Back to Journals » Cancer Management and Research » Volume 12
Molecular Mechanisms of Anticancer Activities of Puerarin
Authors Ahmad B , Khan S , Liu Y, Xue M, Nabi G , Kumar S, Alshwmi M, Qarluq AW
Received 5 October 2019
Accepted for publication 16 December 2019
Published 8 January 2020 Volume 2020:12 Pages 79—90
DOI https://doi.org/10.2147/CMAR.S233567
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Bashir Ahmad,1,* Suliman Khan,2,3,* Yang Liu,2,3 Mengzhou Xue,2,3 Ghulam Nabi,4 Sunjeet Kumar,5 Mohammed Alshwmi,6 Abdul Wakeel Qarluq7
1Department of Pathology and Pathophysiology, College of Basic Medical Sciences, Dalian Medical University, Dalian, Liaoning 116044, People’s Republic of China; 2The Department of Cerebrovascular Diseases, The Second Affiliated Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China; 3Henan Medical Key Laboratory of Translational Cerebrovascular Diseases, Zhengzhou, People’s Republic of China; 4Key Laboratory of Animal Physiology, Biochemistry and Molecular Biology of Hebei Province, College of Life Sciences, Hebei Normal University, Shijiazhuang 050024, People’s Republic of China; 5The Key Laboratory of Aquatic Biodiversity and Conservation, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, People’s Republic of China; 6Department of Clinical Laboratory, The First Affiliated Hospital, Dalian Medical University, Dalian, Liaoning 116044, People’s Republic of China; 7Department of Biochemistry and Molecular Biology, Dalian Medical University, Dalian, Liaoning 116044, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mengzhou Xue; Ghulam Nabi Email [email protected]; [email protected]
Abstract: Medicinal plants are a vital source of natural products (NPs) that can cure cancer through modulation of different pathways, including oxidative stress, extrinsic and intrinsic apoptosis, cell cycle, inflammation, NF-kB, PI3K/AKT/mTOR, AMPK (JNK), MEK/ERK (Raf)-MEK-ERK and autophagy. Puerarin (Pue), an important NP belonging to the isoflavone glycoside group, is derived from Pueraria lobata (Willd.) Ohwi, Pueraria thomsonii Benth, and Pueraria tuberosa (Willd.). This NP was approved by the Chinese Ministry of Health for the treatment of different diseases in 1993, but it was also later reported to exhibit anticancer activity. Pue causes cancer cells death through modulation of different mechanisms including oxidative stress, intrinsic and extrinsic, Survivin and XIAP, PI3K/AKT/mTOR, Ras-Raf-MEK-ERK, JNK, cell cycle, AMPK, NF-kB, inflammation and autophagy pathways. Therefore, this review compiles for the first time the studies about the anticancer mechanism of Pue and provides comprehensive information about the anticancer effects of Pue. This review may serve as a basis for future research and clinical treatment.
Keywords: medicinal plants, natural products, Puerarin, Pueraria lobata, Pueraria thomsonii, Pueraria tuberosa
Introduction
Cancer is a major cause of mortality worldwide; therefore, the development of cancer treatment is highly important. Natural products (NPs) are widely used in cancer treatment because of their low toxicity and high level of success.1 Medicinal plants proven to get active compounds contain NPs.2 In the recent era, medicinal plants have been explored for the treatment of a wide spectrum of physiological diseases.3–8 Common medicinal plant-derived NPs are isoflavones with a 3-phenylchroman skeleton.9 Legumes of the Leguminosae and Fabaceae families, including lupine, kudzu, barley, cauliflower, soy, and fava beans, are a major source of plant-based isoflavones.9,10 Puerarin (Pue), depicted in Figure 1, is an important bioactive isoflavone glycoside.11,12 Pue has been isolated from several leguminous plants of the genus Pueraria, including Pueraria tuberosa (Willd.),13–15 Pueraria lobata (Willd.) Ohwi (Gegen in Chinese),16,17 and Pueraria thomsoniiBenth.13,18,19 Pueraria plants have been interlinked with Asian culture because of their use in decoration, cooking, and disease treatment.13 Systematic biology is an emerging approach that focuses on molecular interactions with biological systems.20 Pue has molecular weight 416,21 possesses several pharmacological activities against osteoporosis,22 cardiovascular diseases,23 fever,24 neurological dysfunction,25 liver injury,26 and hangover, and they have been used in clinical treatments and experimental research.27 Pue injections are used extensively in China,16,28,29 but their effects on human health remain unclear to date.16 Pue was approved for clinical treatment in 1993 by the Chinese Ministry of Health, and it was initially used for the treatment of cardiovascular diseases; however, Pue was later reported to have anticancer activity.30 Furthermore, Li et al (2006) reported the tissue distribution and pharmacokinetics after oral administration of high dose of Pue and a complex of Pue and phospholipids (400 mg/kg) through high-performance liquid chromatography (HPLC). Although Pue in high dose has effects of saturation and change metabolism, but have no evidence for the presence in tissue.31 The excretion of Pue also remains unclear, whether they excrete with bile, following enterohepatic circulation32 or excreted through urine. Recently, it is reported that the Pue eliminated very rapidly from the body.33 Although the level of Pue was very low after 6 h of administration, the excretion in feces and urine remained unchanged till 24 h (45.33%).33,34 These studies also clear that the Pue excrete through urine and feces. Pue is an NP against cancer, and a number of reviews about this topic are available. However, a review of its anticancer activity remains lacking. Therefore, this review summarizes the available studies on the anticancer activity of Pue to encourage future research and clinical trials on Pue. The following are the mainly reported mechanisms through which Pue inhibits cancer cell proliferation and induces death.
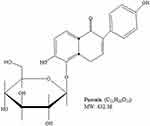 |
Figure 1 Chemical Structure of Puerarin. |
Pue Anticancer Molecular Mechanisms
Pue possesses anticancer effect through different molecular mechanisms which are listed below.
Oxidative Stress
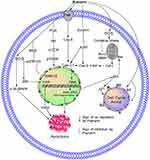
Multiple studies show that the high oxidative stress in cancer cells increases cell proliferation, survival, metastasis, and angiogenesis; disrupts cell death signaling; and promotes drug resistance.35–37 Increased generation of reactive oxygen species (ROS) and inhibition of mitochondrial membrane potential (MMP) lead to oxidative stress.13 ROS play a vital role in different types of cellular processes, including gene expression, cell survival, proliferation, differentiation, enzyme regulation, and eliminating foreign particles and pathogens.38,39 ROS promote tumor growth, but recent studies suggest that this property of ROS can be beneficial for cancer therapy. Various in vitro and in vivo experiments showed that phytochemicals induce exogenous ROS generation above a threshold level in cancer cells and decrease the MMP40 that selectively kills these cancer cells.35,37,41,42 Pue is a potential NP that inhibits the proliferation and induces the apoptosis of SMMC7721 cells through oxidative stress via ROS generation and MMP dissipation.43 Oxidative stress activates the intrinsic apoptosis pathway as shown in Table 1 and Figure 2.
 |
Table 1 Potential Mechanisms of Puerarin in Different Cancer Cell Lines Through Different Pathways |
Intrinsic Apoptosis Pathway
Mitochondrial-dependent apoptosis is an important pathway for the induction of apoptosis, and disturbance in this pathway can inhibit apoptosis. The intrinsic pathway is controlled by the B-cell lymphoma 2 (Bcl-2) family proteins, which either increase or decrease the mitochondrial membrane permeability for the release of cytochrome-c and other apoptotic proteins.44 Furthermore, caspase activation and DNA fragmentation are the main features of induced apoptosis.45–47 Plant-derived NPs induce apoptosis in cancer cells through the mitochondrial-dependent pathway.48,49
Pue significantly induces apoptosis in HT-29 colon cancer cells,50 human mental cell lymphoma (MCL) Z138,51 SMMC7721,43 MDA-MB-231,52 MCF-7,52 NB4,53 A549 cells53 and HS578T52 cell lines through the intrinsic apoptosis pathway.43,50–55 In this pathway, Pue downregulates Bcl-2,50,51,53–55 which causes Bax50–53,55 upregulation and increases the permeability of the mitochondria to apoptosis-inducing factor (AIF),43 which is then released from the mitochondria to the cytosol. In the cytosol, AIF43 activates caspase-3,43,50,52–55 −7,53 and −9,43,52,53 which trigger DNA fragmentation and ultimately induce apoptosis,43,50–55 as illustrated in Table 1 and Figure 2.
Survivin and X-Linked Inhibitor of Apoptosis Protein (XIAP) Pathway
Survivin, which belongs to the inhibitor of apoptosis protein (IAP) family, is one of the top 5 cancer-associated genes. In different types of cancer, survivin is upregulated and is associated with resistance to radiotherapy and chemotherapy along with poor prognosis.56 Overexpression of survivin in tumors decreases apoptosis and increases cell division. XIAP also belongs to the family of IAP, and it is upregulated in different cancers, including lung, breast, bladder, and renal carcinoma.57–60 A variety of NPs reduce the expression levels of survivin and XIAP in different cancers.61,62 Pue inhibits the expression of survivin in NB4 cells54 and XIAP51 in Z13851 cells, which further activate caspase-3 and −9, as shown in Table 1 and Figure 2.
Extrinsic Apoptosis Pathway
The extrinsic pathway is activated through the tumor necrosis factor (TNF) family proteins, including Fas or TNF receptor-1 (TNFR1).63 Fas or TNFR1 activates caspase-8 via Fas-associated death domain protein, creating a death-inducing signaling complex that triggers caspase-3 activation and cell death.64,65 The NPs also modulate the extrinsic apoptosis pathway in different cancers.66,67
Pue induces the extrinsic apoptosis pathway in NB4 and SMMC772143 cells through activation of Fasl,54 which further activates caspase-8;43,54 activated caspase-8 then activates caspase-338,54 and causes apoptotic cell death.43,54 This pathway is further summarized in Table 1 and Figure 2.
Phosphatidylinositol-3-Kinase/Protein Kinase B/Mammalian Target of Rapamycin (PI3K/AKT/mTOR) Pathway
The PI3K/AKT/mTOR signaling pathway increases cell growth and survival through various mechanisms.68,69 The PI3K/AKT/mTOR pathway is activated in a number of human cancers through different mechanisms.70–73 For example, phosphorylation of the two residues of AKT, including threonine (Thr 308) and serine 473 (Ser 473), leads to AKT activation.74 Following activation, AKT enters the nucleus, where they affect the activity of transcription-regulating factors. PI3K/AKT signaling increases the expression of mTOR, and this expression of mTOR is associated with poor prognosis. NPs reportedly inhibit the PI3K/AKT/mTOR pathway in cancer cells.75
A natural Pue induces apoptosis in bladder cancer T24, EJ cells and human mental lymphoma Z13851 cells through the PI3K/AKT/mTOR51,76 pathway by downregulating PI3K,51 Akt,76 p-mTOR,76 and p-p70S6K.51,76 This PI3K/AKT/mTOR pathway is further summarized in Table 1 and Figure 2.
MAPK/ERK (Ras-Raf-MEK-ERK) Pathway
The mitogen-activated protein kinase/extracellular signal-regulated kinases (MAPK/ERK) pathway are also known as Rat Sarcoma (Ras)-Rapidly Accelerated Fibrosarcoma (Raf)-MEK-ERK pathway.77 The MAPK/ERK pathway possesses different cascades and is mostly deregulated in human cancers.77 It regulates several cellular processes, such as cell growth, proliferation, differentiation, migration, senescence, and apoptosis.78 The molecules of the MAPK/ERK pathway become activated through its phosphorylation. The activated ERK enters the nucleus, where it activates transcription factors. The activated transcription factors then bind to various genes, including growth factors and cytokines. Such genes are responsible for the increase in cell proliferation and decrease in apoptosis.79 Disturbance of the normal signaling of this pathway causes senescence, drug resistance, and tumorigenesis.78,80,81 Failure of this pathway has been detected in various cancers.82,83 NPs trigger the death of cancer cells through the MAPK/ERK pathway.84 Pue induces apoptosis in SMMC772143,85 cells through ROS43-mediated p-38 MAPK85 and ERK143 activation.43,85 In NSCLC cells, Pue inhibits the IL-4-induced activation of MEK and ERK1/2 and its nuclear translocation.86 In addition, Pue reverses the oxidized low-density lipoprotein (ox-LDL)-induced increase in VSM cell proliferation through ERK1/2 and proliferating cell nuclear antigen activation,87 as depicted in Table 1 and Figure 2.
c-Jun NH2 Terminal Kinase (JNK) Pathway
The JNK pathway controls different physiological processes, such as cell survival, death, differentiation, proliferation, inflammation, and protein expression. Dysregulation of the JNK pathway is linked with different diseases, including auto-immune disease, cardiac hyper therapy, asthma, diabetes, and cancer.88 JNK is involved in oncogenic changes. The JNK signaling pathway is involved in apoptosis elimination through suppression of Ras transformation.89 Aromatase P450 is an enzyme that is expressed in different parts of the body, and changes in its level in the body cause different diseases.90 Different NPs induce apoptosis in cancer cells through the regulation of the JNK pathway.91–93
The oxidative stress generated in SMMC7721 cells with Pue treatment due to ROS causes JNK activation, which causes c-jun inhibition and cell apoptosis.43 Another study revealed that Pue alone or in combination with arsenic trioxide induces apoptosis in NB4 cells through JNK activation.54 In endometriosis, uterine fibroblast and endometrial cancer Aromatase P450 (P450 (arom)) is overexpressed, which is downregulated by Pue in RL95-2 and Ishikawa cell lines at both protein and mRNA levels.94 Furthermore, with Pue treatment, c-jun regulates the P450 (arom) expression and activity, which was confirmed by c-jun knockdown through siRNA. Therefore, inhibition of P450 (arom) activity and expression with Pue may be linked with transcription factor AP-1 or c-jun down-regulation,94 as shown in Table 1 and Figure 2.
Cell Cycle
Cell growth is controlled by the cell cycle. Cells are regulated at different checkpoints by the interactions of various cyclins with their exact cyclin-dependent kinases (CDKs) to make active complexes. The process of each checkpoint completes accurately before the progression to the next phase of the cell cycle.95 Among CDKs, p21 regulates cell cycle at different checkpoints.96,97 In cell cycle regulation, p53 plays a key component role. It is activated in a wide range of damage and stresses.98,99 Recently, NPs have attracted the attention of researchers because of their potential to reverse cancer through the cell cycle.100
Pue induces cell cycle arrest in T24,76 EJ,76 NB4,101 Kasumi-1,101 U937,101 HL-60,101,102 MDA-MB-231,52 MCF-7,52 HS578T,52 vascular smooth muscle cells (VSMCs)87 and bladder cancer cells at the G1/G0,76,101 sub-G1,103 G1S1,87 and G2/M52 phases through p5352 upregulation, which further increases p2152 expression and triggers cell apoptosis,52,76,87,102 as illustrated in Table 1 and Figure 2.
AMP-Activated Protein Kinase (AMPK) Pathway
AMPK plays a role as a key sensor for cellular energy because it phosphorylates and activates enzymes, including acetyl-CoA carboxylase (ACC).103 The glycogen synthase kinase-3beta (GSK-3b) causes the phosphorylation of cAMP-responsive element-binding protein (CREB),104,105 and inhibition of GSK-3b increases multi-drug resistance 1 (MDR1) gene expression.106 The overexpression of MDR1, which has been reported in several cancers, lowers drug efficacy.107 A variety of anticancer natural compounds derived from vegetables, fruits, herbs, and oilseed107 regulate MDR1 activity.108,109
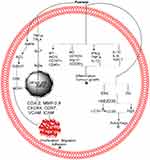
In the human breast cancer cell line MCF-7/adriamycin (MCF-7/adr), plant-derived Pue activates AMPK, ACC, and GSK-3b, which lead to the inhibition of CREB and MDR1.110 Pue-induced suppression of MDR1 can be reversed by inhibitor of AMPK (compound C).110 Furthermore, both protein kinase A/CRE inhibitor (H89) and Pue inhibit the transcriptional activities of both cAMP-responsive element (CRE) and MDR1 protein.110 These results show that Pue inhibits the expression of MDR1 through CRE transcriptional activity-dependent upregulation of AMPK in MCF-7/adr cells,110 as depicted in Table 1 and Figure 3A.
Nuclear Factor kB (NF-kB) Pathway
NF-kB is a transcription factor complex consisting of hetero- and homodimers of five members of a Reticuloenotheliosis oncogene cellular homolog (Rel) family, including RelA (p65), RelB, c-Rel, NF-kB1 (p50/p105), and NF-kB2 (p52/p100).111 In cancer, the functions of NF-kB are mostly dysregulated.112 Active NF-kB has been reported in several cancers, including colon, liver, breast, pancreas, prostrate, ovarian, leukemia, and lymphoma cancers.113–115 NF-kB becomes activated when the DNA becomes damaged, which consequently activates a number of NF-kB-targeted genes, including inducible nitric oxide synthase (iNOS)116 and COX-2.117 Furthermore, binding of TNFα to TNFR leads to homotrimerization of receptors and adaptor proteins, resulting in cell proliferation and survival by increasing the expression of NF-kB and activator protein 1 target genes, including vascular cell adhesion molecule-1 (VCAM-1).118–120 NF-kB activation triggers the activation of chemokines and its related receptors, including C-X-C chemokine receptor 4 (CXCR4)121 and CCR7,122 which play important roles in the migration of cancer cells to target organs.118 These genes play pivotal roles in pro-survival anti-apoptosis. Therefore, NF-kB is a candidate for therapeutic resistance in different cancers. Different NPs have potential therapeutic efficacy against cancer by inhibiting NF-kB pathway activation in cancer cells.123
In lipopolysaccharide-induced MDA-MB-231,124 MCF-7/adriamycin (MCF-7/adr),110 MCF-7124 cells, Z138 cells,51 T24,125 and THP1126 cells, Pue inhibits proliferation51 and negates adhesion,124 migration,124 and invasion124 by regulating the NF-kB51,110,124,125 pathway.51,110,124–126 In the NF-kB pathway, Pue inhibits the expression of inflammatory factors TNF-α and IL-6124 and abolishes NF-kB activation through inhibition of Phospho-IκBα/IκBα110,124,126 IkkappaB,110,124 and p65124 and upregulation of miR16.125 Pue also inhibits the NF-kB nuclear translocation,125 which leads to the inhibition of COX-2, MMP-2,9 CXCR4, CCR7, VCAM, and ICAM at the mRNA and protein levels,124 as shown in Table 1 and Figure 3A.
Inflammation Pathway
Inflammation is often associated with the progression and development of cancer. Inflammation leads to tumorigenesis via two basic ways, including extrinsic and intrinsic.127 There are many factors which cause tumor-extrinsic inflammation, including viral and bacterial infections, obesity, autoimmune diseases, asbestos exposure, excessive alcohol consumption and tobacco smoking.127 On the other hand, cancer intrinsic inflammation can be triggered through cancer-initiating mutations and increase tumor growth through the activation of inflammatory cells.127 These information show that both the extrinsic and intrinsic inflammations are providing a good background for cancer progression.127 Those cells which are responsible for cancer-causing inflammation are stable genetically and did not cause rapid emergence of drug resistance, therefore targeting of inflammation represents a good strategy for cancer prevention and therapy.127 Here, we focus on cancer-elicited intrinsic inflammation.
Macrophages play important roles in different types of inflammatory diseases and cancer progression.128 M1 and M2 are markers of inflammation, in which M1 macrophages intimate tumorigenesis through reactive oxygen and intermediates of nitrogen, while M2 macrophages increase tumor progression.129,130 Next, NF-kB is considered a central mediator in inflammation process while identification of its kinship with v-Rel oncogene, shows that the NF-kB is involved in cancer development.131 The dual role of Macrophages and NF-kB has made its therapeutic targeting a challenge. Therefore, the understanding of such interaction between immune signaling pathways and cellular metabolism can provide us the clues to develop potential therapeutic strategies for the treatment of inflammatory diseases, including cancer. Recent studies have reported that NPs target both cancer and inflammation.132
Pue inhibits the tumor volume and its growth in the NSCLC xenograft model by upregulating M1 markers (such as iNOS+, cluster of differentiation 197, and CD40+) and decreasing M2 markers (including CD163+, Arginase 1, and CD206+).86 Furthermore, Pue increases TNF-α, pro-inflammatory cytokine interferon-γ, and interleukin-12 while reducing pro-tumor cytokines IL-4, IL-10 and transforming growth factor-β.86 Pue inhibits the change of pure macrophages from polarized to M2 phenotype without other auxiliary cell involvement.86 In THP1 macrophages, Pue dose-dependently inhibits the expression of oxLDL-activated pro-inflammatory genes, including toll-like receptor 4, and the ratio of Phospho-IκBα/IκBα.126 Furthermore, Pue inhibits the formation of foam cells and lipid deposition induced by oxLDL, which are associated with scavenger receptor CD36 downregulation. Therefore, these results reveal that Pue acts as an antiatherogenic and anti-inflammatory agent by downregulating CD36 expression and inhibiting the TLR4/NF-κB pathway.126 All these results show that the Pue inhibits the cancer progression through inhibition of inflammation. The inflammation pathway is summarized further in Table 1 and Figure 3B.
Autophagy Pathway
In normal cells, autophagy suppresses tumor growth by maintaining genomic stability. Once the tumor forms, the unbalance in autophagy contributes to tumor growth. In the complexity of autophagy, the ERK/MAPK, PI3K/MAPK, and other signaling pathways play important roles.133 Furthermore, the light chain 3 (LC3) and autophagy protein 5 (Atg5) are considered essential for autophagy.134 The NP modulates autophagy in different diseases, including cancer.135 In NCI-H441 cells, Pue induces autophagy through the PI3K/Akt and ERK pathway via extreme inhibition of Akt and ERK phosphorylation, which was further inhibited through BEZ235, an inhibitor of PI3K/Akt. Activation of the PI3K/Akt pathway further increases Atg5 expression, but no obvious effect was observed in LC3I conversion to LC3II. However, rapamycin, an inhibitor of mTOR, increases the expression of Atg5 and LC3II,55 as depicted in Table 1 and Figure 3C.
Conclusion
Pue has potential anticancer activity. Available studies show that Pue might be a very good therapeutic drug for the treatment of different cancers because it is a good inducer of apoptosis. Additional preclinical and clinical studies must be designed and conducted to determine the definite dose of Pue for each type of cancer and establish a specific pathway or gene. The available anticancer information about Pue is summarized in Table 1 and Figures 2 and 3.
Abbreviations
NPs, Natural products; Pue, Puerarin; ROS, reactive oxygen species; MMP, mitochondrial membrane potential; Bcl-2, B-cell lymphoma 2; AIF, apoptosis-inducing factor; IAP, inhibitors of apoptosis protein; XIAP, X-linked inhibitor of apoptosis protein; TNF, tumor necrosis factor; TNFR1, Fas or TNF receptor-1; CDKs, cyclin-dependent kinases; iNOS+, inducible nitric oxide synthase; TNF-α, tumor necrosis factor; oxLDL, oxidized low-density lipoprotein; NF-kB, Nuclear factor kB; Rel, Reticuloenotheliosis oncogene cellular homolog; VCAM-1, vascular cell adhesion molecule-1; CXCR4, C-X-C chemokine receptor 4; PI3K/AKT/mTOR, Phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin; MAPK/ERK, Mitogen-activated protein kinase/extracellular signal-regulated kinases; Ras, Rat Sarcoma; Raf, Rapidly Accelerated Fibrosarcoma; AMPK, AMP-activated protein kinase; ACC, acetyl-CoA carboxylase; GSK-3b, glycogen synthase kinase-3beta; CREB, cAMP-responsive element-binding protein; MDR1, multi-drug resistance 1; MCF-7/adr, MCF-7/adriamycin; CRE, cAMP-responsive element; JNK, c-Jun NH2 terminal kinase; P450 (arom), Aromatase P450; LC3, light chain 3; Atg5, autophagy protein 5.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
The project was supported by the National Science Foundation of China (Grant Numbers 31870917, 81471174, 81520108011, and 81870942), the National Key Research and Development Program of China (grant no: 2018YFC1312200), the Innovation Scientists and Technicians Troop Constructions Projects of Henan Province of China for MX, and China Postdoctoral Science Foundation (2016M602291).
Disclosure
The authors declare no conflict of interest related to this work.
References
1. Pengyu S, Ahmad B, Lijuan Z. Natural β-elemene: advances in targeting cancer through different molecular pathways. North Am J Academic Res. 2018;1(4):27.
2. Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, et al. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv. 2015;33(8):1582–1614. doi:10.1016/j.biotechadv.2015.08.001
3. Bui TT, Nguyen TH. Natural product for the treatment of alzheimer’s disease. J Basic Clin Physiol Pharmacol. 2017;28(5):413–423. doi:10.1515/jbcpp-2016-0147
4. Hirsch GE, Viecili PRN, de Almeida AS, et al. Natural products with antiplatelet action. Curr Pharm Des. 2017;23(8):1228–1246. doi:10.2174/1381612823666161123151611
5. Jahan S, Kumar D, Chaturvedi S, et al. Therapeutic targeting of NLRP3 inflammasomes by natural products and pharmaceuticals: a novel mechanistic approach for inflammatory diseases. Curr Med Chem. 2017;24(16):1645–1670. doi:10.2174/0929867324666170227121619
6. Kohansal MH, Nourian A, Rahimi MT, Daryani A, Spotin A, Ahmadpour E. Natural products applied against hydatid cyst protoscolices: a review of past to present. Acta Trop. 2017;176:385–394. doi:10.1016/j.actatropica.2017.09.013
7. Parvez MK. Natural or plant products for the treatment of neurological disorders: current knowledge. Curr Drug Metab. 2018;19(5):424–428. doi:10.2174/1389200218666170710190249
8. Rios JL, Francini F, Schinella GR. Natural products for the treatment of type 2 diabetes mellitus. Planta Med. 2015;81(12–13):975–994. doi:10.1055/s-0035-1546131
9. Dixon RA, Sumner LW. Legume natural products: understanding and manipulating complex pathways for human and animal health. Plant Physiol. 2003;131(3):878–885. doi:10.1104/pp.102.017319
10. Prasad S, Phromnoi K, Yadav VR, Chaturvedi MM, Aggarwal BB. Targeting inflammatory pathways by flavonoids for prevention and treatment of cancer. Planta Med. 2010;76(11):1044–1063. doi:10.1055/s-0030-1250111
11. Zhang Z, Lam TN, Zuo Z. Radix puerariae: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2013;53(8):787–811. doi:10.1002/jcph.96
12. Cherdshewasart W, Subtang S, Dahlan W. Major isoflavonoid contents of the phytoestrogen rich-herb pueraria mirifica in comparison with pueraria lobata. J Pharm Biomed Anal. 2007;43(2):428–434. doi:10.1016/j.jpba.2006.07.013
13. Maji AK, Pandit S, Banerji P, Banerjee D. Pueraria tuberosa: a review on its phytochemical and therapeutic potential. Nat Prod Res. 2014;28(23):2111–2127. doi:10.1080/14786419.2014.928291
14. Maji AK, Maity N, Banerji P, Banerjee D. A validated RP-HPLC-UV method for quantitative determination of puerarin in pueraria tuberosa DC tuber extract. Pharm Methods. 2012;3(2):79–83. doi:10.4103/2229-4708.103879
15. Chauhan SK, Singh B, Agrawal S. Determination of puerarin from pueraria tuberosa dc by HPLC. Anc Sci Life. 2004;23(3):22–25.
16. Wong KH, Li GQ, Li KM, Razmovski-Naumovski V, Chan K. Kudzu root: traditional uses and potential medicinal benefits in diabetes and cardiovascular diseases. J Ethnopharmacol. 2011;134(3):584–607. doi:10.1016/j.jep.2011.02.001
17. Chen R, Xue J, Xie M. Puerarin prevents isoprenaline-induced myocardial fibrosis in mice by reduction of myocardial TGF-beta1 expression. J Nutr Biochem. 2012;23(9):1080–1085. doi:10.1016/j.jnutbio.2011.05.015
18. Wei SY, Chen Y, Xu XY. Progress on the pharmacological research of puerarin: a review. Chin J Nat Med. 2014;12(6):407–414. doi:10.1016/S1875-5364(14)60064-9
19. Wang J, Yang ZR, Guo XF, et al. Synergistic effects of puerarin combined with 5- fluorouracil on esophageal cancer. Mol Med Rep. 2014;10(5):2535–2541. doi:10.3892/mmr.2014.2539
20. Kitano H. Systems biology: a brief overview. Science. 2002;295(5560):1662–1664. doi:10.1126/science.1069492
21. Luo CF, Yuan M, Chen MS, Liu SM, Ji H. Metabolites of puerarin identified by liquid chromatography tandem mass spectrometry: similar metabolic profiles in liver and intestine of rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(3–4):363–370. doi:10.1016/j.jchromb.2009.12.002
22. Wong R, Rabie B. Effect of puerarin on bone formation. Osteoarthritis Cartilage. 2007;15(8):894–899. doi:10.1016/j.joca.2007.02.009
23. Pan ZY, Bao ZS, Wu ZM, et al. The myocardial protective effects of puerarin on STZinduced diabetic rats. Fen Zi Xi Bao Sheng Wu Xue Bao. 2009;42(2):137–144.
24. Yao XJ, Yin JA, Xia YF, et al. Puerarin exerts antipyretic effect on lipopolysaccharide induced fever in rats involving inhibition of pyrogen production from macrophages. J Ethnopharmacol. 2012;141(1):322–330. doi:10.1016/j.jep.2012.02.038
25. Lin F, Xie B, Cai F, Wu G. Protective effect of puerarin on beta-amyloid-induced neurotoxicity in rat hippocampal neurons. Arzneimittelforschung. 2012;62(4):187–193. doi:10.1055/s-0031-1299763
26. Zhao M, Du YQ, Yuan L, Wang NN. Protective effect of puerarin on acute alcoholic liver injury. Am J Chin Med. 2010;38(2):241–249. doi:10.1142/S0192415X10007816
27. Penetar DM, Toto LH, Farmer SL, et al. The isoflavone puerarin reduces alcohol intake in heavy drinkers: a pilot study. Drug Alcohol Depend. 2012;126(1–2):251–256. doi:10.1016/j.drugalcdep.2012.04.012
28. Wang Q, Wu T, Chen X, et al. Puerarin injection for unstable angina pectoris. Cochrane Database Syst Rev. 2006;3:Cd004196.
29. Tan Y, Liu M, Wu B. Puerarin for acute ischaemic stroke. Cochrane Database Syst Rev. 2008;1:Cd004955.
30. le Sage C, Agami R. Immense promises for tiny molecules: uncovering miRNA functions. Cell Cycle. 2006;5(13):1415–1421. doi:10.4161/cc.5.13.2890
31. Li Y, Pan WS, Chen SL, Xu HX, Yang DJ, Chan AS. Pharmacokinetic, tissue distribution, and excretion of puerarin and puerarin-phospholipid complex in rats. Drug Dev Ind Pharm. 2006;32(4):413–422. doi:10.1080/03639040600559123
32. Sfakianos J, Coward L, Kirk M, Barnes S. Intestinal uptake and biliary excretion of the isoflavone genistein in rats. J Nutr. 1997;127(7):1260–1268. doi:10.1093/jn/127.7.1260
33. Luo C, Yuan M, Chen M, Liu S, Zhu L, Liu X. Determination of puerarin in rat plasma by HPLC with fluorescence detection and its application to pharmacokinetic studies. Lat Am J Pharm. 2009;28:351–357.
34. Luo C, Yuan M, Chen M, Xiong W, Tian J. Study on the correlation between excretion of puerarin and administration routes in rats. Strait Pharm J. 2009;21(4):41–44.
35. Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8(7):579–591. doi:10.1038/nrd2803
36. Hong YH, Uddin MH, Jo U, et al. ROS accumulation by PEITC selectively kills ovarian cancer cells via UPR-mediated apoptosis. Front Oncol. 2015;5:167. doi:10.3389/fonc.2015.00167
37. Zhu L, Ren L, Chen Y, Fang J, Ge Z, Li X. Redox status of high-mobility group box 1 performs a dual role in angiogenesis of colorectal carcinoma. J Cell Mol Med. 2015;19(9):2128–2135. doi:10.1111/jcmm.12577
38. Gorlach A, Dimova EY, Petry A, et al. Reactive oxygen species, nutrition, hypoxia and diseases: problems solved? Redox Biol. 2015;6:372–385. doi:10.1016/j.redox.2015.08.016
39. Gorlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: a mutual interplay. Redox Biol. 2015;6:260–271. doi:10.1016/j.redox.2015.08.010
40. Sun DK, Wang L, Zhang P. Antitumor effects of chrysanthemin in PC-3 human prostate cancer cells are mediated via apoptosis induction, caspase signalling pathway and loss of mitochondrial membrane potential. Afr J Tradit Complement Altern Med. 2017;14(4):54–61. doi:10.21010/ajtcam.v14i4.7
41. Wei C, Xiao Q, Kuang X, Zhang T, Yang Z, Wang L. Fucoidan inhibits proliferation of the SKM-1 acute myeloid leukaemia cell line via the activation of apoptotic pathways and production of reactive oxygen species. Mol Med Rep. 2015;12(5):6649–6655. doi:10.3892/mmr.2015.4252
42. Seo KH, Ryu HW, Park MJ, et al. Mangosenone F, a furanoxanthone from Garciana mangostana, induces reactive oxygen species-mediated apoptosis in lung cancer cells and decreases xenograft tumor growth. Phytother Res. 2015;29(11):1753–1760. doi:10.1002/ptr.v29.11
43. Zhang WG, Liu XF, Meng KW, Hu SY. Puerarin inhibits growth and induces apoptosis in SMMC-7721 hepatocellular carcinoma cells. Mol Med Rep. 2014;10(5):2752–2758. doi:10.3892/mmr.2014.2512
44. Wu CC, Bratton SB. Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid Redox Signal. 2013;19(6):546–558. doi:10.1089/ars.2012.4905
45. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi:10.1080/01926230701320337
46. Ferreira CG, Epping M, Kruyt FA, Giaccone G. Apoptosis: target of cancer therapy. Clin Cancer Res. 2002;8(7):2024–2034.
47. Ouyang L, Shi Z, Zhao S, et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45(6):487–498. doi:10.1111/cpr.2012.45.issue-6
48. Yang Y, Zong M, Xu W, et al. Natural pyrethrins induces apoptosis in human hepatocyte cells via Bax- and Bcl-2-mediated mitochondrial pathway. Chem Biol Interact. 2017;262:38–45. doi:10.1016/j.cbi.2016.12.006
49. Plitzko B, Kaweesa EN, Loesgen S. The natural product mensacarcin induces mitochondrial toxicity and apoptosis in melanoma cells. J Biol Chem. 2017;292(51):21102–21116. doi:10.1074/jbc.M116.774836
50. Yu Z, Li W. Induction of apoptosis by puerarin in colon cancer HT-29 cells. Cancer Lett. 2006;238(1):53–60. doi:10.1016/j.canlet.2005.06.022
51. Gan M, Yin X. Puerarin induced in mantle cell lymphoma apoptosis and its possible mechanisms involving multi-signaling pathway. Cell Biochem Biophys. 2015;71(1):367–373. doi:10.1007/s12013-014-0207-y
52. Lin Y-J, Hou YC, Lin C-H, et al. Puerariae radix isoflavones and their metabolites inhibit growth and induce apoptosis in breast cancer cells. Biochem Biophys Res Commun. 2009;378(4):683–688. doi:10.1016/j.bbrc.2008.10.178
53. Chen T, Chen H, Wang Y, Zhang J. In vitro and in vivo antitumour activities of puerarin 6ʹʹ-O-xyloside on human lung carcinoma A549 cell line via the induction of the mitochondria-mediated apoptosis pathway. Pharm Biol. 2016;54(9):1793–1799. doi:10.3109/13880209.2015.1127980
54. Tang YH, Zhu HQ, Zhang YC, et al. Apoptosis of NB4 cells induced by flavonoids of puerarin in vitro. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010;18(2):326–329.
55. Hu Y, Li X, Lin L, Liang S, Yan J. Puerarin inhibits non-small cell lung cancer cell growth via the induction of apoptosis. Oncol Rep. 2018;39(4):1731–1738. doi:10.3892/or
56. Yamamoto H, Ngan CY, Monden M. Cancer cells survive with survivin. Cancer Sci. 2008;99(9):1709–1714. doi:10.1111/j.1349-7006.2008.00870.x
57. Li M, Song T, Yin ZF, Na YQ. XIAP as a prognostic marker of early recurrence of nonmuscular invasive bladder cancer. Chin Med J (Engl). 2007;120(6):469–473. doi:10.1097/00029330-200703020-00007
58. Mizutani Y, Nakanishi H, Li YN, et al. Overexpression of XIAP expression in renal cell carcinoma predicts a worse prognosis. Int J Oncol. 2007;30(4):919–925.
59. Tamm I, Kornblau SM, Segall H, et al. Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin Cancer Res. 2000;6(5):1796–1803.
60. Foster FM, Owens TW, Tanianis-Hughes J, et al. Targeting inhibitor of apoptosis proteins in combination with ErbB antagonists in breast cancer. Breast Cancer Res. 2009;11(3):R41. doi:10.1186/bcr2328
61. Wang Y, Wu X, Zhou Y, Jiang H, Pan S, Sun B. Piperlongumine suppresses growth and sensitizes pancreatic tumors to gemcitabine in a xenograft mouse model by modulating the NF-kappa B pathway. Cancer Prev Res (Phila). 2016;9(3):234–244. doi:10.1158/1940-6207.CAPR-15-0306
62. Kong Y, Li F, Nian Y, et al. KHF16 is a leading structure from cimicifuga foetida that suppresses breast cancer partially by inhibiting the NF-kappaB signaling pathway. Theranostics. 2016;6(6):875–886. doi:10.7150/thno.14694
63. Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104(4):487–501. doi:10.1016/S0092-8674(01)00237-9
64. Ashkenazi A. Targeting the extrinsic apoptosis pathway in cancer. Cytokine Growth Factor Rev. 2008;19(3–4):325–331. doi:10.1016/j.cytogfr.2008.04.001
65. Fulda S. Targeting extrinsic apoptosis in cancer: challenges and opportunities. Semin Cell Dev Biol. 2015;39:20–25. doi:10.1016/j.semcdb.2015.01.006
66. Fatehchand K, Santhanam R, Shen B, et al. Active hexose-correlated compound enhances extrinsic-pathway-mediated apoptosis of acute myeloid leukemic cells. PLoS One. 2017;12(7):e0181729.
67. Kang TH, Yoon G, Kang IA, Oh HN, Chae JI, Shim JH. Natural compound licochalcone B induced extrinsic and intrinsic apoptosis in human skin melanoma (A375) and squamous cell carcinoma (A431) cells. Phytother Res. 2017;31(12):1858–1867.
68. Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28(6):1075–1083. doi:10.1200/JCO.2009.25.3641
69. Steelman LS, Chappell WH, Abrams SL, et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy implications for cancer and aging. Aging (Albany NY). 2011;3(3):192–222. doi:10.18632/aging.v3i3
70. Wong KK, Engelman JA, Cantley LC. Targeting the PI3K signaling pathway in cancer. Curr Opin Genet Dev. 2010;20(1):87–90. doi:10.1016/j.gde.2009.11.002
71. Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304(5670):554. doi:10.1126/science.1096502
72. Samuels Y, Velculescu VE. Oncogenic mutations of PIK3CA in human cancers. Cell Cycle. 2004;3(10):1221–1224. doi:10.4161/cc.3.10.1164
73. Kang S, Denley A, Vanhaesebroeck B, Vogt PK. Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase. Proc Natl Acad Sci U S A. 2006;103(5):1289–1294. doi:10.1073/pnas.0510772103
74. Vincent EE, Elder DJ, Thomas EC, et al. Akt phosphorylation on Thr308 but not on Ser473 correlates with Akt protein kinase activity in human non-small cell lung cancer. Br J Cancer. 2011;104(11):1755–1761. doi:10.1038/bjc.2011.132
75. Moselhy J, Srinivasan S, Ankem MK, Damodaran C. Natural products that target cancer stem cells. Anticancer Res. 2015;35(11):5773–5788.
76. Jiang K, Chen H, Tang K, et al. Puerarin inhibits bladder cancer cell proliferation through the mTOR/p70S6K signaling pathway. Oncol Lett. 2018;15(1):167–174. doi:10.3892/ol.2017.7298
77. Santarpia L, Lippman SM, El-Naggar AK. Targeting the MAPK-RAS-RAF signalling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16(1):103–119. doi:10.1517/14728222.2011.645805
78. Chang F, Steelman LS, Lee JT, et al. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003;17(7):1263–1293.
79. McCubrey JA, Steelman LS, Abrams SL, et al. Targeting survival cascades induced by activation of Ras/Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways for effective leukemia therapy. Leukemia. 2008;22(4):708–722. doi:10.1038/leu.2008.27
80. Martelli AM, Evangelisti C, Chiarini F, et al. The emerging role of the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin signaling network in normal myelopoiesis and leukemogenesis. Biochim Biophys Acta. 2010;1803(9):991–1002. doi:10.1016/j.bbamcr.2010.04.005
81. Martelli AM, Evangelisti C, Chiarini F, McCubrey JA. The phosphatidylinositol 3- kinase/Akt/mTOR signaling network as a therapeutic target in acute myelogenous leukemia patients. Oncotarget. 2010;1(2):89–103. doi:10.18632/oncotarget.114
82. Samatar AA, Poulikakos PI. Targeting RAS-ERK signalling in cancer: promises and challenges. Nat Rev Drug Discov. 2014;13(12):928–942. doi:10.1038/nrd4281
83. Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26(22):3279–3290. doi:10.1038/sj.onc.1210421
84. Kong AN, Yu R, Chen C, Mandlekar S, Primiano T. Signal transduction events elicited by natural products: role of MAPK and caspase pathways in homeostatic response and induction of apoptosis. Arch Pharm Res. 2000;23(1):1–16. doi:10.1007/BF02976458
85. Zhang WG, Yin XC, Liu XF, et al. Puerarin induces hepatocellular carcinoma cell apoptosis modulated by MAPK signaling pathways in a dose-dependent manner. Anticancer Res. 2017;37(8):4425–4431. doi:10.21873/anticanres.11837
86. Kang H, Zhang J, Wang B, et al. Puerarin inhibits M2 polarization and metastasis of tumor-associated macrophages from NSCLC xenograft model via inactivating MEK/ERK 1/2 pathway. Int J Oncol. 2017;50(2):545–554. doi:10.3892/ijo.2017.3841
87. Hu Y, Liu K, Bo S, et al. Inhibitory effect of puerarin on vascular smooth muscle cells proliferation induced by oxidised low-density lipoprotein via suppressing ERK ½ phosphorylation and PCNA expression. Pharmazie. 2016;71(2):89–93.
88. Kumar A, Singh UK, Kini SG, et al. JNK pathway signaling: a novel and smarter therapeutic targets for various biological diseases. Future Med Chem. 2015;7(15):2065–2086. doi:10.4155/fmc.15.132
89. Kennedy NJ, Davis RJ. Role of JNK in tumor development. Cell Cycle. 2003;2(3):199–201.
90. Garcia-Barrado MJ, Blanco EJ, Iglesias-Osma MC. Relation among aromatase P450 and tumoral growth in human prolactinomas. Int J Mol Sci. 2017;18(11):2299.
91. Yan L, Liu X, Yin A, Wei Y, Yang Q, Kong B. Huaier aqueous extract inhibits cervical cancer cell proliferation via JNK/p38 pathway. Int J Oncol. 2015;47(3):1054–1060. doi:10.3892/ijo.2015.3094
92. Li X, Lao Y, Zhang H, et al. The natural compound guttiferone F sensitizes prostate cancer to starvation induced apoptosis via calcium and JNK elevation. BMC Cancer. 2015;15:254. doi:10.1186/s12885-015-1292-z
93. Zhai T, Hei Z, Ma Q, et al. Shikonin induces apoptosis and G0/G1 phase arrest of gallbladder cancer cells via the JNK signaling pathway. Oncol Rep. 2017;38(6):3473–3480. doi:10.3892/or.2017.6038
94. Yu C, Li Y, Chen H, Yang S, Xie G. Decreased expression of aromatase in the Ishikawa and RL95-2 cells by the isoflavone, puerarin, is associated with inhibition of c-jun expression and AP-1 activity. Food Chem Toxicol. 2008;46(12):3671–3676. doi:10.1016/j.fct.2008.09.045
95. Khan M, Rasul A, Yi F, Zhong L, Ma T. Jaceosidin induces p53-dependent G2/M phase arrest in U87 glioblastoma cells. Asian Pac J Cancer Prev. 2011;12(12):3235–3238.
96. Lu MC, Yang SH, Hwang SL, et al. Induction of G2/M phase arrest by squamocin in chronic myeloid leukemia (K562) cells. Life Sci. 2006;78(20):2378–2383. doi:10.1016/j.lfs.2005.09.048
97. Yang G, Chang B, Yang F, et al. Aurora kinase A promotes ovarian tumorigenesis through dysregulation of the cell cycle and suppression of BRCA2. Clin Cancer Res. 2010;16(12):3171–3181. doi:10.1158/1078-0432.CCR-09-3171
98. Horn HF, Vousden KH. Coping with stress: multiple ways to activate p53. Oncogene. 2007;26(9):1306–1316. doi:10.1038/sj.onc.1210263
99. Bourougaa K, Naski N, Boularan C, et al. Endoplasmic reticulum stress induces G2 cell-cycle arrest via mRNA translation of the p53 isoform p53/47. Mol Cell. 2010;38(1):78–88. doi:10.1016/j.molcel.2010.01.041
100. Newman DJ, Cragg GM, Holbeck S, Sausville EA. Natural products and derivatives as leads to cell cycle pathway targets in cancer chemotherapy. Curr Cancer Drug Targets. 2002;2(4):279–308. doi:10.2174/1568009023333791
101. Shao HM, Tang YH, Jiang PJ, et al. Inhibitory effect of flavonoids of puerarin on proliferation of different human acute myeloid leukemia cell lines in vitro. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010;18(2):296–299.
102. Peng XY, Qi ZH, Chen HP. Study on the differentiation and apoptosis of HL-60 cell line induced by Puerarin. Hunan Yi Ke Da Xue Xue Bao. 2001;26(2):126–128.
103. Carling D, Clarke PR, Zammit VA, Hardie DG. Purification and characterization of the AMP-activated protein kinase. Copurification of acetyl-CoA carboxylase kinase and 3- hydroxy-3-methylglutaryl-CoA reductase kinase activities. Eur J Biochem. 1989;186(1–2):129–136. doi:10.1111/ejb.1989.186.issue-1-2
104. Horike N, Sakoda H, Kushiyama A, et al. AMP-activated protein kinase activation increases phosphorylation of glycogen synthase kinase 3beta and thereby reduces cAMP-responsive element transcriptional activity and phosphoenolpyruvate carboxykinase C gene expression in the liver. J Biol Chem. 2008;283(49):33902–33910. doi:10.1074/jbc.M802537200
105. Fiol CJ, Williams JS, Chou CH, Wang QM, Roach PJ, Andrisani OM. A secondary phosphorylation of CREB341 at Ser129 is required for the cAMP-mediated control of gene expression. A role for glycogen synthase kinase-3 in the control of gene expression. J Biol Chem. 1994;269(51):32187–32193.
106. Lim JC, Kania KD, Wijesuriya H, et al. Activation of beta-catenin signalling by GSK-3 inhibition increases p-glycoprotein expression in brain endothelial cells. J Neurochem. 2008;106(4):1855–1865. doi:10.1111/j.1471-4159.2008.05537.x
107. Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer: mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci. 2000;11(4):265–283.
108. Takano M, Kakizoe S, Kawami M, et al. Modulation of P-glycoprotein function and multidrug resistance in cancer cells by Thai plant extracts. Pharmazie. 2014;69(11):823–828.
109. Sreenivasan S, Ravichandran S, Vetrivel U, Krishnakumar S. Modulation of multidrug resistance 1 expression and function in retinoblastoma cells by curcumin. J Pharmacol Pharmacother. 2013;4(2):103–109. doi:10.4103/0976-500X.110882
110. Hien TT, Kim HG, Han EH, Kang KW, Jeong HG. Molecular mechanism of suppression of MDR1 by puerarin from pueraria lobata via NF-kappaB pathway and cAMP-responsive element transcriptional activity-dependent up-regulation of AMP activated protein kinase in breast cancer MCF-7/adr cells. Mol Nutr Food Res. 2010;54(7):918–928. doi:10.1002/mnfr.200900146
111. Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46(5):705–716. doi:10.1016/0092-8674(86)90346-6
112. Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8(1):49–62. doi:10.1038/nrm2083
113. Arkan MC, Greten FR. IKK- and NF-kappaB-mediated functions in carcinogenesis. Curr Top Microbiol Immunol. 2011;349:159–169. doi:10.1007/82_2010_97
114. Basseres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25(51):6817–6830. doi:10.1038/sj.onc.1209942
115. Prasad S, Ravindran J, Aggarwal BB. NF-kappaB and cancer: how intimate is this relationship. Mol Cell Biochem. 2010;336(1–2):25–37. doi:10.1007/s11010-009-0267-2
116. Park JH, Jeong YJ, Won HK, Choi SY, Park JH, Oh SM. Activation of TOPK by lipopolysaccharide promotes induction of inducible nitric oxide synthase through NFkappaB activity in leukemia cells. Cell Signal. 2014;26(5):849–856. doi:10.1016/j.cellsig.2014.01.004
117. Yamamoto K, Arakawa T, Ueda N, Yamamoto S. Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J Biol Chem. 1995;270(52):31315–31320. doi:10.1074/jbc.270.52.31315
118. Müller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50. doi:10.1038/35065016
119. Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol. 2016;12(1):49–62. doi:10.1038/nrrheum.2015.169
120. Brenner D, Blaser H, Mak TW. Regulation of tumour necrosis factor signalling: live or let die. Nat Rev Immunol. 2015;15(6):362–374. doi:10.1038/nri3834
121. Ali A, Kim SH, Kim MJ, et al. O-GlcNAcylation of NF-kappaB promotes lung metastasis of cervical cancer cells via upregulation of CXCR4 expression. Mol Cells. 2017;40(7):476–484. doi:10.14348/molcells.2017.2309
122. Hopken UE, Foss HD, Meyer D, et al. Up-regulation of the chemokine receptor CCR7 in classical but not in lymphocyte-predominant hodgkin disease correlates with distinct dissemination of neoplastic cells in lymphoid organs. Blood. 2002;99(4):1109–1116. doi:10.1182/blood.V99.4.1109
123. Nabekura T, Hiroi T, Kawasaki T, Uwai Y. Effects of natural nuclear factor-kappa B inhibitors on anticancer drug efflux transporter human P-glycoprotein. Biomed Pharmacother. 2015;70:140–145. doi:10.1016/j.biopha.2015.01.007
124. Liu X, Zhao W, Wang W, Lin S, Yang L. Puerarin suppresses LPS-induced breast cancer cell migration, invasion and adhesion by blockage NF-kappaB and Erk pathway. Biomed Pharmacother. 2017;92:429–436. doi:10.1016/j.biopha.2017.05.102
125. Liu X, Li S, Li Y, Cheng B, Tan B, Wang G. Puerarin inhibits proliferation and induces apoptosis by upregulation of miR-16 in bladder cancer cell line T24. Oncol Res. 2018;26(8):1227–1234. doi:10.3727/096504018X15178736525106
126. Zhang H, Zhai Z, Zhou H, et al. Puerarin inhibits oxLDL-induced macrophage activation and foam cell formation in human THP1 macrophage. Biomed Res Int. 2015;2015:403616. doi:10.1155/2015/403616
127. Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med. 2019;18(3):121–126. doi:10.4103/aam.aam_56_18
128. Na YR, Je S, Seok SH. Metabolic features of macrophages in inflammatory diseases and cancer. Cancer Lett. 2018;413:46–58. doi:10.1016/j.canlet.2017.10.044
129. Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7(3):211–217. doi:10.1016/j.ccr.2005.02.013
130. Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6(5):447–458. doi:10.1016/j.ccr.2004.09.028
131. DiDonato JA, Mercurio F, Karin M. NF-kappaB and the link between inflammation and cancer. Immunol Rev. 2012;246(1):379–400. doi:10.1111/j.1600-065X.2012.01099.x
132. Qin J, Wang W, Zhang R. Novel natural product therapeutics targeting both inflammation and cancer. Chin J Nat Med. 2017;15(6):401–416. doi:10.1016/S1875-5364(17)30062-6
133. Liu L, Liao JZ, He XX, Li PY. The role of autophagy in hepatocellular carcinoma: friend or foe. Oncotarget. 2017;8(34):57707–57722. doi:10.18632/oncotarget.17202
134. Arakawa S, Honda S, Yamaguchi H, Shimizu S. Molecular mechanisms and physiological roles of Atg5/Atg7-independent alternative autophagy. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(6):378–385. doi:10.2183/pjab.93.023
135. Wang P, Zhu L, Sun D, et al. Natural products as modulator of autophagy with potential clinical prospects. Apoptosis. 2017;22(3):325–356. doi:10.1007/s10495-016-1335-1
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.