, we examined the protein expression levels of phosphorylated (p)-JAK2 and p-STAT3 in dorsal skin by immunohistochemistry staining to explore whether the JAK2/STAT3 pathway is involved in the anti-AD effects of luteolin (Figures 10A and B). Compared with the normal group, the protein expression of p-JAK2 and p-STAT3 in paraffin section of skin biopsies was noticeably increased about 1.36-fold and 1.40-fold in the DNFB group (p < 0.01, Figures 10C), while luteolin prominently reduced the increased levels of p-JAK2 and p-STAT3 (p < 0.05 or p < 0.01, Figure 10C).
Discussion
AD is one of the most common skin diseases. Recurrent eczematous lesions and intense pruritus seriously affect social interactions and quality of life of AD patients.1,2 The standard therapy for AD in clinic mainly involves the corticosteroids or immunosuppressive agents. However, there is still an urgent need for more effective and fewer side effects anti-AD therapeutics because of their limited efficacy and unwanted additional adverse effects.4,27 To date, various studies have shown that luteolin possesses pharmacological activities such as anti-inflammatory, antioxidant and anti-allergic, which exhibits its great potential in the treatment of AD.9–11 Zhang et al28 previously demonstrated that luteolin could effectively diminish collagen production and attenuate skin fibrosis via the inhibition of TGF-β/Smads signaling mediated by binding to ALK5. Moreover, it has been reported that topical application of luteolin significantly inhibited the scratching behavior associated with allergic cutaneous anaphylaxis.12 However, the effects and underlying mechanisms of luteolin against AD have not been currently defined. In the present study, we firstly conducted a systemic study via a combination of network pharmacology molecular docking and in vivo studies to explore the possible mechanism of luteolin in treating AD.
31 overlapping targets of luteolin against AD were obtained and PPI network result indicated luteolin may need to simultaneously regulate multiple anti-inflammatory or immune-regulatory targets to attenuate AD-like symptoms rather than single target. Twenty core gene targets were screened out by topology analysis, and they were MMP9, IL6, TNF, IL10, VEGFA, IL4, ICAM1, NFKBIA, IFNG, etc. Among them, IL6, IL10, IL2, and IL4 belong to the interleukin family, which play critical role in regulating immune and inflammatory processes.29,30 MAPK8 and MAPK1, members of the MAPK family, are reported to mediate rapid responses to abiotic stress stimuli.31,32 The targets closely related to the chemotaxis and adhesion function of leukocytes are ICAM1 and MPO.33,34 Targets closely related to immune regulation are IL10, IFNG, IL-4, IL2 and CD40LG, AHR.35,36 Targets closely related to angiogenesis are EGFR and VEGFA.37,38 Immune disorder, leukocyte chemotaxis and angiogenesis are reported to play critical roles in the acute and chronic phases of AD.39,40 Altogether, the above results showed that luteolin could effectively improve AD-like symptoms through multiple targets and pathological process.
To further reveal the biological functions of luteolin in AD treatment, we performed GO and KEGG enrichment analysis of the common targets. GO analysis results showed revealed that these targets were correlated with the regulation of cytokines, cytokine-mediated signaling pathway, inflammatory response, and other biological processes. Additionally, KEGG pathway enrichment analysis suggested that common targets were enriched mainly in IL-17 signaling pathway, T cell receptor signaling pathway, Th17 cell differentiation, TNF signaling pathway, JAK/STAT signaling pathway, Th1 and Th2 cell differentiation, etc., which were the critical mechanism of luteolin against AD. Studies have confirmed that AD is a typical chronic inflammatory skin disease, largely mediated by T cells. Various T-cell subsets, such as Th1, Th2, Th17, and CD4+ T cells are critical in immune pathogenesis and chronic inflammatory responses in AD.41,42 The dominance of Th2 over Th1 type response is recognized as a main hallmark of AD. A biased Th2-mediated immune response can lead to Th1/Th2 immune imbalance and skin barrier disruption, which triggers the occurrence and development of AD.41 Besides, Th17 cells and IL-17 signaling have also been proved to be implicated in both the initiation and maintenance of AD.24,41 Th17-related cytokines, IL-17, IL-6 and TNF-α, can activate Th2 immune response and impair skin barrier function.43 Consistent with the results predicted from network pharmacology analysis, our results demonstrated that topical luteolin treatment effectively reduced the secretion of Th2-related cytokine (IL-4) and Th17-related cytokines (IL-17, TNF-α and IL-6) and elevated the level of Th1-type cytokine (IFN-γ) in dorsal skin, thus restoring the Th1/Th2 immune balance in AD.
The JAK/STAT signaling pathway, a widely expressed intracellular signal transduction pathway, is reported to involve in many immune and inflammatory diseases, among which the JAK2/STAT3 pathway has been proposed as a well-accepted therapeutic target against AD.44,45 Previous studies reported that activated JAK2/STAT3 signaling could upregulate the Th2 immune response and induce the secretion of excessive inflammatory cytokines and IgE, thus exacerbating skin inflammation.46,47 In the present research, KEGG pathway analysis predicted that JAK/STAT signaling could be the crucial mechanism of luteolin against AD and the core targets, such as IFNG, IL4, IL6 etc., were significantly enriched in the JAK/STAT signaling pathway. Taking the JAK2/STAT3 signaling as the breakthrough, the protein levels of p-JAK2 and p-STAT3 in skin tissues were further examined. In this study, we found that the expression of p-JAK2 and p-STAT3 were significantly increased in the dorsal skin of AD mice, exhibiting the activation of JAK2/STAT3 signaling pathway, while this phenomenon was reversed by topical application of luteolin. We here concluded that luteolin exhibits protective effects on AD-like skin injury potentially by inhibiting the JAK2/STAT3 signaling, thereby significantly reducing expression of inflammatory cytokines and alleviating the inflammatory responses.
Conclusion
To summarize, this study has identified the core targets and key pathways of luteolin against AD through network pharmacology combined with the experimental validation. Our study suggests that luteolin can ameliorate the AD-like lesions through the synergistic interaction between multiple core targets and pathways, and Th1/Th2/Th17-related cytokines and JAK2/STAT3 signaling pathway are the possible core targets. Although the in-depth mechanism is warranted, the current research provides a basis for the clinical application and basic research of luteolin in the treatment of AD.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by the Fundamental Research Funds for the Central Universities, grant number 2042022kf1077.
Disclosure
The authors report no conflict of interest in this work.
References
1. Li H, Zhang Z, Zhang H, Guo Y, Yao Z. Update on the pathogenesis and therapy of atopic dermatitis. Clin Rev Allergy Immunol. 2021;61(3):324–338. doi:10.1007/s12016-021-08880-3
2. Saini S, Pansare M. New insights and treatments in atopic dermatitis. Immunol Allergy Clin North Am. 2021;41(4):653–665. doi:10.1016/j.iac.2021.07.005
3. Reed B, Blaiss MS. The burden of atopic dermatitis. Allergy Asthma Proc. 2018;39(6):406–410. doi:10.2500/aap.2018.39.4175
4. Moniaga CS, Tominaga M, Takamori K. The pathology of type 2 inflammation-associated itch in atopic dermatitis. Diagnostics. 2021;11(11):2090. doi:10.3390/diagnostics11112090
5. Souwer Y, Szegedi K, Kapsenberg ML, De Jong EC. IL-17 and IL-22 in atopic allergic disease. Curr Opin Immunol. 2010;22(6):821–826. doi:10.1111/j.1600-065X.2011.01027.x
6. Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242(1):233–246. doi:10.1111/j.1600-065X.2011.01027.x
7. Zheng T, Fan M, Wei Y, et al. Huangbai liniment ameliorates skin inflammation in atopic dermatitis. Front Pharmacol. 2021;12:726035. doi:10.3389/fphar.2021.726035
8. Wu S, Pang Y, He Y, et al. A comprehensive review of natural products against atopic dermatitis: flavonoids, alkaloids, terpenes, glycosides and other compounds. Biomed Pharmacother. 2021;140:111741. doi:10.1016/j.biopha.2021.111741
9. Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets. 2008;8(7):634–646. doi:10.2174/156800908786241050
10. Pandurangan AK, Esa NM. Luteolin, a bioflavonoid inhibits colorectal cancer through modulation of multiple signaling pathways: a review. Asian Pac J Cancer Prev. 2014;15(14):5501–5508. doi:10.7314/APJCP.2014.15.14.5501
11. Gendrisch F, Esser PR, Schempp CM, Wölfle U. Luteolin as a modulator of skin aging and inflammation. Biofactors. 2021;47(2):170–180. doi:10.1002/biof.1699
12. Baolin L, Weiwei W, Ning T. Topical application of luteolin inhibits scratching behavior associated with allergic cutaneous reaction in mice. Planta Med. 2005;71(5):424–428. doi:10.1055/s-2005-864137
13. Kibble M, Saarinen N, Tang J, Wennerberg K, Mäkelä S, Aittokallio T. Network pharmacology applications to map the unexplored target space and therapeutic potential of natural products. Nat Prod Rep. 2015;32(8):1249–1266. doi:10.1039/c5np00005j
14. Noor F, Tahir Ul Qamar M, Ashfaq UA, Albutti A, Alwashmi ASS, Aljasir MA. Network pharmacology approach for medicinal plants: review and assessment. Pharmaceuticals. 2022;15(5):572. doi:10.3390/ph15050572
15. Mandlik DS, Mandlik SK, Patel SS. Sarsasapogenin and fluticasone combination improves DNFB induced atopic dermatitis lesions in BALB/c mice. Immunopharmacol Immunotoxicol. 2021;43(6):767–777. doi:10.1080/08923973.2021.1981375
16. Leung DY, Hirsch RL, Schneider L, et al. Thymopentin therapy reduces the clinical severity of atopic dermatitis. J Allergy Clin Immunol. 1990;85(5):927–933.
17. Han NR, Moon PD, Yoo MS, Ryu KJ, Kim HM, Jeong HJ. Regulatory effects of chrysophanol, a bioactive compound of AST2017-01 in a mouse model of 2,4-dinitrofluorobenzene-induced atopic dermatitis. Int Immunopharmacol. 2018;62:220–226. doi:10.1016/j.intimp.2018.06.046
18. Hsin KY, Ghosh S, Kitano H. Combining machine learning systems and multiple docking simulation packages to improve docking prediction reliability for network pharmacology. PLoS One. 2013;8(12):e83922. doi:10.1371/journal.pone.0083922
19. Lu L, Guan QX, Tian YX, Lin JX, Liang SW, Wang SM. Simulation and prediction of material foundation of Rhei Radix et Rhizoma for ischemic stroke based on molecular docking technology. Zhong Yao Cai. 2015;38(4):781–785.
20. Xu SN, Zhuang L, Zhai YY, et al. Material basis and mechanism of erzhi pill for preventing osteoporosis based on network pharmacology. Chinese Pharm J. 2018;24:1913–1920.
21. Motoyama K, Tanida Y, Sakai A, Higashi T, Kaneko S, Arima H. Anti-allergic effects of novel sulfated polysaccharide sacran on mouse model of 2,4-Dinitro-1-fluorobenzene-induced atopic dermatitis. Int J Biol Macromol. 2018;108:112–118. doi:10.1016/j.ijbiomac.2017.11.155
22. Tang L, Li XL, Wan LP, et al. Ameliorative effect of orally administered different linoleic acid/α-linolenic acid ratios in a mouse model of DNFB-induced atopic dermatitis. J Funct Foods. 2020;65:103754. doi:10.1016/j.jff.2019.103754
23. Glatz M, Jo JH, Kennedy EA, et al. Emollient use alters skin barrier and microbes in infants at risk for developing atopic dermatitis. PLoS One. 2018;13(2):e0192443. doi:10.1371/journal.pone.0192443
24. Choi EJ, Debnath T, Tang Y, Ryu YB, Moon SH, Kim EK. Topical application of Moringa oleifera leaf extract ameliorates experimentally induced atopic dermatitis by the regulation of Th1/Th2/Th17 balance. Biomed Pharmacother. 2016;84:870–877. doi:10.1016/j.biopha.2016.09.085
25. Batista DI, Perez L, Orfali RL, et al. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J Eur Acad Dermatol Venereol. 2015;29(6):1091–1095. doi:10.1111/jdv.12753
26. Kaczynska A, Klosinska M, Janeczek K, Zarobkiewicz M, Emeryk A. Promising immunomodulatory effects of bacterial lysates in allergic diseases. Front Immunol. 2022;13:907149. doi:10.3389/fimmu.2022.907149
27. Salvati L, Cosmi L, Annunziato F. From emollients to biologicals: targeting atopic dermatitis. Int J Mol Sci. 2021;22(19):10381. doi:10.3390/ijms221910381
28. Zhang Y, Wang J, Zhou S, et al. Flavones hydroxylated at 5, 7, 3’ and 4’ ameliorate skin fibrosis via inhibiting activin receptor-like kinase 5 kinase activity. Cell Death Dis. 2019;10(2):124. doi:10.1038/s41419-019-1333-7
29. Mertowska P, Mertowski S, Smarz-Widelska I, Grywalska E. Biological role, mechanism of action and the importance of interleukins in kidney diseases. Int J Mol Sci. 2022;23(2):647. doi:10.3390/ijms23020647
30. Peluzzo AM, Autieri MV. Challenging the paradigm: anti-inflammatory interleukins and angiogenesis. Cells. 2022;11(3):587. doi:10.3390/cells11030587
31. Ruan X, Du P, Zhao K, et al. Mechanism of Dayuanyin in the treatment of coronavirus disease 2019 based on network pharmacology and molecular docking. Chin Med. 2020;15:62. doi:10.1186/s13020-020-00346-6
32. Aihaiti Y, Song Cai Y, Tuerhong X, et al. Therapeutic effects of naringin in rheumatoid arthritis: network pharmacology and experimental validation. Front Pharmacol. 2021;12:672054. doi:10.3389/fphar.2021.672054
33. Kristjánsson S, Shimizu T, Strannegård IL, Wennergren G. Eosinophil cationic protein, myeloperoxidase and tryptase in children with asthma and atopic dermatitis. Pediatr Allergy Immunol. 1994;5(4):223–229. doi:10.1111/j.1399-3038.1994.tb00244.x
34. Wiesolek HL, Bui TM, Lee JJ, et al. Intercellular adhesion molecule 1 functions as an efferocytosis receptor in inflammatory macrophages. Am J Pathol. 2020;190(4):874–885. doi:10.1016/j.ajpath.2019.12.006
35. Grobe W, Bieber T, Novak N. Pathophysiology of atopic dermatitis. J Dtsch Dermatol Ges. 2019;17(4):433–440. doi:10.1111/ddg.13819
36. Chu M, Tsang MS, He R, Lam CW, Quan ZB, Wong CK. The active compounds and therapeutic mechanisms of pentaherbs formula for oral and topical treatment of atopic dermatitis based on network pharmacology. Plants. 2020;9(9):1166. doi:10.3390/plants9091166
37. Agha-Majzoub R, Becker RP, Schraufnagel DE, Chan LS. Angiogenesis: the major abnormality of the keratin-14 IL-4 transgenic mouse model of atopic dermatitis. Microcirculation. 2005;12(6):455–476. doi:10.1080/10739680591003297
38. Jung MK, Hur DY, Song SB, et al. Tannic acid and quercetin display a therapeutic effect in atopic dermatitis via suppression of angiogenesis and TARC expression in Nc/Nga mice. J Invest Dermatol. 2010;130(5):1459–1463. doi:10.1038/jid.2009.401
39. Furue M, Chiba T, Tsuji G, et al. Atopic dermatitis: immune deviation, barrier dysfunction, IgE autoreactivity and new therapies. Allergol Int. 2017;66(3):398–403. doi:10.1016/j.alit.2016.12.002
40. Guttman-Yassky E, Waldman A, Ahluwalia J, Ong PY, Eichenfield LF. Atopic dermatitis: pathogenesis. Semin Cutan Med Surg. 2017;36(3):100–103. doi:10.12788/j.sder.2017.036
41. Tokura Y, Hayano S. Subtypes of atopic dermatitis: from phenotype to endotype. Allergol Int. 2022;71(1):14–24. doi:10.1016/j.alit.2021.07.003
42. Tsai YC, Tsai TF. Overlapping Features of psoriasis and atopic dermatitis: from genetics to immunopathogenesis to phenotypes. Int J Mol Sci. 2022;23(10):5518. doi:10.3390/ijms23105518
43. Hofmann MA, Fluhr JW, Ruwwe-Glösenkamp C, Stevanovic K, Bergmann KC, Zuberbier T. Role of IL-17 in atopy-A systematic review. Clin Transl Allergy. 2021;11(6):e12047. doi:10.1002/clt2.12047
44. Xiong X, Huang C, Wang F, et al. Qingxue jiedu formulation ameliorated DNFB-induced atopic dermatitis by inhibiting STAT3/MAPK/NF-κB signaling pathways. J Ethnopharmacol. 2021;270:113773. doi:10.1016/j.jep.2020.113773
45. Zeng H, Zhao B, Zhang D, et al. Viola yedoensis Makino formula alleviates DNCB-induced atopic dermatitis by activating JAK2/STAT3 signaling pathway and promoting M2 macrophages polarization. Phytomedicine. 2022;103:154228. doi:10.1016/j.phymed.2022.154228
46. Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2(3):e24137. doi:10.4161/jkst.24137
47. Nakashima C, Yanagihara S, Otsuka A. Innovation in the treatment of atopic dermatitis: emerging topical and oral Janus kinase inhibitors. Allergol Int. 2022;71(1):40–46. doi:10.1016/j.alit.2021.10.004
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.