Back to Journals » Infection and Drug Resistance » Volume 13
Molecular Epidemiology and Mechanisms of High-Level Resistance to Meropenem and Imipenem in Pseudomonas aeruginosa
Authors Hassuna NA , Darwish MK , Sayed M, Ibrahem RA
Received 8 October 2019
Accepted for publication 16 January 2020
Published 30 January 2020 Volume 2020:13 Pages 285—293
DOI https://doi.org/10.2147/IDR.S233808
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Noha Anwar Hassuna, 1 Marwa K Darwish, 2 Mohamed Sayed, 1 Reham Ali Ibrahem 3
1Medical Microbiology and Immunology Department, Faculty of Medicine, Minia University, Minia, Egypt; 2Chemistry Department (Biochemistry Branch), Faculty of Science, Suez University, Suez, Egypt; 3Microbiology and Immunology Department, Faculty of Pharmacy, Minia University, Minia, Egypt
Correspondence: Noha Anwar Hassuna
Medical Microbiology and Immunology Department, Faculty of Medicine, Minia University, Minia, Egypt
Tel +20 862342813
Email [email protected]
Purpose: Pseudomonas aeruginosa possesses a large number of resistance mechanisms to different antimicrobials with carbapenems being the most powerful in treating resistant P. aeruginosa. Hence, it is imperative to explore different mechanisms of carbapenems-resistance in P. aeruginosa to achieve successful treatment through the design of new drugs acting on this interaction to combat against antimicrobial resistance.
Strains and Methods: A total of 634 P. aeruginosa clinical isolates were collected from various patient sources and their MIC levels were measured. Molecular evaluation of carbapenem resistance was assessed by investigating the presence of blaIMP1, blaIMP2, blaVIM1, blaVIM2, blaSPM and blaNDM genes and the gene expression of the following multi-drug efflux pump systems: MexAB-OprM, MexCD-OprJ, MexEF-OprN and MexXY-OprM and its correlation with MIC. Isolates were typed by Random Amplified Polymorphic DNA (RAPD)-typing.
Results: Carbapenem resistance was detected in 32 (5%) isolates, which were all imipenem resistant (of which 29 were meropenem resistant). High-level resistance (≥ 64mg/mL) to imipenem was found in 27 (84.3%) isolates, and to meropenem in 28 (96.5%) isolates. The carbapenemase blaVIM-1 was found in 31 isolates, while blaNDM was detected in 4 isolates. None of the isolates possessed either bla-VIM-2, blaIMP-1, blaIMP-2 or blaSPM. The majority of the isolates displayed over-expression of MexCD-OprJ (75%) followed by MexXY-OprM efflux pump (62%), while MexAB-OprM and MexEF-OprN efflux pumps were overexpressed in 21.8% and 18.7% of the isolates, respectively, with no down-regulation of oprD in any of the isolates. A strong correlation was found between CDJ efflux pump expression and meropenem, imipenem resistance (r=0.532, 0.654, p< 0.001, < 0.001) respectively. Four major clusters were detected by RAPD-typing: group 1(10 isolates), group 3 (9 isolates), group 2 (8 isolates) while the fourth group (4) included 4 isolates (12.5% polymorphism).
Conclusion: High-level carbapenem resistance reported in this study was allied to multiple mechanisms including carbapenemase production and efflux-pump over-expression. Threatening cross-infection is possible inside the hospital and stringent infection control measures are crucial.
Keywords: carbapenems, efflux pump, OprD and metallo-β-lactamases
Introduction
Pseudomonas aeruginosa is a gram-negative opportunistic organism that causes severe global healthcare-associated infections such as respiratory tract infections, sepsis, urinary tract infections and surgical site infections, especially in immunocompromised patients.1 The development of multidrug-resistant (MDR) strains that display resistance to nearly all antibiotics except for one or two classes is becoming an uppermost public health problem, increasing morbidity, mortality and length of hospital stay.2 Unfortunately, P.aeruginosa harbors a wide array of MDR mechanisms, including the existence of outer-membrane barriers (porin OprD), over-expression of multidrug efflux pumps and endogenous antimicrobial inactivation.3 Accordingly, the choice of an appropriate treatment is a formidable problem in hospitals in many regions worldwide.4
Imipenem, meropenem, and doripenem are members of the carbapenems (β lactam class) and are routinely used to manage P. aeruginosa infections.3 The mechanism of action of carbapenems is the inhibition of the peptidoglycan-assembling transpeptidases (penicillin-binding proteins [PBP]) placed on the outer part of the plasma membrane.5 Although this class of antibiotics has a great efficacy in treatment of MDR P. aeruginosa infections, an expansion of carbapenem-resistant strains has been detected in the recent years.4
Carbapenems resistance in P. aeruginosa could be attributed to a reduction in the outer membrane permeability, up-regulated expression of the efflux pumps genes and production of metallo-β-lactamases (MBL), which inactivate these drugs efficiently.6
The outer membrane protein OprD permits the transport of amino acids, peptides and carbapenems. Downregulation of oprD generally causes resistance to imipenem and meropenem.7 Another major mechanism of Carbapenem resistance in P. aeruginosa is decreasing the antibiotic concentration through efflux systems belonging to the resistance-nodulation- division (RND) family (MexAB-OprM, MexCD-OprJ, MexEF-OprN, MexXY-OprM, MexJK and MexVW).8 Meropenem is affected by high regulation of MexAB-OprM, MexCD-OprJ, and MexXY-OprM, whilst imipenem is not affected.9 MexAB-OprM and MexXY-OprM pumps are the principal means of natural resistance against antimicrobial and disinfectant compounds. The MexAB-OprM system confers resistance against a wide range of antibiotics including tetracycline, chloramphenicol quinolones, trimethoprim and most β lactams and MexXY-OprM contributes to the resistance against aminoglycosides, tetracycline, and erythromycin.10
Carbapenems withstand relatively well hydrolysis by most of the beta lactamases; however, metallo-b-lactamases (MBL) can inactivate them.11 This group of enzymes include imipenemase (IMP), Verona imipenemase (VIM), New Delhi MBL (NDM), Seoul imipenemase (SIM), Sao Paulo MBL (SPM), German imipenemase (GIM), Adelaide imipenemase (AIM), and Dutch imipenemase (DIM).12 The genes that encode the production of MBLs are typically part of class 1 integron structures and are transmitted by mobile genetic elements.13 Other non-metallo carbapenemases also exist, including Ambler class A β-lactamases: K. pneumonia carbapenemase (KPC) and Guiana Extended spectrum (GES) as well as class D β-lactamases: Oxa-48.14
The aims of this study were to evaluate the frequency of carbapenem resistance among P. aeruginosa isolated from different clinical specimens (urine, sputum, pus, etc.) in Minia governorate, Egypt and to investigate the correlation between the MICs obtained and overexpression of certain efflux pump genes, downregulation of outer membrane porin, OprD and the presence of metallo-b-lactamases (MBL) genes.
Materials and Methods
This study was conducted at the Minia University Hospital (the largest tertiary hospital in Minia-Upper Egypt, receiving referrals from 9 districts and serving a population of more than 5 million) in the period between December 2017 to November 2018.
Bacterial Isolates
A total of 634 P. aeruginosa isolates were collected from different clinical specimens (160 urine, 103 sputum, 227 wound, 116 tracheal aspirates, 88 bloodstream infections, and 56 bronco-alveolar lavage samples) as part of the routine hospital laboratory procedure. Isolates were identified using standard laboratory methods including bacteriological and biochemical tests such as Gram stain, oxidase test, growth on cetrimide agar medium, the ability to grow at 42°C, O/F (Oxidation-Fermentation) test and pigment production. The identified P. aeruginosa isolates were stored at −70°C in trypticase soy broth (Merck, Darmstadt, Germany) supplemented with 10% glycerol until further investigation. P. aeruginosa ATCC 27853 was used as the reference strain throughout the study. A set of 32 carbapenem-sensitive clinical isolates were used as negative controls.
Antimicrobial Susceptibility Testing
The minimum inhibitory concentrations (MICs) of meropenem and imipenem were measured on Muller Hinton Agar plates (Oxoid, Basingstoke, UK) by the agar dilution method and interpreted according to CLSI breakpoint.15 Meropenem and Imipenem were purchased from Himedia, Mumbai (India). Antimicrobial profile against different anti-pseudomonas drugs was done for the carbapenem-resistant isolates using disc-diffusion method according to CLSI.15
Detection of Carbapenemase Genes
DNA Extraction and PCR
Total DNA from P. aeruginosa isolates was extracted by boiling method according to Rostami et al.16 The tubes were stored at −20°C prior to being used in PCR amplification as a DNA template.10
All carbapenem-resistant P. aeruginosa isolates were screened by PCR for the blaIMP1, blaIMP2, blaVIM1, blaVIM2, blaSPM and blaNDM genes using specific primers and conditions listed in Table 1.
 |
Table 1 Primers Used in This Study |
Efflux Pump and Porin Expression
RNA Extraction and Reverse Transcription to cDNA
For total RNA extraction, P. aeruginosa isolates were cultured in 1.5 mL LB broth and placed in a shaking incubator (180 rpm) at 37°C for 18–24 hrs. Tubes were incubated overnight at 37°C and then were centrifuged (8000rpm) at 4°C for 7 mins; afterwards the pellet was processed according to the manufacturer’s instructions using the GeneJET RNA Purification Kit (Thermo-ScientificTM, USA). To prepare cDNA, 5 µL of extracted RNA was added to15 µL deionized water and converted to cDNA in a total volume of 20µL according to the manufacturer’s instructions using Revert Aid First Strand cDNA synthesis Kit (Thermo-ScientificTM, USA). The cDNAs were stored at −20°C and were used within 1 week.
Real-Time (RT)-PCR to Measure mexA, mexC, mexE, mexY and oprD Expression Levels
The expression of mexA, mexC, mexE, mexY and oprD in the P. aeruginosa isolates that showed resistance to carbapenems (imipenem and meropenem) was determined by real-time PCR (RT-PCR). Primers that were used for the amplification of mexA, mexC, mexE, mexY, oprD and rpsL are specified in Table 1 and the housekeeping gene rpsL was used as the normalizing gene. Expression levels of the above genes for clinical isolates of P. aeruginosa were compared to P. aeruginosa PAO1.
The quantification of transcripts was carried out by SYBRGreen PCR Master Mix (Thermo-ScientificTM, USA). Applied Biosystems 7500-Fast Real-Time PCR system (Thermo-ScientificTM-USA). The relative gene expression was calculated using the ΔΔCt method.26 Expression of the house-keeping gene rpsL was determined in parallel to normalize the transcriptional levels of target genes and they were furthermore standardized against target gene expression by the wild-type reference strain PAO1. Overexpression of the efflux systems MexAB-OprM, MexCD-OprJ, MexEF-OprN and MexXY-OprM was considered when mexA, mexC, mexE or mexY transcriptional levels were at least two-, two-, 10-and 4-fold higher than that of PAO1, respectively.27,28 All the experiments were repeated three times.
Random Amplified Polymorphic DNA (RAPD) PCR
The isolated P. aeruginosa strains were sent for RAPD-genotyping using primer 272-AGCGGGCCAA as previously designated by Mahenthiralingam and co-workers.29 The master mixture was prepared using 2.5 µL 10x PCR buffer; 2.5mM MgCl2; 300µM of dNTPs; 1.7U Taq DNA polymerase; and 3 µL genomic DNA (40 ng) in a final volume of 25 µL using the following thermocycler conditions: denaturation 5 mins at 95°C, annealing 5 mins at 36°C, and elongation 5 mins at 72°C, for 4 cycles and 31 cycles consisting of 94°C for 1 min, 45°C for 1min, and 72°C for 2 mins, followed by a final extension at 72°C for 10 mins. For reproducibility, each reaction was performed in triplicate. For analysis of RAPD products, PCR amplicons were separated on 1.% (w/v) agarose gels and the DNA band size was determined in comparison to 1Kb DNA ladders (250 bp–10,000 bp band sizes). Similarity between isolates was assessed according to Dice similarity coefficient and Unweighted Average Pair Group Method (UPGMA), using FreeTree and TreeView software. Only major reproducible bands of intensity were put into consideration for similarity matrix calculation. Cut-off values of ≥80% were used for determination of potential clonal relatedness.30
Statistical Analysis
Data were analyzed using SPSS statistical package version 20 (Chicago, USA). The following had been done accordingly: mean (X~) and standard deviation (SD) for quantitative data, Chi-squared test for categorical data and Bivariate Pearson correlation analysis for association analysis. For all tests, probability (p) was considered: non-significant if ≥ 0.05, significant if < 0.05, Highly significant if < 0.01 and very highly significant if <0.001. Grade of correlation or association were calculated as follows: 0.00 to 0.24: weak or no association, 0.25 to 0.49: fair association, 0.50 to 0.74: moderate association and 0.75+: strong association.
Results
Out of 634 P. aeruginosa isolates, 32 (5%) were imipenem resistant of which 29 were meropenem resistant. High-level resistance (≥64mg/mL) to imipenem was found in 27 isolates (84.3%) out of 32 isolates, while high-level resistance (≥64mg/mL) to meropenem was found in 28 (96.5%) out of the 29 meropenem-resistant isolates. The antimicrobial profile to different antimicrobials used for treating P. aeruginosa infection is illustrated in Table S1.
Carbapenemase genes were found as follows: 31 isolates were positive for blaVIM-1, while blaNDM was detected in 4 isolates, which co-harbored blaVIM-1 also. None of the isolates possessed either bla-VIM-2, blaIMP-1, blaIMP-2 or blaSPM.
Regarding gene expression, most of the isolates showed over-expression of CDJ (75%) followed by XY efflux pump (62%), while ABM and EFN efflux pumps were overexpressed in 21.8% and 18.7% of the isolates, respectively. None of the isolates showed low expression of oprD (Table 2). There was a significant difference between sensitive and resistant isolates regarding XY and CDJ efflux pump expression, which were overexpressed in 14.3, 22.9% vs 62.1, 79.3% among sensitive and resistant groups, respectively (p <0.001) (Table 3). Only three of the isolates showed over-expression of the four tested efflux pumps. Expression levels of different genes are demonstrated in Table S2.
 |
Table 2 MIC and Characterization of Efflux Pump Overexpression and Carbapenemase Genes in the Carbapenem-Resistant P.aeruginosa Isolates |
 |
Table 3 Correlation of Efflux Pump Gene Expression with Carbapenem-Resistant and Sensitive P.aeruginosa Isolates |
A highly significant fair association was found between XY efflux pump expression with meropenem, imipenem resistance (r=0.363, 0.327, p=0.003, 0.008) respectively. In addition, there was a very highly significant moderate correlation between CDJ efflux pump expression and meropenem, imipenem resistance (r=0.532, 0.654, p<0.001, <0.001) respectively (Table 4).
 |
Table 4 Bivariate Correlation Analysis Between Meropenem, Imipenem Resistance and Efflux Pump Over-Expression |
The carbapenem-resistant P. aeruginosa isolates were typed by RAPD-PCR analysis and Figure 1 illustrates their clustering. RAPD fingerprinting produced 6–10 fragments with band sizes between 250 bp and 3 kb. There were three major groups: group 1(10 isolates), group 3 (9 isolates), group 2 (8 isolates) while the fourth group (4) consisted of 4 isolates. Only one isolate (PA-15) was a unique isolate forming a separate cluster.
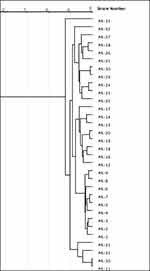 |
Figure 1 Dendrogram based on RAPD analysis of 32 P.aeruginosa isolates. The dendrogram was developed using gelJ software. Abbreviation: PA, Pseudomonas aeruginos. |
Discussion
In this study, carbapenem resistance was detected in 5% of P. aeruginosa isolates, with the majority showing high-level resistance to both imipenem and meropenem. Regarding the incidence of carbapenem resistance, our findings are lower than previously reported in the same region,31 probably due to an improvement in abiding to infection control measures. Nevertheless, this is the first report of high-level resistance to carbapenems in Egypt, which is disturbingly snowballing in clinical P.aeruginosa isolates world-wide.32 We further investigated the mechanisms of this high-level carbapenem resistance among the isolates and interestingly we found that 31 of the isolates (>96%) harbored at least one carbapenemase gene. This was in agreement with previous reports in Egypt,33,34 which are all in agreement with finding that MBLs can overtake efflux pump up-regulation or loss of oprD as principal elements leading to carbapenem resistance.35 However, our results show a dominance of blaVIM-1, which is not in agreement with earlier studies carried out in Egypt showing a dissemination of bla-VIM-2 instead or the universal bla-VIM genes.34,36 Interestingly, we report here for the first time in Upper Egypt the emergence of New Delhi metallo-beta-lactamase −1 gene (bla-NDM) harboring isolates, which possessed bla-VIM-1 as well and showed high-level resistance. There are no previous reports of bla-NDM in Egypt, except a case report of two isolates recovered from a tertiary hospital in 2012.37 The patients who harbored the bla-NDM isolates had no reported contact with a foreign population or a history of travelling to or from India or any foreign country. The presence of this gene could be explained as described earlier by Zafer and co-workers as a result of antimicrobial abuse or unreported contact and cross-contamination with a positive case37 (perhaps a contact with the previously described cases in Cairo could be the source, although not confirmed).
This occurrence of MBL-producing isolates can produce grave infections that are problematic to treat and their existence in different sources in Egypt is of great countrywide importance.
Different efflux pumps are described in P. aeruginosa, among which MexAB-OprM, MexCD-OprJ, MexEF-OprN and MexXY-OprM are the best-characterized systems that are correlated with antibiotic resistance in clinical P. aeruginosa isolates.38 In our study, the majority of the isolates demonstrated over-expression of MexCD-OprJ and MexXY-OprM efflux pumps. A strong correlation was found between MexCD-OprJ over-expression and both meropenem and imipenem resistance, concurring with Bubonja-Sonje and co-workers.39
Although the MexXY-OprM system is known as a substantial element in aminoglycoside resistance in only P. aeruginosa,40 it is also over-expressed in multi-drug resistant P. aeruginosa41 and similar reports to our study have shown its over-expression in carbapenem-resistant P. aeruginosa strains.7,42,43
MexAB-OprM over-expression was observed in 21.8% of the isolates, concurring with Xavier and co-workers.44 In contrast, Dantas et al7 and Chalhoub et al32 reported a much higher frequency of MexAB-OprM over-expression in P. aeruginosa isolates. Surprisingly, no down-regulation of oprD was detected in this study, which could be claimed to the presence of mutations eg, in loops L2 and L3 as described by Muderris et al.45 However, Chalhoub et al found no role for oprD mutation in high-level resistance compared to efflux pump overexpression.32
Finding bacterial genetic similarity and relatedness is crucial for cross-infection assessment, for which the value of various genotyping techniques has been recognized.46,47 Of those techniques, RAPD-PCR is advantageous in being fast, simple, and reproducible in addition to having a high discriminatory power.47
In the present study, we detected 4 distinct RAPD clusters among 32 isolates (12.5% of polymorphisms), which unfortunately suggests possible hospital cross-contamination.
Conclusion
Our results demonstrate that high-level carbapenems resistance reported in this region is attributable to carbapenemase production as well as to concomitant efflux-pump over-production. A high probability of cross-acquisition is concluded from phylogenetic results suggesting an infection control problem, which necessitates more strict procedures.
Limitations: Studying the mutation of oprD was not done in this study due to lack of funding. There was no positive control for the over-producers.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Micek ST, Wunderink RG, Kollef MH, et al. An international multicenter retrospective study of Pseudomonas aeruginosa nosocomial pneumonia: impact of multidrug resistance. Crit Care. 2015;19:219. doi:10.1186/s13054-015-0926-5
2. Castanheira M, Deshpande LM, Costello A, Davies TA, Jones RN. Epidemiology and carbapenem resistance mechanisms of carbapenem-non-susceptible Pseudomonas aeruginosa collected during 2009-11 in 14 European and Mediterranean countries. J Antimicrob Chemother. 2014;69(7):1804–1814. doi:10.1093/jac/dku048
3. Morita Y, Tomida J, Kawamura Y. Responses of Pseudomonas aeruginosa to antimicrobials. Front Microbiol. 2014;4:422. doi:10.3389/fmicb.2013.00422
4. Lee JY, Ko KS. OprD mutations and inactivation, expression of efflux pumps and AmpC, and metallo-beta-lactamases in carbapenem-resistant Pseudomonas aeruginosa isolates from South Korea. Int J Antimicrob Agents. 2012;40(2):168–172. doi:10.1016/j.ijantimicag.2012.04.004
5. Ocampo-Sosa AA, Cabot G, Rodriguez C, et al. Alterations of OprD in carbapenem-intermediate and -susceptible strains of Pseudomonas aeruginosa isolated from patients with bacteremia in a Spanish multicenter study. Antimicrob Agents Chemother. 2012;56(4):1703–1713. doi:10.1128/AAC.05451-11
6. Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev. 2009;22(4):582–610. doi:10.1128/CMR.00040-09
7. Dantas RCC, Silva RTE, Ferreira ML, et al. Molecular epidemiological survey of bacteremia by multidrug resistant Pseudomonas aeruginosa: the relevance of intrinsic resistance mechanisms. PLoS One. 2017;12(5):e0176774. doi:10.1371/journal.pone.0176774
8. Li XZ, Barre N, Poole K. Influence of the MexA-MexB-oprM multidrug efflux system on expression of the MexC-MexD-oprJ and MexE-MexF-oprN multidrug efflux systems in Pseudomonas aeruginosa. J Antimicrob Chemother. 2000;46(6):885–893. doi:10.1093/jac/46.6.885
9. Riera E, Cabot G, Mulet X, et al. Pseudomonas aeruginosa carbapenem resistance mechanisms in Spain: impact on the activity of imipenem, meropenem and doripenem. J Antimicrob Chemother. 2011;66(9):2022–2027. doi:10.1093/jac/dkr232
10. Masuda N, Sakagawa E, Ohya S, et al. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-oprM efflux pumps in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2000;44(12):3322–3327. doi:10.1128/AAC.44.12.3322-3327.2000
11. Khosravi AD, Mihani F. Detection of metallo-beta-lactamase-producing Pseudomonas aeruginosa strains isolated from burn patients in Ahwaz, Iran. Diagn Microbiol Infect Dis. 2008;60(1):125–128. doi:10.1016/j.diagmicrobio.2007.08.003
12. Cornaglia G, Giamarellou H, Rossolini GM. Metallo-beta-lactamases: a last frontier for beta-lactams? Lancet Infect Dis. 2011;11(5):381–393. doi:10.1016/S1473-3099(11)70056-1
13. Palzkill T. Metallo-beta-lactamase structure and function. Ann N Y Acad Sci. 2013;1277:91–104. doi:10.1111/j.1749-6632.2012.06796.x
14. Poirel L, Walsh TR, Cuvillier V, Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Inf Dis. 2011;70(1):119–123. doi:10.1016/j.diagmicrobio.2010.12.002
15. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. PA: Clinical and Laboratory Standards Institute. 27th M100: 2018.
16. Rostami S, Farajzadeh Sheikh A, Shoja S, et al. Investigating of four main carbapenem-resistance mechanisms in high-level carbapenem resistant Pseudomonas aeruginosa isolated from burn patients. J Chin Med Assoc. 2018;81(2):127–132. doi:10.1016/j.jcma.2017.08.016
17. Senda K, Arakawa Y, Ichiyama S, et al. PCR detection of metallo-beta-lactamase gene (blaIMP) in gram-negative rods resistant to broad-spectrum beta-lactams. J Clin Microbiol. 1996;34(12):2909–2913. doi:10.1128/JCM.34.12.2909-2913.1996
18. Shibata N, Doi Y, Yamane K, et al. PCR typing of genetic determinants for metallo-beta-lactamases and integrases carried by gram-negative bacteria isolated in Japan, with focus on the class 3 integron. J Clin Microbiol. 2003;41(12):5407–5413. doi:10.1128/JCM.41.12.5407-5413.2003
19. Tsakris A, Pournaras S, Woodford N, et al. Outbreak of infections caused by Pseudomonas aeruginosa producing VIM-1 carbapenemase in Greece. J Clin Microbiol. 2000;38(3):1290–1292. doi:10.1128/JCM.38.3.1290-1292.2000
20. Poirel L, Naas T, Nicolas D, et al. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-beta-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob Agents Chemother. 2000;44(4):891–897. doi:10.1128/AAC.44.4.891-897.2000
21. Nordmann P, Poirel L, Carrer A, Toleman MA, Walsh TR. How to detect NDM-1 producers. J Clin Microbiol. 2011;49(2):718–721. doi:10.1128/JCM.01773-10
22. Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev. 2007;20(3):440–458. doi:10.1128/CMR.00001-07
23. Quale J, Bratu S, Gupta J, Landman D. Interplay of efflux system, ampC, and oprD expression in carbapenem resistance of Pseudomonas aeruginosa clinical isolates. Antimicrob Agents Chemother. 2006;50(5):1633–1641. doi:10.1128/AAC.50.5.1633-1641.2006
24. Yoneda K, Chikumi H, Murata T, et al. Measurement of Pseudomonas aeruginosa multidrug efflux pumps by quantitative real-time polymerase chain reaction. FEMS Microbiol Lett. 2005;243(1):125–131. doi:10.1016/j.femsle.2004.11.048
25. Shigemura K, Osawa K, Kato A, et al. Association of overexpression of efflux pump genes with antibiotic resistance in Pseudomonas aeruginosa strains clinically isolated from urinary tract infection patients. J Antibiot (Tokyo). 2015;68(9):568–572. doi:10.1038/ja.2015.34
26. Cabot G, Ocampo-Sosa AA, Tubau F, et al. Overexpression of AmpC and efflux pumps in Pseudomonas aeruginosa isolates from bloodstream infections: prevalence and impact on resistance in a Spanish multicenter study. Antimicrob Agents Chemother. 2011;55(5):1906–1911. doi:10.1128/AAC.01645-10
27. Rodriguez-Martinez JM, Poirel L, Nordmann P. Molecular epidemiology and mechanisms of carbapenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2009;53(11):4783–4788. doi:10.1128/AAC.00574-09
28. Takata I, Yamagishi Y, Mikamo H. Association of the exoU genotype with a multidrug non-susceptible phenotype and mRNA expressions of resistance genes in Pseudomonas aeruginosa.. J Infect Chemother. 2018;24(1):45–52. doi:10.1016/j.jiac.2017.08.018
29. Mahenthiralingam E, Campbell ME, Foster J, Lam JS, Speert DP. Random amplified polymorphic DNA typing of Pseudomonas aeruginosa isolates recovered from patients with cystic fibrosis. J Clin Microbiol. 1996;34(5):1129–1135. doi:10.1128/JCM.34.5.1129-1135.1996
30. Vaez H, Moghim S, Nasr Esfahani B, Ghasemian Safaei H. Clonal relatedness among imipenem-resistant Pseudomonas aeruginosa isolated from ICU-hospitalized patients. Crit Care Res Pract. 2015;2015:5.
31. Marian RR, Mohamed S, Hazim AR, Noha Anwar H. High incidence of MBL-mediated imipenem resistance among Pseudomonas aeruginosa from surgical site infections in Egypt. J Infect Developing Country. 2018;12:07.
32. Chalhoub H, Saenz Y, Rodriguez-Villalobos H, et al. High-level resistance to meropenem in clinical isolates of Pseudomonas aeruginosa in the absence of carbapenemases: role of active efflux and porin alterations. Int J Antimicrob Agents. 2016;48(6):740–743. doi:10.1016/j.ijantimicag.2016.09.012
33. Fam N, Gamal D, El Said M, et al. Occurrence of VIM-2 Metallo-ß-Lactamases in imipenem resistant and susceptible Pseudomonas aeruginosa clinical isolates from Egypt. Afr J Microbiol Res. 2013;7:4465–4472.
34. Zafer MM, Al-Agamy MH, El-Mahallawy HA, Amin MA, El Din Ashour S, El Din Ashour S. Dissemination of VIM-2 producing Pseudomonas aeruginosa ST233 at tertiary care hospitals in Egypt. BMC Infect Dis. 2015;15(1):122. doi:10.1186/s12879-015-0861-8
35. Tsakris A, Poulou A, Kristo I, et al. Large dissemination of VIM-2-metallo-[4]-lactamase-producing Pseudomonas aeruginosa strains causing health care-associated community-onset infections. J Clin Microbiol. 2009;47(11):3524–3529. doi:10.1128/JCM.01099-09
36. Gaballah A, Elbaradei A, Elsheredy A, Kader O. Emergence of blaVEB and blaGES among VIM-producing Pseudomonas aeruginosa clinical isolates in Alexandria, Egypt. Acta Microbiolog Immunolog Hungar. 2019;66(1):131–142. doi:10.1556/030.65.2018.044
37. Zafer M, Amin M, El-Mahallawy H, Ashour S, Al-agamy M. First report of NDM-1-producing Pseudomonas aeruginosa in Egypt. Int J Infect Dis. 2014;29C:80–81. doi:10.1016/j.ijid.2014.07.008
38. Nikaido H, Pages JM. Broad-specificity efflux pumps and their role in multidrug resistance of gram-negative bacteria. FEMS Microbiol Rev. 2012;36(2):340–363. doi:10.1111/j.1574-6976.2011.00290.x
39. Bubonja-Sonje M, Matovina M, Skrobonja I, Bedenic B, Abram M. Mechanisms of carbapenem resistance in multidrug-resistant clinical isolates of Pseudomonas aeruginosa from a Croatian hospital. Microb Drug Resist. 2015;21(3):261–269. doi:10.1089/mdr.2014.0172
40. Poole K. Efflux-Mediated Antimicrobial Resistance. In: Dougherty TJ, Pucci MJ, editors. Antibiotic Discovery and Development. Boston, MA: Springer US; 2012:349–395.
41. Morita Y, Tomida J, Kawamura Y. MexXY-OprM multidrug efflux system of Pseudomonas aeruginosa. Front Microbiol. 2012;3:408. doi:10.3389/fmicb.2012.00408
42. Rostami S, Farajzadeh Sheikh A, Shoja S, et al. Investigating of four main carbapenem-resistance mechanisms in high-level carbapenem resistant Pseudomonas aeruginosa isolated from burn patients. J Chin Med Ass. 2018;81(2):127–132. doi:10.1016/j.jcma.2017.08.016
43. Tuba M, Rıza D, Birsen O, et al. Role of efflux pump and OprD porin expression in carbapenem resistance of Pseudomonas aeruginosa clinical isolates. J Inf Develop Country. 2018;12:01. doi:10.3855/jidc.9486
44. Xavier DE, Picão RC, Girardello R, Fehlberg LC, Gales AC. Efflux pumps expression and its association with porin down-regulation and β-lactamase production among Pseudomonas aeruginosa causing bloodstream infections in Brazil. BMC Microbiol. 2010;10(1):217. doi:10.1186/1471-2180-10-217
45. Muderris T, Durmaz R, Ozdem B. et al. Role of efflux pump and OprD porin expression in carbapenem resistance of Pseudomonas aeruginosa clinical isolates. J Infect Dev Ctries. 2018;12(1):1–8. doi:10.3855/jidc.9486
46. Onteniente L, Brisse S, Tassios PT, Vergnaud G. Evaluation of the polymorphisms associated with tandem repeats for Pseudomonas aeruginosa strain typing. J Clin Microbiol. 2003;41(11):4991–4997. doi:10.1128/JCM.41.11.4991-4997.2003
47. Dawson SL, Fry JC, Dancer BN. A comparative evaluation of five typing techniques for determining the diversity of fluorescent pseudomonads. J Microbiol Methods. 2002;50(1):9–22. doi:10.1016/S0167-7012(02)00003-9
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
