Back to Journals » Infection and Drug Resistance » Volume 16
Molecular and Clinical Characteristics of Carbapenem-Resistant Klebsiella pneumoniae Isolates at a Tertiary Hospital in Wuhan, China
Authors Hu F, Lin ML, Mou JL, Feng JH, Huang K, Lao YJ, Cheng J, Lin J
Received 17 November 2022
Accepted for publication 23 December 2022
Published 5 January 2023 Volume 2023:16 Pages 65—76
DOI https://doi.org/10.2147/IDR.S397975
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Fan Hu,1,2,* Meng-Lu Lin,1,2,* Juan-Li Mou,1,2,* Jia-Hui Feng,1,2 Kai Huang,1,2 Yao-Jia Lao,1,2 Jie Cheng,1,2 Jun Lin1,2
1Department of Gastroenterology/Hepatology, Zhongnan Hospital of Wuhan University, Wuhan, People’s Republic of China; 2The Hubei Clinical Center & Key Laboratory of Intestinal & Colorectal Diseases, Wuhan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jun Lin, Email [email protected]
Background: Carbapenem resistant Klebsiella pneumoniae (CRKP) is an independent risk factor for nosocomial infection which poses a serious threat to human health. How to prevent and suppress CRKP infection and explore its drug resistance mechanisms have become a huge challenge and possesses immediate significance.
Methods: A total of 45 CRKP strains isolated from hospitalized patients in Zhongnan Hospital of Wuhan University were collected from August 2018-December 2020. The strain’s identification and antimicrobial susceptibility tests were performed using the VITEK 2 automated identification instrument. Single molecule DNA sequencing of 45 CRKP isolates was performed by the third generation high-throughput sequencing technology.
Results: The results were analyzed by multi locus sequence typing (MLST) and phylogenetic analysis. Antimicrobial susceptibility showed that 45 CRKP isolates were multi-drug resistant strains, and the resistance rates to common antibiotics were as high as 68%. Whole genome sequencing results showed that the CRKP strains carried multiple drug resistance genes and virulence factors. MLST analysis found two different sequence types (ST), of which 44 were ST11 and 1 was ST1049.
Conclusion: Through whole genome sequencing (WGS), we found multiple drug-resistant genes and virulence factors, and there was obvious dominant microbiota. The source was mainly related to nosocomial infection. The ST11-KPC Klebsiella pneumoniae was the main type, which was consistent with the most common type in China. We identified several dominant microbiotas which may serve as a target in the clinical prevention and treatment of severe bacterial infections. Our finding may have a role for guiding clinical antibiotic choosing.
Keywords: Klebsiella pneumoniae, carbapenemases, whole-genome sequencing, resistance genes, virulence factor
Introduction
Klebsiella pneumoniae (KP) is one of the most common Gram-negative pathogens, which is a common source for community and hospital-acquired infections.1,2 There are three types of strains, including opportunistic strains, hypervirulent strains and multidrug-resistant (MDR) strains, which are often dominated by multi-drug resistant strains, especially carbapenem resistance,3 and its MDR characteristics are closely related to the plasmid-encoded resistance genes (ARG).4 Carbapenems and others β-lactam antibiotics are commonly used to treat infections caused by KP and are normally thought to be the last resort for treating multidrug resistant gram-negative pathogens.5,6 In case of long-term antibiotics application due to serious infection or severe basic diseases, ARG can be accumulated, resulting in the emergence of extremely resistant strains.7
Carbapenem resistant Klebsiella pneumoniae (CRKP) was first reported in the 1990s. Due to the expansion of antibiotic resistant phenotype and through mobile genetic elements such as plasmids and transposons, its mortality rates are significantly higher than that caused by infection with carbapenem-susceptible Klebsiella pneumoniae. This pathogen seriously threatens human health,8 and is an independent risk factor for nosocomial infection and death.9 The spread of MDR strains becomes an urgent problem to be solved, particularly for CRKP. How to prevent and suppress CRKP infection and explore its drug resistance mechanisms have become a current challenge and research hotspot. Whole genome sequencing (WGS) is widely used in the genetic evolution, ethnic migration and epidemic analysis of pathogenic bacteria and plays an increasingly important role in contagious disease prevention and control.10 Previous studies have used WGS to characterize CRPK isolates from local hospitals and their susceptibility to commonly used antibiotics are urgently needed for improved clinical guidance.11–13 However, the reports from Wuhan China are limited. Our study explored the antibiotic resistance genes, virulence factors and action mechanisms of CRKP through antimicrobial susceptibility tests and WGS technology, to screen common antibiotic resistance genes, so as to further guide the rational and standardized use of antibiotics in the clinic, which hopefully to effectively reduce and control CRKP occurrence.
Materials and Methods
Bacterial Strains
The 45 non-repeatability K. pneumoniae isolates were consecutively collected from various clinical specimens at the different departments in Zhongnan Hospital of Wuhan University from August 2018 to December 2020. The study sample was composed of 12 women and 33 men, with an age average of 69 years old. The strains from different specimens of the same patient or samples collected from the same patient at different times were all regarded as repetitive strains. Only the first strain was selected for follow-up study. All strains were identified by VITEK 2 automated identification instrument.
Antimicrobial Susceptibility Test
The strains were verified by mass spectrometer and the antimicrobial susceptibility tests were determined using the K-B diffusion method. Antimicrobials used for the tests include ampicillin (AMP), levofloxacin (LVX), ciprofloxacin (CIP), cefoxitin (FOX), ceftriaxone (CRO), aztreonam (ATM), amoxicillin–clavulanate (AMC), imipenem (IMP), piperacillin-tazobactam (TZP), cefepime (FEP), gentamicin (GEN), amikacin (AMK), tobramycin (TOB), sulfamethoxazole/trimethoprim (SXT), tigecycline (TGC), and polymyxin B (PB). The results of the antibiotic sensitivity test were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) and the susceptibility to tigecycline and polymyxin B was interpreted according to the 2018 European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints, with Escherichia coli ATCC 25922 used as a quality control strain.
Whole-Genome Sequencing
Single molecule DNA sequencing of 45 CRKP isolates was performed on the third generation high-throughput sequencing technology sequencer (Promethion, Oxford nanopore Technology). Genomic DNA was extracted and tested using QIAamp DNA Extraction kit (Qiagen, Valencia, CA, United States). The BluePippin automatic nucleic acid glue-cutting instrument was used to recover DNA of specific fragment size, and then the DNA after glue-cutting was repaired by damage and end repair. After magnetic bead purification, the NBD103 and NBD114 kits were used to connect the barcode tag at the DNA end. Real-time single-molecule sequencing was performed using PromethION to generate complete genome sequences.
Genome Assembly and Annotation
After quality control, the second-generation data and third-generation data were mixed and assembled with Unicycler V0.4.8, and corrected with Pilon V1.23 or NextPolish V1.4.13 combined with the second-generation sequencing data. The corrected genome uses its own script to detect whether the loop is formed. After removing the redundant part, circlator V1.5.1 is used to move the origin of the looped sequence to the replication starting point of the genome, so as to obtain the final genome sequence.
The coding genes were predicted by Prodial V2.6.3, and the complete CDS was retained; tRNA gene was predicted by TrnasCAN-SE V2.0; rRNA gene was predicted by RNAmmer V1.2; Other ncRNAs use Infernal V1.1.3 to search the Rfam database for prediction and keep results with a predicted length >80% of the sequence length in the database. CRISPR was predicted with MinCED V0.3.0, and gene island was predicted with Islander V1.2. The prediction results were integrated with our own scripts and assigned locus_tag numbers according to gene order for subsequent analysis.
After extracting genome-encoded proteins, Interproscan V5.25–64.0 was used for annotation, and the annotation information of TIGRFAMs Pfam and GO database was extracted. The encoded proteins were compared with BLAST + V3.10.0 + to KEGG, Refseq and COG databases, and the best results with coverage greater than 30% were retained as annotation results.
CARD and VFDB database annotation analysis using Abricate V1.0.1; Blast + V3.10.0 + was used to compare the encoded proteins to the PHI database, and the best results with coverage greater than 30% were retained as the PHI data annotation results.
Multilocus Sequence Typing (MLST) Analysis
Upload the generated sequence file to the Pasteur database (https://bigsdb.pasteur.fr/), and obtain the ST type of each strain. Upload the data to BacWGSTdb 2.0 database (http://bacdb.cn/BacWGSTdb/), using HS11286_CO4P003200_ST11 as a reference genome to obtain a phylogenetic tree and SNP count matrix heat map based on SNP analysis.
Statistical Analysis
All statistical analyses were performed using SPSS 25.0 software. We used the chi-square test or Fisher’s exact test for comparing categorical variables. The p-value <0.05 was considered statistically significant.
Results
Clinical Characteristics of 45 CRKP Isolates
The 45 inpatient samples were isolated from 10 different clinical departments. There were 33 males (73.33%) and 12 females (26.67%). Thirty-six strains (80.00%) came from sputum samples and 9 strains (20.00%) from lavage fluid samples. Among the 45 patients, 43 (95.56%) had been admitted to ICU during hospitalization, and most of them were complicated with basic diseases such as hypertension, diabetes, coronary heart disease, tumor, etc. Most of these patients were seriously ill and older, and some of them had underwent surgery or invasive ventilation, catheterization, deep vein catheterization, central vein catheterization and other invasive operations, with high risk of infection (Table 1).
 |
Table 1 Clinical Characteristics of CRKP Patients |
Multilocus Sequence Typing and Phylogenetic Analysis
Genomic information of 45 CRKP isolates was uploaded to Pasteur database to obtain MLST typing. Two different ST types were found, 44 strains were ST11 and 1 strain was ST1049. The phylogenetic tree and SNP count matrix heat map of CRKP were obtained by BacWGSTdb 2.0 database analysis (Figure 1).
Antimicrobial Susceptibility Testing
The 45 CRKP strains showed high resistance to most tested antibiotics, especially ampicillin, imipenem, levofloxacin, ciprofloxacin, cefoxitin, ceftriaxone, amoxicillin/clavulanic, piperacillin/tazobactam, aztreonam (100%). Meanwhile, these strains were highly resistant to cefepime (81.7%). The resistance rates to amikacin, gentamicin, tobramycin were 86.67%, and the resistance rates to sulfamethoxazole/trimethoprim was 68.89%. Antimicrobial susceptibility testing results showed that 45 CRKP isolates in this study were resistant to a variety of antimicrobial agents, showing extensive drug resistance and high resistance, namely MDR-KP (multidrug-resistant Klebsiella pneumoniae), which are described in Table 2.
 |
Table 2 Antimicrobial Susceptibility |
Resistance Gene, Virulence Gene Profiles
Resistance Gene
Forty-five CRKP isolates were detected by WGS, and a total of 29 resistant genes were found. The specific distributions are as follows:
- Among the 10 β-lactam resistance genes, 43 strains (95.56%) carried blaKPC-1, and many of them also carried extend-spectrum β-lactamases (ESBLs) resistance genes, mainly were blactx-m-65 and blaTEM-1, with 41 (91.11%) and 40 (88.89%) strains, respectively. In addition, 40 strains carried blaSHV-182 (88.89%), and 11 strains carried blaSHV-134 (24.44%). Only one strain carried blaCTX-M-15, blaCTX-M-55, blaCTX-M-3, bkaSHV-172 and TEM-141 (2.22%).
- Among the 7 aminoglycoside resistance genes, 39 strains of rmtB (86.67%), 36 strains of aadA2 (80.00%) and 19 strains of APH(3ʹ) -IA (42.22%) were the main ones.
- There were 2 kinds of sulfa resistance genes, 37 strains carried sul1 (82.22%) and 11 strains carried sul2 (24.44%).
- There were 5 quinolone resistance genes, among which 11 strains carried QnrS1 (24.44%), 7 strains carried both oqxA and oqxB (15.56%), and 1 strain each carried QnrB1 and QnrB2 (2.22%).
- All 45 strains carried fosfomycin resistance gene FosA6. Twenty strains carried streptomycin resistance gene mphA (44.47%). In addition, tetracycline resistance gene TET (A) was detected in 12 strains (26.67%), and chloramphenicol resistance gene floR was detected in 2 strains (4.44%) (Table 3).
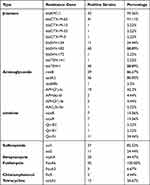 |
Table 3 Resistance Genes of 45 CRKP Isolates |
In order to explore the relationship between antibiotic resistance genes and clinical data such as age, length of hospital stay, basic diseases, invasive ventilation, antibiotic use and whether or not to undergo surgery. The clinical information of these 45 patients with CRKP was collected and grouped according to Table 1, the proportion of various antibiotic resistance genes was calculated, and the statistical difference of antibiotic resistance genes among the groups was compared (Supplementary Table 1).
According to the analysis of clinical data, the P values of β-lactam resistance gene blaTEM-1 in age group and hospital stay group were all <0.05, which was statistically significant. In addition, there were significant differences in β-lactam resistance gene blactX-M-65 in operation group, blaTEM-1 in length of hospital stay group, blaSHV-134 in type of antibiotic use group, and blaSHV-182 in underlying diseases group. The P value of aminoglycoside resistance gene APH(3ʹ) -IA in age group was also <0.05. It can be seen that age, length of hospital stay, comorbidities, types of antibiotics used, and whether or not surgery have certain effects on antibiotic resistance genes, and β-lactam resistance genes are significantly affected.
Virulence Gene
The genome sequencing results of 45 CRKP isolates were compared with VFDB. The results showed that 35 virulence genes in three categories were screened out, among which 9 were adhesive (25.71%), 25 were iron absorbent (71.43%) and 1 was invasive (2.86%).
The virulence gene annotation results of CRKP are shown as follows:
- Adhesive virulence gene: FimA, yagV/ecpE, yagW/ecpD, yagX/ecpC, yagY/ecpB, yagZ/ecpA, ykgK/ecpR genes were detected in 45 strains (100.00%), and fimE genes were detected in 17 strains (37.78%). FimB gene was detected in 1 strain (2.22%).
- Iron-absorption virulence gene: Genes of fepD, fepG, fepC, fepA, fepB, entA, entB, entE and entS were detected in 45 strains (100.00%). Genes of ybtE, ybtT, ybtU, ybtA, ybtP, ybtQ, ybtX and ybtS were detected in 44 strains (97.78%). IucD, iucC, iucB, iucA and iroN genes were detected in 10 strains (22.22%), and iroB, iroC and iroD genes were detected in 1 strain (2.22%).
- Invasive virulence gene: OmpA gene was detected in all 45 strains (100.00%) (Table 4).
 |
Table 4 Virulence Genes of 45 CRKP Isolates |
The results of this study showed that in the whole genome of clinical CRKP strains classified in our hospital, the virulence factors of adhesion virulence genes included Type I fimbriae (fimA/E/B), E. coli pilus ECP (yagV/ecpE, yagW/ecpD, yagX/ecpC, yagY/ecpB, yagZ/ecpA) ykgK/ecpR). Virulence factors expressed by iron-absorption virulence genes include enterobactin (fepA/B/C/D/G, entA/B/E/S), aerbacittin (iucA/B/C/D), salmonella (iroB/C/D/N), yersiniabactin (ybtA/E/P/Q/S/T/U/X). The virulence factor of invasive virulence gene was ompA.
According to the collected clinical data, the correlation between virulence factors and clinical information was analyzed (Supplementary Table 2). There was no significant correlation between various virulence factors and clinical information, such as age, length of hospital stay, underlying diseases, invasive ventilation, antibiotic use and surgery.
Discussion
KP is one of the most common opportunistic pathogens of nosocomial infection, which can cause lung infection, urinary tract infection, digestive tract infection, blood system infection and other diseases. Among them, one kind of KP which produces carbapenemase, is the most common carbapenem-resistant Enterobacteriaceae (CRE). It is the main cause of high mortality of nosocomial infection.14 According to China’s 2020 CHINET test report, through continuous bacterial resistance monitoring and hospital infection prevention measures, although the antibiotic resistance rate of KP slightly decreased in 2019 and 2020, the resistance rate of KP to imipenem has rapidly increased from 16.1% in 2016 to 23.3% in 2020,15 and it is still not optimistic. At present, the number of antibiotic that can be used to treat CRKP is limited, because the etiology of CRKP infection is caused by multiple factors, including lack of effective antibacterial agents, delay in starting effective treatment measures, and serious underlying and/or concomitant and concurrent diseases. Although high-dose meropenem, colistin, fosfomycin, tigecycline and aminoglycosides are applied in the clinic, the optimal treatment scheme for CRKP infection has not been determined.16
The 45 strains of CRKP collected in this study were mainly from neurosurgical ICU and respiratory department. Patients in these departments were old and had many underlying diseases and severe conditions, and had multiple risk factors for CRKP infection, including the use of central venous catheter, mechanical ventilation, tracheotomy, carbapenems and aminoglycosides antibiotics.6
The 45 CRKP strains in this study showed multidrug resistance phenotype. They were not sensitive to carbapenems, second-generation cephalosporins, third-generation cephalosporins, quinolones and β-lactam/β-lactamase inhibitor combination, and showed high resistance rate to aminoglycosides (86.67%) and to sulfonamides (68.89%).
CRKP has two main resistance mechanisms: (1) producing carbapenemases that hydrolyze carbapenems; (2) extended-spectrum β-lactamases (ESBLs) or AmpC enzymes combined with structural mutations, such as the lack of outer membrane proteins and high expression of effluence pump genes or changes in penicillin-binding proteins.17 At present, carbapenemase production is the main mechanism of CRKP resistance to carbapenems.2,18 KPC resistance gene is the most common cause of carbapenem resistance in K. pneumoniae in China.19,20
In this study, antibiotic resistance gene detection by WGS showed that 45 strains of MDR-KP in our hospital carried multiple antibiotic resistance genes, and 43 strains (95.56%) detected KPC-1, the most common carbapenem gene in China,21 which may be the main mechanism leading to carbapenem antibiotic resistance of this strain in our hospital. However, other major carbapenem resistance genes in Enterobacteria, such as NDM,22 IMP,23 VIM,24 OXA-48,25 were not detected, suggesting that these resistance genes were not widely spread in the strains of our hospital. In addition, many other antibiotic resistance genes were detected, such as ESBLs resistance genes TEM-1 and CTX-M-65; encoding aminoglycoside resistant rmtB, aadA2 and APH (3ʹ)-Ia; encoding fluoroquinolone resistant oqxA and oqxB; encoding sulfonamides resistant sul1 and sul2; encoding macrolide resistant mphA; encoding fosfomycin resistant fosA; and tetA, which encodes tetracycline resistance. These resistance genes may be the main reasons for the multi-drug resistance phenotype of KP. Most strains carry a variety of ESBLs resistance genes, some carry aminoglycosides and quinolones resistance genes, so cephalosporins, aminoglycosides and quinolones may fail in clinical treatment of CRKP. In addition, although the antimicrobial susceptibility test did not detect fosfomycin resistance, WGS detection showed that all strains carried fosfomycin resistance gene fosA, so fosfomycin may not be effective in the treatment of clinical CRKP in our hospital too. For this multi-drug-resistant KP, tigecycline, polymyxin and ceftazidime-avertam are recognized antimicrobials with relatively good efficacy,26 but the antibiotic sensitivity test of the above three drugs requires higher requirements, so it is necessary to establish more unified and perfect detection standards.
In conclusion, the antibiotic resistance spectrum of MDR-KP is broad, the resistance gene is complex and the resistance mechanism needs to be further studied. Clinical laboratories should jointly carry out antibiotic sensitivity tests of a variety of antimicrobials, including fosfomycin, chloramphenicol and sulbactam, as far as possible, in order to find possible effective anti-infective treatments, which are helpful to the accurate clinical use of antibiotic and the prevention and control of bacterial infection in hospitals. According to the clinical data and antibiotic resistance gene analysis, age, length of hospital stay, underlying diseases, types of antibiotics and operation had certain effects on the phenotype of antibiotic resistance genes, especially the difference of β-lactam resistance genes. However, the number of cases included in this study is limited, and it is a single-center study, so further large-sample multicenter studies are needed to explore the correlation between clinical data and antibiotic resistance gene phenotype.
In this study, the results of 45 CRKP MLST showed that 44 ST11 strains were the most dominant ST (97.78%), which was consistent with the domestic research results,27,28 indicating that ST11 is the most common KP MLST typing in China.29 There is only one site difference between ST11 and global epidemic ST258, which belongs to the same clone group.30 It has been reported that ST11 strain has a binding original (conjugative elements, ICEs) ICEKp258.1, which carries a type IV secretory system that may contribute to the transmission of mobile genetic elements, such as plasmids, making ST258 and ST11 become epidemic strains.29 Among the SNP analysis results of the 44 ST11 CRKP strains, some strains had a small number of SNPs, suggesting a similar evolutionary relationship and direct transmission relationship. The phylogenetic tree was constructed by SNPs, and it was found that there were 4 clonal groups in CRKP, one of which was a clonal group. It is the dominant clone group, suggesting that the hospital infection prevention and control department should focus on the strains of this clone group. In the future surveillance work, in addition to the clinical routinely isolated strains, it is also necessary to screen the circulating cloned strains for possible storage environments, find and eliminate the source of infection, and cut off the transmission route, thereby reducing the risk of nosocomial transmission and infection of CRKP.31
CRKP usually carries a variety of virulence factors and can encode some proteins involved in KP adhesion, invasion and anti-phagocytosis.32 By analyzing virulence genes, we found that all strains carried the fimA gene encoding type 1 fimbriae, the genes encoding enterobactin (fepA/B/C/D/G, entA/B/E/S), and the gene encoding yersiniabactin (ybtA/E/P/Q/S/T/U/X). However, none of the strains carried rmpA or rmpA2 genes (Table 3), which encode a high mucinous phenotype and have a high mortality rate,33 indicating that CRKP strains in our hospital are not yet highly lethal and virulent. The fimA gene mainly encodes type 1 pili. Type 1 or type 3 fimbriae are the most important virulence factors that lead to KP adhesion and increase its growth ability in biofilm communities.34 Type 1 fimbriae can help bacteria adhere to host cells, mainly participate in the formation of intracellular bacterial colonies, and easily cause infection.35 Virulence factors expressed by iron absorption genes include enterobactin, aerocin, salmonellin and yersiniabactin, which play an important role in promoting bacterial growth, biofilm maturation, aggravating tissue damage, infection and transmission.36 Enterobactin has a high affinity with iron and promotes bacterial growth mainly by binding iron in plasma transferrin. Yersiniabactin can activate outer membrane protein biofilm formation when the body is iron-deficient.37 Virulence factors expressed by invasive virulence genes can protect bacteria from host immune killing and phagocytosis by macrophages.
In our study, CRKP carried a large number of virulence genes, which may be related to its multi-drug resistance. The more virulence genes carried, the more antibiotic resistant. Due to the small sample size of this study, third-generation high-throughput sequencing technology was used to detect the common virulence factor types carried by MDR-KP, and mainly adhesion virulence genes, iron-absorption virulence genes and invasion virulence genes were detected. However, no capsule-related virulence genes have been detected. Large-scale multicenter studies are still needed to further analyze the relationship between virulence factors and antibiotic resistance.
Conclusion
In conclusion, the CRKP in our hospital presents a multi-drug resistant phenotype and is resistant to a variety of common antibiotics, and the drug resistance situation must be highly valued. CRKP resistance is related to the production of different resistance genes and carrying a variety of virulence factors. It was detected by WGS that blaKPC was the main carbapenemase gene isolated from clinical CRKP in our hospital, and ST11 type was the main type of CRKP. Therefore, ST11-KPC Klebsiella pneumoniae strain is the focus of infection control and clinical research. Strict and effective nosocomial infection management and control measures should be formulated, epidemiological investigation should be conducted in time, antibiotic resistance monitoring should be strengthened. It is extremely necessary to curb the spread and prevalence of CRKP in hospitals. In addition, WGS has a high application prospect in predicting antibiotic resistance phenotypes and detecting virulence genes, which is worthy of clinical promotion and conducive to guiding clinical treatment and control.
Data Sharing Statement
We have upload the data to the repository (GenBank). The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, PRJNA888051.
Ethics Statement
All procedures performed were approved by the Institutional Ethics Board of Zhongnan Hospital of Wuhan University (No. 2019126). The present study was a retrospective study focusing on bacteria and did not contain any sensitive personal information. Therefore, informed consent was not required in line with local legislation.
Acknowledgments
We are grateful to Zhongnan Hospital of Wuhan University for providing clinical CRKP isolates and data.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was funded by the Special Project for Significant New Drug Research and Development in the Major National Science and Technology Projects of China (project no. 2020ZX09201007).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Chen J, Yang Y, Yao H, et al. Prediction of prognosis in adult patients with carbapenem-resistant Klebsiella pneumoniae infection. Front Cell Infect Microbiol. 2022;11:818308. doi:10.3389/fcimb.2021.818308
2. Wang B, Pan F, Wang C, et al. Molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae in a paediatric hospital in China. Int J Infect Dis. 2020;93:311–319. doi:10.1016/j.ijid.2020.02.009
3. Li Y, Shen H, Zhu C, Yu Y. Carbapenem-resistant Klebsiella pneumoniae infections among ICU admission patients in central China: prevalence and prediction model. BioMed Res Int. 2019;2019:9767313. doi:10.1155/2019/9767313
4. Kopotsa K, Mbelle NM, Osei Sekyere J. Epigenomics, genomics, resistome, mobilome, virulome and evolutionary phylogenomics of carbapenem-resistant Klebsiella pneumoniae clinical strains. Microb Genom. 2020;6(12):mgen000474. doi:10.1099/mgen.0.000474
5. Wang YP, Chen YH, Hung IC, et al. Transporter genes and fosA associated with fosfomycin resistance in carbapenem-resistant Klebsiella pneumoniae. Front Microbiol. 2022;13:816806. doi:10.3389/fmicb.2022.816806
6. Zhu WM, Yuan Z, Zhou HY. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection relative to two types of control patients: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2020;9:23. doi:10.1186/s13756-020-0686-0
7. Navon-Venezia S, Kondratyeva K, Carattoli A. Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol Rev. 2017;41(3):252–275. doi:10.1093/femsre/fux013
8. Hu Y, Liu C, Shen Z, et al. Prevalence, risk factors and molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae in patients from Zhejiang, China, 2008–2018. Emerg Microbes Infect. 2020;9(1):1771–1779. doi:10.1080/22221751.2020.1799721
9. Dai G, Xu Y, Kong H, Xie W, Wang H. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection and associated clinical outcomes. Am J Transl Res. 2021;13(6):7276–7281.
10. Whole-genome sequencing of bacterial pathogens: the future of nosocomial outbreak analysis. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5608882/.
11. Wang D, Wang M, He T, et al. Molecular epidemiology and mechanism of Klebsiella pneumoniae resistance to ertapenem but not to other carbapenems in China. Front Microbiol. 2022;13:974990. doi:10.3389/fmicb.2022.974990
12. Hou M, Chen N, Dong L, et al. Molecular epidemiology, clinical characteristics and risk factors for bloodstream infection of multidrug-resistant Klebsiella pneumoniae infections in pediatric patients from Tianjin, China. Infect Drug Resist. 2022;15:7015–7023. doi:10.2147/IDR.S389279
13. Cheng S, Fleres G, Chen L, et al. Within-host genotypic and phenotypic diversity of contemporaneous carbapenem-resistant Klebsiella pneumoniae from blood cultures of patients with bacteremia. mBio. 2022:e02906. doi:10.1128/mbio.02906-22
14. Brink AJ. Epidemiology of carbapenem-resistant gram-negative infections globally. Curr Opin Infect Dis. 2019;32(6):609–616. doi:10.1097/QCO.0000000000000608
15. Li Z, Ding Z, Yang J, et al. Carbapenem-resistant Klebsiella pneumoniae in Southwest China: molecular characteristics and risk factors caused by KPC and NDM producers. Infect Drug Resist. 2021;14:3145–3158. doi:10.2147/IDR.S324244
16. Bassetti M, Akova M, Tumbarello M. Treatment and mortality of Klebslella pneumoniae infections in critically ill patients: should we do and predict them better? Intensive Care Med. 2018;44(11):1982–1984. doi:10.1007/s00134-018-5390-7
17. Lee N-Y, Tsai C-S, Syue L-S. Treatment outcome of bacteremia due to non–carbapenemase-producing carbapenem-resistant Klebsiella pneumoniae bacteremia: role of carbapenem combination therapy. Clin Ther. 2020;42(3):e33–e44. doi:10.1016/j.clinthera.2020.01.004
18. Luan YY, Chen YH, Li X, et al. Clinical characteristics and risk factors for critically ill patients with carbapenem-resistant Klebsiella pneumoniae (CrKP): a cohort study from developing country. Infect Drug Resist. 2021;14:5555–5562. doi:10.2147/IDR.S343489
19. Chi X, Hu G, Xu H, et al. Genomic analysis of A KPC-2-producing Klebsiella Pneumoniae ST11 outbreak from a teaching hospital in Shandong Province, China. Infect Drug Resist. 2019;12:2961–2969. doi:10.2147/IDR.S221788
20. Su S, Li C, Zhao Y, et al. Outbreak of KPC-2–producing Klebsiella pneumoniae ST76 isolates in an intensive care unit and neurosurgery unit. Microb Drug Resist. 2020;26(9):1009–1018. doi:10.1089/mdr.2019.0363
21. Wang Q, Wang X, Wang J, et al. Phenotypic and genotypic characterization of carbapenem-resistant Enterobacteriaceae: data from a longitudinal large-scale CRE study in China (2012–2016). Clin Infect Dis. 2018;67(suppl_2):S196–S205. doi:10.1093/cid/ciy660
22. Zheng R, Zhang Q, Guo Y, et al. Outbreak of plasmid-mediated NDM-1-producing Klebsiella pneumoniae ST105 among neonatal patients in Yunnan, China. Ann Clin Microbiol Antimicrob. 2016;15:10. doi:10.1186/s12941-016-0124-6
23. Chiu SK, Ma L, Chan MC, et al. Carbapenem nonsusceptible Klebsiella pneumoniae in Taiwan: dissemination and increasing resistance of carbapenemase producers during 2012–2015. Sci Rep. 2018;8. doi:10.1038/s41598-018-26691-z
24. Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant enterobacteriaceae: epidemiology and prevention. Clin Infect Dis. 2011;53(1):60–67. doi:10.1093/cid/cir202
25. Lu MC, Chen YT, Tang HL, et al. Transmission and evolution of OXA-48-producing Klebsiella pneumoniae ST11 in a single hospital in Taiwan. J Antimicrob Chemother. 2019:dkz431. doi:10.1093/jac/dkz431
26. Huang W, Zhang J, Zeng L, et al. Carbapenemase production and epidemiological characteristics of carbapenem-resistant Klebsiella pneumoniae in Western Chongqing, China. Front Cell Infect Microbiol. 2022;11:775740. doi:10.3389/fcimb.2021.775740
27. Qin X, Wu S, Hao M, et al. The colonization of carbapenem-resistant Klebsiella pneumoniae: epidemiology, resistance mechanisms, and risk factors in patients admitted to intensive care units in China. J Infect Dis. 2020;221(Supplement_2):S206–S214. doi:10.1093/infdis/jiz622
28. Zhang WX, Chen HY, Chen C, et al. Resistance phenotype and molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae isolates in Shanghai. Microb Drug Resist. 2021;27(10):1312–1318. doi:10.1089/mdr.2020.0390
29. Chen L, Mathema B, Chavda KD, DeLeo FR, Bonomo RA, Kreiswirth BN. Carbapenemase-producing Klebsiella pneumoniae: molecular and genetic decoding. Trends Microbiol. 2014;22(12):686–696. doi:10.1016/j.tim.2014.09.003
30. Liao W, Liu Y, Zhang W. Virulence evolution, molecular mechanisms of resistance and prevalence of ST11 carbapenem-resistant Klebsiella pneumoniae in China: a review over the last 10 years. J Glob Antimicrob Resist. 2020;23:174–180. doi:10.1016/j.jgar.2020.09.004
31. Yu X, Zhang W, Zhao Z, et al. Molecular characterization of carbapenem-resistant Klebsiella pneumoniae isolates with focus on antimicrobial resistance. BMC Genom. 2019;20:822. doi:10.1186/s12864-019-6225-9
32. Lan P, Jiang Y, Zhou J, Yu Y. A global perspective on the convergence of hypervirulence and carbapenem resistance in Klebsiella pneumoniae. J Glob Antimicrob Resist. 2021;25:26–34. doi:10.1016/j.jgar.2021.02.020
33. Russo TA, Olson R, Fang CT, et al. Identification of biomarkers for differentiation of hypervirulent Klebsiella pneumoniae from classical K. pneumoniae. J Clin Microbiol. 2018;56(9):e00776. doi:10.1128/JCM.00776-18
34. Zhang S, Zhang X, Wu Q, et al. Clinical, microbiological, and molecular epidemiological characteristics of Klebsiella pneumoniae-induced pyogenic liver abscess in southeastern China. Antimicrob Resist Infect Control. 2019;8:166. doi:10.1186/s13756-019-0615-2
35. Yan Q, Zhou M, Zou M, Liu WE. Hypervirulent Klebsiella pneumoniae induced ventilator-associated pneumonia in mechanically ventilated patients in China. Eur J Clin Microbiol Infect Dis. 2016;35(3):387–396. doi:10.1007/s10096-015-2551-2
36. Khalil MAF, Hager R, Abd-El Reheem F, et al. A study of the virulence traits of carbapenem-resistant Klebsiella pneumoniae isolates in a Galleria mellonella model. Microb Drug Resist. 2019;25(7):1063–1071. doi:10.1089/mdr.2018.0270
37. Lam MMC, Wick RR, Wyres KL, et al. Genetic diversity, mobilisation and spread of the yersiniabactin-encoding mobile element ICEKp in Klebsiella pneumoniae populations. Microb Genom. 2018;4(9):e000196. doi:10.1099/mgen.0.000196
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

