Back to Journals » Cancer Management and Research » Volume 6
Molecular effects of bioactive fraction of Curcuma mangga (DLBS4847) as a downregulator of 5α-reductase activity pathways in prostatic epithelial cells
Authors Karsono AH , Tandrasasmita O, Tjandrawinata R
Received 21 January 2014
Accepted for publication 30 March 2014
Published 6 June 2014 Volume 2014:6 Pages 267—278
DOI https://doi.org/10.2147/CMAR.S61111
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Agung Heru Karsono, Olivia Mayasari Tandrasasmita, Raymond R Tjandrawinata
Section of Molecular Pharmacology, Research Innovation and Invention, Dexa Laboratories of Biomolecular Sciences, Dexa Medica, Cikarang, Indonesia
Abstract: DLBS4847 is a standardized bioactive fraction of Curcuma mangga. In this study, we used prostate cancer (PC)-3 as the cell line to study the effects of DLBS4847 on prostatic cell viability, as well as related molecular changes associated with the decreased cell number. The observation revealed that DLBS4847 inhibited the growth of PC3 cells through downregulation of the 5α-reductase (5AR) pathway. At the transcription level, 5AR1 and androgen-receptor gene expressions were downregulated in a dose-dependent manner. Furthermore, 5AR-1 and dihydrotestosterone expression were also downregulated at the protein level. A microarray study was also performed to see the effects of DLBS4847 on differential gene expressions in prostate cancer 3 cells. Among others, DLBS4847 downregulated genes related to prostate growth and hypertrophy. Our results suggested that DLBS4847 could potentially become an alternative treatment for prostate disorders, such as benign prostatic hyperplasia. In this regard, DLBS4847 exerts its growth inhibition partially through downregulation of the 5AR pathway.
Keywords: DLBS4847, Curcuma mangga, 5α-reductase inhibitor, benign prostatic hyperplasia (BPH), prostate cancer
Introduction
Benign prostatic hyperplasia (BPH) is one of the serious health problems affecting the prostate gland. It is an age- and male hormone-related condition that often appears in males over the age of 50 years. The prostate size increases due to hyperproliferation of stromal and epithelial cells of the prostate gland. The changes are caused by a complex cellular dysregulation, including changes in proliferation, differentiation, apoptosis, and senescence.1 The increased size of the prostate in BPH usually leads to urinary outflow resistance, which is associated with obstructive and irritative lower urinary tract symptoms. If BPH is not treated well, it may lead to acute urinary retention, renal insufficiency, urinary tract infection, gross hematuria, bladder stones, and renal failure.2,3
It is widely believed that the development of BPH occurs because of a combination of two factors: testicular androgens and aging. Within the prostate gland, testosterone is converted to a more potent androgen – dihydrotestosterone (DHT) – by 5α-reductase (5AR).4 The expression level of 5AR is elevated in BPH, resulting in the enhancement of DHT production.5
In addition to 5AR, BPH is also influenced by androgen receptor (AR) levels. AR plays a vital role in the development of prostate cancer, since it promotes growth and differentiation of the male urogenital structure.6 In the prostate, AR is mediated by androgenic signals that primarily arise from extracellular sources, namely testosterone and adrenal glands. Furthermore, testosterone passively diffuses across the prostate cell membranes, where it is converted into the more active metabolite, DHT, by the 5AR enzyme. DHT binds to the AR ligand–ligand binding domain with high affinity, inducing conformational changes that lead to the activation of prostate growth.7 The other activation of AR is through the phosphoinositide 3-kinase (PI3K) pathway. Usually, phosphatase and tensin homologue that has been deleted on chromosome 10 is mutated in prostate cancer.7,8 Moreover, it leads to the activation of Akt and AR.
In the prostate diseases, there is an imbalance between prostate cell growth and apoptosis. This imbalance is complex, and influenced by factors that stimulate proliferation and minimize cell apoptosis, such as growth factors, cytokines, and steroid hormones.9 BPH seems to be closely related with prostate cancer, because both diseases involve overgrowth of epithelial cells.
Our laboratory have been investigating a number of natural products that can be further developed into medical treatments.10–15 As BPH is a major problem for elderly males, investigations on potential natural products for this treatment have been carried out mostly on Serenoa repens, otherwise known as saw palmetto.16 DLBS4847 is a standardized bioactive fraction of Curcuma mangga, another potential natural product. Here, we report on the molecular effects of a bioactive fraction of DLBS4847 on gene and protein expression related to proliferation and apoptosis in vitro using the prostate cancer (PC)-3 epithelial cell line.
Materials and methods
DLBS4847 preparation and characterization
C. mangga was purchased from CV Pratama, Magelang, Central Java, Indonesia. This plant has been identified by the Herbarium Bogoriense, Research Center of Biology, Indonesian Institute of Sciences, with certificate 1261/IPH.1.02/If.8/XII/2009. The extraction process was carried out by maceration using ethanol 70% (% volume/volume) with ratio 1:8 at temperatures of 50°C for 2–3 hours. Miscella was collected and concentrated by evaporation at low pressure using a rotary evaporator at temperatures of 55°C–60°C to obtain concentrate. The concentrate was subsequently dried at 60°C–70°C to obtain dry extract. This dry extract was referred to as bioactive fraction DLBS4847 for further study.
Cell culture and cell treatment
The PC3 cell line was purchased from the American Type Culture Collection (Manassas, VA, USA). The cell was propagated into F-12 medium containing 10% fetal calf serum, and 1% penicillin/streptomycin (Thermo Fisher Scientific, Waltham, MA, USA). For the in vitro cell-viability assay, 2×105 cells/mL were plated on 96-well plates (Iwaki, Tokyo, Japan), while for ribonucleic acid (RNA) isolation and fluorescence-activated cell-sorting (FACS) analysis, 2×105 cells/mL were plated until confluent. All cells were grown in F-12 medium contain 1% penicillin/streptomycin (Thermo Fisher Scientific) without fetal calf serum. Cells were incubated at 37°C, 5% CO2, 24 hours prior to treatment.
In vitro cell viability
PC3 cells were treated with various concentrations of DLBS4847. After 24 hours of treatment, cell viability was observed using CellTiter 96® Aqueous nonradioactive cell-proliferation assay reagents (Promega, Fitchburg, WI, USA) following the manufacturer’s protocol.
FACS analysis
After 24 hours of treatment, the PC3 cells were harvested. Then, the cell pellets were washed with phosphate-buffered saline. After being washed, cells were fixed with 300 μL phosphate-buffered saline and 700 μL ethanol. The sample was incubated at 4°C for at least 24 hours. After incubation, the fixed cells were harvested to obtain pellets. Propidium iodide (1 mL) was added to the pellets, and these were incubated again for 30 minutes in dark conditions. Subsequently, the sample was run in a FACS machine (Falcon™; BD Biosciences, San Jose, CA, USA) and the result analyzed with the CellQuest™ program (BD Biosciences).
RNA analysis
Total RNA was extracted using TRIzol® reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol. The RNA concentration was quantified using NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific). The expressions of several target genes of this study (5AR1, AR, and PI3) were determined using a quantitative real-time polymerase chain-reaction (PCR) assay in 25 μL reaction consisting of 12.5 μl iQ™ SYBR® Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA), 0.8 μM of each targeted primer, and 0.1 μM of each internal control primer (β-actin). The quantitative real-time PCR was performed using a Mini Opticon MJ Mini™ (Bio-Rad) in optimum conditions for each primer. The primers used in this study were (F, forward; R, reverse): 5AR-1 F, TGCTGATGACTGGGTAACAG; 5AR-1 R, GTTGGCTGCAGTTACGTATTC; AR F, CCTGGCTTCCGC-AACTTACAC; AR R, GGACTTGTGCATGCGGTACTC; PI3 F, CCC-CTCCATCAACTTCTTCA; PI3 R, CGGTTGCCTACTGGTTCAAT; β-actin F, AGA-GGGAAATCGTGCGTGAC, and β-actin R, CAATAGTGATGACCTGGCCGT.
Western blotting of DHT and 5AR-1
Secreted DHT and 5AR-1 in the medium were analyzed by Western blot (Bio-Rad). Protein was isolated using either lysis buffer (150 mM NaCl, 20 mM Tris, 10% glycerol, 0.1% sodium dodecyl sulfate, 1 mM ethylenediaminetetraacetic acid, supplemented with protease-inhibitor cocktail) or using TRIzol. Protein concentration was measured using the Bradford method. Further, the protein was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis using 10% acrylamide gels at 100 V for 150 minutes. The protein in the gel was then transferred onto polyvinylidene difluoride membranes using a blotting system at 500 mA for 75 minutes. Rabbit or goat polyclonal antibodies (1:500) against human DHT (Abcam, Cambridge, UK), 5AR-1 (Santa Cruz Biotechnology, Dallas, TX, USA) and AR (Santa Cruz Biotechnology) were applied to the membrane. As for the internal control, 1:500 antibodies against tubulin or actin were used. After overnight incubation at 4°C, the primary antibodies were removed and replaced with horseradish peroxidase-conjugated secondary antibodies (1:2,500). The DHT, 5AR-1, and AR proteins that specifically bound to the antibodies were detected using luminol chemiluminescence reagent and revealed by film (Fujifilm, Tokyo, Japan) in a dark room or using gel documentation (Bio-Rad). The primary and secondary antibodies, polyvinylidene difluoride membranes, and luminol reagent were purchased from Santa Cruz Biotechnology.
DHT assay
The effects of DLBS4847 on in vitro DHT measurement assay were performed using an enzyme-linked immunosorbent assay (ELISA) kit (Alpha Diagnostic International, San Antonio, TX, USA) according to the manufacturer’s protocol.
Microarray study
Total RNA was isolated from the cells using TRIzol reagent following the manufacturer’s protocol. The isolated RNA concentration was counted using spectrophotometer (BioSpec Mini; Shimadzu, Kyoto, Japan), while RNA quality was inspected using a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Isolated RNA was further processed according to the manufacturers’ protocols of Affymetrix Inc. (Santa Clara, CA, USA) and NuGen Technologies (San Carlos, CA, USA). RNA (100 ng) was reverse-transcribed to hybrid deoxyribonucleic acid (cDNA) and became a template to make double-stranded cDNA with a DNA–RNA heteroduplex in one of its upstream/downstream ends. Amplification was done using single-primer isothermal amplification (SPIA), which produced single-stranded antisense DNA. cDNA from SPIA was fragmented and labeled with biotin, and then it was hybridized using GeneChip® human gene 1.0 ST arrays (Affymetrix) for 18 hours at 45°C with 60 rpm rotation. Arrays were then washed and stained using the FS450_007 fluidics protocol and scanned using an Affymetrix 3000 76 scanner. Data analysis to observe the changes in gene expressions was done using Partek® Express™ (Partek, St Louis, MO, USA). Those changes were then sorted from highest to lowest. The highest and lowest 50 genes were sorted, selected, and analyzed to determine the relationship of genes and their biological pathways (done using the Reactome database).
Statistical analysis
The statistical differences between the test and control samples were determined by Student’s t-test using the StatView software package (Abacus Concepts, Piscataway, NJ, USA). Values are expressed as means ± standard deviation for at least two independent experiments.
Results
DLBS4847 reduces prostate cell viability, and its effect on phases of the cell cycle
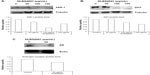
BPH involves hyperplasia (increase in cell numbers) of prostatic stromal and epithelial cells, resulting in the formation of large, fairly discrete nodules in the periurethral region of the prostate. In order to study the activity of DLBS4847 as a growth inhibitor, cell viability assay using a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium reagent assay was performed on PC3 cells. PC3 cells that had been grown on 96-well plates were treated in a serum-free condition, and then DLBS4847 with dosages of 50, 100, 150, and 200 μg/mL was added to the cells. A cell-viability assay showed a dose-dependent growth inhibition of PC3 cells (Figure 1). Significant cell-growth inhibition (P<0.05) started to be seen at the dose of 50 μg/mL. Cell numbers started to plateau at the dose of 200 μg/mL DLBS4847, at which dose cell numbers were still decreased 72% when compared to the control. These data suggested that DLBS4847 conferred a significant growth-inhibitory capacity in PC3 cells.
Following the investigation of cell viability, phases of the cell cycle for cells treated with DLBS4847 were also observed. PC3 cells were treated with various concentrations of DLBS4847, ranging from 50 to 200 μg/mL. Cell cycles were investigated using FACS apparatus after 24 hours’ treatment. Cell-cycle distribution analysis showed that the distribution of the G0 phase was increased by the administration of DLBS4847 in a dose-dependent manner (Figure 2). The complete cell-cycle phase was still seen with 50 μg/mL DLBS4847; however, accumulation of cells in the G0 phase started to appear during this condition. At higher doses of DLBS4847, the G1, S, and G2/M phases disappeared, while at 200 μg/mL only cells in the G0 phase were seen to accumulate. The fact that the G0 phase increased through dosages of 0–200 μg/mL of DLBS4847 indicated that the number of cells undergoing apoptosis had increased, and at the same time inhibition of PC3 cell growth also occurred.
DLBS4847 downregulates 5AR, AR, and PI3K genes in transcription levels
In order to investigate the 5AR downregulating activity pathways, the effects of DLBS4847 on 5AR1, AR, and PI3K gene expressions were studied. DLBS4847 was administered to PC3 cells at various concentrations: 0, 50, 100 and 150 μg/mL. After being treated for 24 hours, RNA was isolated and used for real-time PCR analysis. The result revealed that gene expression of 5AR1 decreased along with the increase in DLBS4847 concentrations (Figure 3A). Decreased expression of 0.44-fold occurred at the concentration of 50 μg/mL of DLBS4847, and continued to decrease to 0.19-fold at the concentration of 150 μg/mL of DLBS4847 compared to the control. In addition, AR gene expression was also observed, and it was found that this expression decreased in a dose-dependent manner (Figure 3B). AR gene expression at the concentration of 50 μg/mL of DLBS4847 decreased by 0.24-fold compared to control, and it was lost at the concentration of 150 μg/mL of DLBS4847. The decreasing pattern of 5AR1 and AR gene expressions indicated that DLBS4847 inhibited the development of prostate via the 5AR downregulating pathway by reducing the formation of DHT and DHT-AR.
Besides the testosterone pathway, observation was continued in normal cell-growth pathways that might be affected by DLBS4847. In order to study these possibilities, PI3K gene expression was observed, and it was found that gene expression was also decreased (Figure 3C). The observation was done using the same protocol of the 5AR1 and AR experiments. Decreased expression of 0.39-fold also occurred at the concentration 50 μg/mL DLBS4847, and decreased to 0.19-fold at the concentration of 150 μg/mL DLBS4847 compared to the control. In this study, the results indicated that DLBS4847 inhibits the development of the prostate through testosterone and normal cell-growth pathways.
DLBS4847 decreases DHT levels
To study the activity of DLBS4847 as a 5AR downregulating pathway in protein levels, qualitative and quantitative protein assays were studied. Qualitative studies were done using Western blot analysis. Three types of proteins were investigated: 5AR-1, DHT, and AR. PC3 cells were treated with various concentrations of DLBS4847 ranging from 0 to 150 μg/mL for 24 hours. The protein assay was conducted using Western blot analysis. Analysis revealed that the expression level of 5AR-1 was reduced. Expression levels were significantly reduced in the sample treated with 100 μg/mL of DLBS4847 (Figure 4A). Furthermore, 5AR-1 protein is an enzyme that converts testosterone to DHT. To confirm those reductions by 5AR-1 activity, DHT protein levels were also observed. We found that DHT protein was reduced as well (Figure 4B). Moreover, AR (DHT receptor) protein levels were also observed. Observations revealed that with 50 μg/mL of DLBS4847, AR levels were also reduced (Figure 4C). Furthermore, AR was not detected in the sample treated with 100 μg/mL of DLBS4847 (data not shown). These results showed that the DLBS4847 could reduce the production of DHT via downregulating the 5AR-1 protein and also its receptor (AR).
DLBS4847 was studied to look into inhibition of the production of DHT; DHT levels were observed quantitatively using an ELISA kit. PC3 cells were treated with 8.8 μg/mL testosterone as control, 8.8 μg/mL testosterone with 150 μg/mL DLBS4847 as the test substance, and 8.8 μg/mL of testosterone with 2 μg/mL finasteride as a positive control. Untreated PC3 was also used as a negative control. Treatment was carried out for 24 hours. Proteins were isolated from the PC3 cells and used as samples in the DHT ELISA assay. The assay was conducted to investigate DHT levels within cells after treatment with DLBS4847. PC3 cells that had been treated with testosterone had increased DHT levels: mean 995.63 pg/mL. PC3 cells that were treated with DLBS4847 had lower DHT levels – mean 46.84 pg/mL (Figure 5) – whereas the mean DHT level of PC3 cells that were treated with finasteride was 217.34 pg/mL. These observations indicated that downregulation of the 5AR pathway by DLBS4847 not only occurred in gene-expression levels, but also in protein levels.
Microarray
In order to confirm the activity of DLBS4847 as a 5AR downregulator, microarray analysis was conducted. The treated group was divided into two groups: DLBS4847-treated PC3 cells with doses of 100 μg/mL and 150 μg/mL. Cells were treated for 24 hours, and then the RNA was subsequently subjected to microarray analysis. Analysis revealed that the 5AR1 gene was not downregulated. However, the other 5AR gene, ie, 5AR2, and the AR gene were downregulated insignificantly: only −1.12 fold (P=0.05) and −1.03-fold (P=0.05), respectively. The other genes that were observed with microarray analysis were genes involved in MAPK pathways. Those genes were GF, GFR, PI3K, RAS, RAF, AKT, and MEK. Those genes were also downregulated, though not significantly (P=0.05) (Table 1). Therefore, from this microarray study, it can be concluded that the 5AR and MAPK pathway genes were not downregulated significantly. These results might be due to the number of samples. Samples were carried out in duplicate, and thus if the number of samples was increased, the result could be more representative.
  | Table 1 Fold change (control versus treated) of 5AR and MAPK gene pathways (cutoff value for 5% significance =0.00296) |
The microarray study revealed that there were other pathways that might be affected by DLBS4847. All genes were sorted by fold-change rank from highest to lowest. Furthermore, the 20 highest and lowest gene-expression levels were taken. Hereafter, the list was sorted to only several genes that contained certain pathways and then analyzed using Reactome (http://www.reactome.org) to find pathway relations. The sorted list revealed that there were several genes that up- and downregulated but have not been observed further. Upregulated genes were NPY1R, NET1, and F2RL1 (GPCR downstream pathway), MGAT5 (asparagine N-linked glycosylation), SLC14A1 (SLC-mediated membrane transport), SPARC (hemostasis), SKP2 (APC/C-mediated degradation of cell-cycle proteins), and MAT2A (biological oxidations). The downregulated genes were HMOX1 (iron uptake and transport), DDIT, IL8, and ATF3 (diabetes pathway), SC4MOL, HMGFCS1 (cholesterol biosynthesis), CDC6 (cell-cycle checkpoints), ZFP36 and HSPA1B (messenger RNA [mRNA] stability), PLIN2 (metabolism of lipids and lipoproteins), HGF (hemostasis), CXCL2 (GPCR downstream signaling), UGDH and GCLC (biological oxidations), IL6 (cytokine signaling in immune system), and UPP1 (metabolism of nucleotides). A list of most upregulated and downregulated genes can be seen in Tables 2 and 3, respectively. This interesting observation from the microarray study could lead to another mechanism that occurs during DLBS4847 treatment. These findings may even lead to the possibility of using DLBS4847 for other indications.
  | Table 3 Genes most downregulated by DLBS4847 |
Discussion
Nowadays, drugs from natural sources are being considered as an alternative approach for chemical drugs and medical therapies. Research and development of these naturally sourced drugs is getting more and more popular. Common medical treatments for BPH currently available are medical therapies or surgical action; therefore, we attempted to make an alternative drug to cure BPH using natural sources. Medical therapies are administered using α-blockers and 5AR inhibitors, while surgical action has likely become the last option, since most patients prefer to undergo medical therapies first.17 However, an alternative treatment for BPH using phytotherapy is getting more attention, due to the fact that it is nonhormonal, nonneuropharmacological, and nontoxic.18 Phytotherapeutic drugs including Pygeum africanum, Serenoa repens, β-sitosterol, and Cernilton are widely used to treat BPH. Therefore, this study was conducted to find a medical drug from a phytotherapeutic perspective as another alternative treatment for BPH, besides chemical drugs and medical therapies.
We demonstrated that DLBS4847 acts as a growth inhibitor of BPH. Growth-inhibiting activity was demonstrated with decreased numbers of PC3 cells after DLBS4847 administration (Figure 1). This activity was also shown in cell-cycle analysis, when the G0 phase increased in a dose-dependent manner (Figure 2). These two results suggest that DLBS4847 as a bioactive fraction could be used as BPH treatment via growth inhibition. In BPH, the prostate gland is swollen and interrupts the urinary system. DLBS4847 treatment was seen to reduce BPH symptoms and the size of the prostate, which in the end could improve patient quality of life. Moreover, the administration of DLBS4847 to PC3 cells indicated that DLBS4847 could also be a potential treatment to fight against prostate cancer through inhibition of cell growth. This potential activity could become an alternative for patients with prostate cancer instead of undergoing prostate gland castration. More studies are required to observe DLBS4847 for prostate cancer therapy.
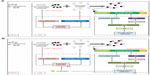
In this study, BPH treatment was our main focus; therefore, growth-inhibiting activity via 5AR was studied. The growth and maintenance of the prostate cell is regulated by the potent androgen 5α-DHT.19 DHT is the converted form of testosterone when it is taken up by the cell. The conversion is needed, because the testosterone in DHT form has a greater affinity for the AR.20 The conversion is done by 5AR isozymes.21 Our study revealed that the growth-inhibiting activity occurred via downregulation of 5AR enzymes. There are two types of 5AR enzymes; 5AR-1 and 5AR-2. These two isozymes have different optimum pH and gene regulators. Moreover, the genetic expressions of those two enzymes are also different. Their genes are expressed more in a BPH model compared to a normal model, but in prostate cancer only 5AR-1 is expressed more than 5AR-2.20,22,23 In this study, PC3 cells were used as a cell model; therefore, 5AR-1 expression was observed for mRNA analysis. Investigation of the expression of 5AR-1 revealed that expression was reduced by DLBS4847 at both the mRNA and protein levels (Figures 3A and 4A). DHT (product of 5AR enzymes) activity levels were reduced as well (Figure 5). This result suggests that DLBS4847 could be a substitute for 5AR-inhibiting drugs.
A recent study showed that 5AR inhibitors are one of the drugs that could reduce prostate-related disease risk. Within the prostate, testosterone was converted to the more potent androgen, DHT, by 5AR.4 Expression levels of 5AR are elevated in BPH and prostate cancer, resulting in the enhancement of DHT production and overexpression of AR,5 and thus the consequence is the prostate could overgrow. 5AR inhibitors could block DHT production, leading to reduced cell growth. Finasteride is an example of a 5AR-inhibitor drug.
In this study, we used finasteride, an approved medicine for BPH treatment, as a positive control. It was shown that DLBS4847 worked similarly to finasteride, and worked even better than finasteride (Figure 5). In the Prostate Cancer Prevention Trial, finasteride was observed to be effective in preventing prostate cancer. The study showed that finasteride reduced the risk of prostate cancer by 24.8%.24–26 Furthermore, since DLBS4847 has similar activity to finasteride, this study indicates that there is a possibility for DLBS4847 to prevent prostate cancer and be developed as a novel agent for such treatment.
Since the 5AR pathway ends in DHT binding to the AR to initiate prostate growth, we analyzed AR gene expression as well. AR also plays a vital role in the development of prostate cancer. It promotes the growth and differentiation of the male urogenital structure.6 DHT binds to the AR ligand–ligand binding domain with high affinity, inducing conformational changes that lead to activation.7 We showed that DLBS4847 downregulated AR gene and protein expression (Figures 3B and 4C). This observation also suggests that DLBS4847 could inhibit DHT production and reduce the opportunity for DHT to bind to the AR. Moreover, with less DHT–AR binding, the activation of cell growth would be reduced as well. This double mechanism of action of DLBS4847 in the 5AR pathway could be effective in inhibiting excessive prostate growth.
DLBS4847 was further studied to find other possible pathways that could downregulate AR. In order to observe other possibilities, cell growth via the PI3K pathway was investigated. In this initiation study, it was shown that DLBS4847 reduced PI3K gene expression (Figure 3C). A previous study revealed that AR gene expression was regulated by Akt,7 which is involved in such cellular processes as proliferation, and activated by PI3K. Unchecked PI3K could lead to continuous activation of Akt, prompting survival/apoptosis evasion, proliferation, and cell growth. PI3K activity was checked via the phosphatase and tensin homologue, which is most frequently mutated in human cancers.8 The result suggests that DLBS4847 could help to reduce PI3K activity, which could lead to downregulated AR gene expression. In addition, DLBS4847 inhibited cell growth via downregulation of 5AR that was linked with downregulation of the PI3K pathway.
We also conducted a microarray analysis to do further study on the mechanisms of action of DLBS4847 in PC3 cells. This analysis revealed that most genes that are involved in the 5AR and PI3K pathways were downregulated, but not significantly. This may have been due to the limited sample numbers used. Although most of the 5AR and PI3K genes were insignificantly downregulated, we found that there were certain other genes in other pathways that were up/downregulated by DLBS4847 (Tables 2 and 3). Those genes are related with other pathways, including hemostasis, diabetes, and the cell-signaling immune system. This result suggests that DLBS4847 might be used to treat other diseases.
However, we also found interesting gene expression that we were not aware of previously. Those genes are IL6 and IL8, and were downregulated by DLBS4847. IL-6 is a cytokine that is produced by prostate cells in vitro and in vivo.27 A recent study suggested that DLBS4847 may work specifically for the prostate gland. The study also revealed that AR was activated by IL-6.28 This observation suggested that downregulation of AR gene expressions may occur with the downregulation of IL-6 expression, affected by DLBS4847. Meanwhile, in another study, IL-6 may also have caused phosphorylation of STAT3, MAPK, and PI3K.29 This recent study may correlate with our results of PI3K genes being downregulated in real-time PCR analysis. Downregulation of those PI3K genes may happen because IL-6 was downregulated. This observation suggests that IL-6 was affected, so that PI3K genes were further affected, which led to downregulation of AR expression.
Another recent study also revealed that IL-8 promoted AR activation.29 Microarray analysis showed that IL8 gene expression was also downregulated significantly. This result indicates that the downregulation of AR genes may occur through IL-6 and IL-8 being affected by DLBS4847. Therefore, more studies about the interactions of IL-6, IL-8, and DLBS4847 are needed. The IL6 and IL8 genes were also correlated with prostate cancer: they were found elevated in sera from patients with prostate cancer.29 Therefore, DLBS4847 could be a good potential approach for prostate cancer treatment.
In conclusion, DLBS4847 has potential for BPH treatment. This study has demonstrated that DLBS4847 downregulates 5AR (Figure 6). DLBS4847 also reduced prostate proliferation by downregulating AR gene expression. Since DLBS4847 has two mechanisms of action, inhibition of excessive prostate growth could be more effective. In addition, DLBS4847 also shows that it is a promising candidate for prostate cancer and other hemostasis-, diabetes-, cell-signaling, and immune system-related diseases. However, further study is needed to ensure the safety of this substance before it can be applied for human use.
Acknowledgments
The authors would like to thank Dr James S Sinambela, Priska Hardadi, and Ujiatmi Dwi Marlupi for preparing DLBS4847, Florensia Nailufar for performing the animal study, and Audrey Clarissa and Destrina Grace Simanjuntak for their assistance in editing the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Penna B, Fibbi B, Amuchastegui S, et al. Human benign prostatic hyperplasia stromal cells as inducers and targets of chronic immuno-mediated inflammation. J Immunol. 2009;182(7):4056–4064. | |
Beckman TJ, Mynderse LA. Evaluation and medical management of benign prostatic hyperplasia. Mayo Clin Proc. 2005;80(10):1356–1362. | |
Fine AR, Ginsberg P. Alpha-adrenergic receptor antagonists in older patients with benign prostatic hyperplasia: issues and potential complications. J Am Osteopath Assoc. 2008;108(7):333–337. | |
Mostaghel EA, Geng L, Holcomb I, et al. Variability in the androgen response of prostate epithelium to 5α-reductase inhibition: Implications for prostate cancer chemoprevention. Cancer Res. 2010;70(4):1286–1295. | |
Das K, Lorena PD, Shen L, et al. Differential expression of steroid 5α-reductase isozymes and association with disease severity and angiogenic genes predict their biological role in prostate cancer. Endocr Relat Cancer. 2012;17(3):757–770. | |
Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst. 2001;93(22):1687–1697. | |
Lamont KR, Tindall DJ. Minireview: Alternative activation pathways for the androgen receptor in prostate cancer. Mol Endrocinol. 2011;25(6):897–907. | |
Ha S, Ruoff R, Kahoud N, Franke TF, Logan SK. Androgen receptor levels are upregulated by Akt in prostate cancer. Endocr Relat Cancer. 2011;18(2):245–255. | |
Sciarra A, Mariotti G, Salciccia S, et al. Prostate growth and inflammation. J Steroid Biochem Mol Biol. 2008;108(3–5):254–260. | |
Trisina J, Sunardi F, Suhartono MT, Tjandrawinata RR. DLBS1033, a protein extract from Lumbricus rubellus, possesses antithrombotic and thrombolytic activities. J Biomed Biotech. 2011;2011:519652. | |
Tjandrawinata RR, Nofiarny D, Sutanto L, Hendri P, Clarissa A. Symptomatic treatment of premenstrual syndrome and/or primary dysmenorrhea with DLBS1442, a bioactive extract of Phaleria macrocarpa. Int J Gen Med. 2011;4:465–476. | |
Tjandrawinata RR, Arifin PF, Tandrasasmita OM, Rahmi D, Aripin A. DLBS1425, a Phaleria macrocarpa (Scheff.) Boerl extract confers anti proliferative and proapoptosis effects via eicosanoid pathway. J Exp Ther Oncol. 2010;8(3):187–201. | |
Tandrasasmita OM, Lee JS, Baek SH, Tjandrawinata RR. Induction of cellular apoptosis in human breast cancer by DLBS1425, a Phaleria macrocarpa compound extract, via down-regulation of PI3-kinase/AKT pathway. Cancer Biol Ther. 2010;10(8):814–823. | |
Tjandrawinata RR, Nailufar F, Arifin PF. Hydrogen potassium adenosine triphosphatase activity inhibition and downregulation of its expression by bioactive fraction DLBS2411 from Cinnamomum burmanii in gastric parietal cells. Int J Gen Med. 2013;2013(6):807–815. | |
Tandrasasmita OM, Wulan DD, Nailufar F, Sinambela J, Tjandrawinata RR. Glucose-lowering effect of DLBS3233 is mediated through phosphorylation of tyrosine and upregulation of PPARγ and GLUT4 expression. Int J Gen Med. 2011;2011(4):345–357. | |
Fagelman E, Lowe FC. Saw palmetto berry as a treatment for BPH. Rev Urol. 2001;3(3):134–138. | |
Gjertson CJ, Walmsley K, Kaplan SA. Benign prostatic hyperplasia: now we can begin to tailor treatment. Cleve Clin J Med. 2004;71(11):857–880. | |
Yamanishi T, Yasuda K, Kamai T, et al. Single-blind, randomized controlled study of the clinical and urodynamic effects of an α-blocker (naftopidil) and phytotherapy (eviprostat) in the treatment of benign prostatic hyperplasia. Int J Urol. 2004;11(7):501–509. | |
Bauman DR, Steckelbroeck S, Peehl DM, Penning TM. Transcript profiling of the androgen signal in normal prostate, benign prostatic hyperplasia, and prostate cancer. Endrocinology. 2006;147(12):5806–5816. | |
Söderström TG, Bjelfman C, Brekkan E, et al. Messenger ribonucleic acid levels of steroid 5-alpha-reductase-2 in human prostate predict the enzyme activity. J Clin Endrocinol Metab. 2001;86(2):855–858. | |
Zhu YS, Cai LQ, You X, Cordero JJ, Huang Y, Imperato-Mcginley J. Androgen-induced prostate-specific antigen gene expression is mediated via dihydrotestosterone in LNCaP cells. J Androl. 2003;24(5):681–687. | |
Schmidt LJ, Murillo H, Tindall DT. 2004. Gene expression in prostate cancer cells treated with the dual 5 alpha-reductase inhibitor dutasteride. J Androl. 2004;25(6):944–953. | |
Smith CM, Ballard SA, Worman N, Buettner R, Masters JR. 5α-Reductase expression by prostate cancer cell lines and benign prostatic hyperplasia in vitro. J Clin Endocrinol Metab. 1996;81(4):1361–1366. | |
Hamilton RJ, Kahwati LC, Kinsinger LS. Knowledge and use of finasteride for the prevention of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2164–2171. | |
Akaza H, Kanetake H, Tsukamoto T, et al. Efficacy and safety of dutasteride on prostate cancer risk reduction in Asian men: the results from the REDUCE study. Jpn J Clin Oncol. 2011;41(3):417–423. | |
Svatek RS, Lotan Y. Cost utility of prostate cancer chemoprevention with dutasteride in men with an elevated prostate specific antigen. Cancer Prev Res (Phila). 2011;4(2):277–283. | |
Papatsoris AG, Karamouzis MV, Papavassilou AG. The power and promise of “rewiring” the mitogen-activated protein kinase network in prostate cancer therapeutics. Mol Cancer Ther. 2007;6(3):811–819. | |
Ghosh R, Graham H, Rivas P, et al. Phellodendron amurense bark extract prevents progression of prostate tumors in transgenic adenocarcinoma of mouse prostate: potential for prostate cancer management. Anticancer Res. 2010;30(3):857–865. | |
Deeb D, Jiang H, Gao X, et al. Curcumin sensitizes prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L by inhibiting nuclear factor-κB through suppression of IκBα phosphorylation. Mol Cancer Ther. 2004;3(7):803–812. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.