Back to Journals » Cancer Management and Research » Volume 11
Modified Glasgow prognostic score as a prognostic factor for renal cell carcinomas: a systematic review and meta-analysis
Authors Hu X , Wang Y, Yang WX, Dou WC, Shao YX, Li X
Received 15 March 2019
Accepted for publication 6 June 2019
Published 4 July 2019 Volume 2019:11 Pages 6163—6173
DOI https://doi.org/10.2147/CMAR.S208839
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Xu Hu,*,1 Yan Wang,*,1 Wei-Xiao Yang,*,1 Wei-Chao Dou,1 Yan-Xiang Shao,1 Xiang Li2
1West China School of Medicine/West China Hospital, Sichuan University, Chengdu 610041, People’s Republic of China; 2Department of Urology, West China Hospital, West China Medical School, Sichuan University, Chengdu 610041, People’s Republic of China
*These authors contributed equally to this work
Objective: The modified Glasgow prognostic score (mGPS), a combination of C-reactive protein (CRP) and albumin levels, reflects systemic inflammation and nutritional status. This score has been shown to have prognosis value for various tumors. In the present study, we evaluated the prognostic value of mGPS for patients with renal cell carcinoma (RCC).
Methods: Literature search was conducted based on PubMed, Embase, and Cochrane Central Register of Controlled Trials up to December 2018. We pooled HRs and 95% CIs to evaluate the correlation between mGPS and survival in patients with RCC.
Results: Twelve studies comprising 2,391 patients were included in the present study for quantitative synthesis. Our studies demonstrated that higher mGPS was significantly correlated to poor overall survival (HR=4.31; 95%CI, 2.78–6.68; P<0.001), cancer-specific survival (HR=5.88; 95%CI, 3.93–8.78; P<0.001), recurrence-free survival (HR=3.15; 95%CI, 2.07–4.79; P<0.001), and progression-free survival (HR=1.91; 95%CI, 1.27–2.89; P=0.002). Subgroup analyses also confirmed the overall results.
Conclusion: mGPS could serve as a predictive tool for the survival of patients with RCC. In the different subgroups, the results are also consistent with previous results. In conclusion, pretreatment higher mGPS is associated with poorer survival in patients with RCC. Further external validations are necessary to strengthen this concept.
Keywords: modified Glasgow prognostic score, renal cell carcinoma, prognosis, meta-analysis
Introduction
Renal cell carcinoma (RCC) accounts for 2–3% of all cancers, with an estimated 403,262 new cases in the world in 2018.1 The occurrence of RCC has been increasing over the past three decades. Approximately 67% of newly diagnosed patients are localized disease, 16% of patients present regional or distant metastases.2 Although the development of treatment for localized RCC, such as radical nephrectomy or partial nephrectomy, approximately 20–40% of patients will have local or distal recurrence.3 Treatments for metastatic RCC also have developed rapidly, including cytoreductive nephrectomy, immunotherapy, or target therapy, but the clinical outcome is not encouraging.4
Stratifications of patients based on the risk of recurrence are important to aid decision-making, to identify further follow-up and to judge whether patients should be enrolled in adjuvant clinical trials.5 TNM stage and Fuhrman nuclear grade are widely served as prognostic factors, but these factors have limited accuracy.6,7 Currently, more evidence suggested that systemic inflammation is associated with disease development and progression.8,9 Reportedly, an increasing number demonstrated that inflammation biomarkers, such as C-reactive protein (CRP), the neutrophil-to-lymphocyte ratio (NLR), and the platelet-to-lymphocyte ratio (PLR) could predict prognosis of RCC.10,11 Glasgow prognostic score (GPS), a combination of CRP and albumin levels, reflects systemic inflammation and nutritional status. This score has been shown to have prognosis value for various tumors including gastric cancer, non-small cell lung cancer, esophageal cancer, as well as RCC.12–15 Modifying GPS as modified Glasgow prognostic score (mGPS), giving a score of 1 only for an elevated CRP, has been considered as a prognostic factor for several cancers such as non-small cell lung cancer, esophageal cancer, hepatocellular carcinoma, as well as RCC.13,16–18 However, the prognostic value of mGPS for RCC remains unclear, and the findings of different studies are inconsistent. Therefore, it is necessary to evaluate the prognostic value of mGPS for patients with RCC by conducting a systematic review and meta-analysis.
In the present study, we evaluated the association between pretreatment mGPS and prognosis by searching available literature and pooling all outcome data.
Methods
Literatures search
We performed the study according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement.19 Data for this study was collected by searching PubMed, PubMed (from inception to 2018 December 15), Embase (1974 to 2018 December 14), and Cochrane Central Register of Controlled Trials (from inception to November 2018). Literature search was conducted using items about renal cell carcinoma (or carcinoma, renal cell) and mGPS (or modified Glasgow prognostic score, C-reactive protein and albumin) as keywords and Mesh terms. Moreover, we screened the reference lists of eligible studies to ensure comprehensive search. Two reviewers searched the database independently (any conflicts resolved by discussion or consulting with another one).
Inclusion and exclusion criteria
Relevant studies were required to enroll according to inclusion criteria, which are as follows: 1) population-based studies; 2) involved patients with RCC; 3) evaluated the association between mGPS and clinical outcome; 4) reported the clinical outcome, such as overall survival (OS), cancer-specific-survival (CSS), recurrence-free survival (RFS), or progression-free survival (PFS). We excluded the following studies: 1) non-English language; 2) did not evaluate pretreatment mGPS value; 3) did not report the data of survival; 4) non-human studies. Conference abstracted were also included if data could be extracted. While for duplicated patients data, we only enrolled the most informative and recent study for analysis.
Data extraction and quality assessment
Two reviewers independently extracted the following baseline information from included studies: the name of author, study location and enrollment data, study design, treatment, number of patients, age, disease, the mGPS value, and follow-up. As for the outcome, we extracted the HR and 95% CI. The discrepancy in the data extraction was resolved by discussion or consulting with another one. For non-randomized studies, we used Newcastle–Ottawa Quality Assessment Scale for quality assessment. Studies were evaluated on three aspects including selection, comparability, and exposure/outcome. Studies with a score of no less than 7 were deemed as good quality. Studies from conference abstracts were considered as low quality.
Statistical analysis
For analysis of survival, we used HRs and 95%CI, which is the most appropriate for time-to-data events. HRs and 95%CI were extracted from included studies according to multivariable analysis. If HRs were not reported, we could estimate HRs from Kaplan–Meier curve based on the method presented by Tierney.20 Heterogeneity among studies was evaluated using the Q and I2 statistics. If I2 statistics presented P<0.10 or I2>50%, we used a random-effect model for analysis.21 To further investigate the stability of pooled results, we conducted sensitivity analyses. The possibility of publication bias was evaluated by Egger’s test and Begg’s test. If evidence for publication was shown, we further performed trim and fill method to estimate missing studies.22 A two-sided P-value <0.05 was indicated a significant difference. All statistical analyses were performed using STATA version 12 (StataCorp, College Station, TX, USA).
Results
Search results
Our initial search strategy identified 278 studies, 18 of which were duplicated articles. A 187 studies were excluded based on titles and abstracts, the remaining 73 studies were further evaluated. Ultimately, 12 studies including 2,391 patients were enrolled for final analysis after excluding irrelevant studies.18,23–33 A flowchart depicting the process of search strategy was illustrated in Figure 1.
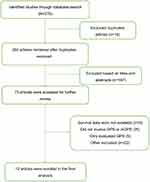 |
Figure 1 Flowchart of literature search. |
Characteristics of enrolled studies
Of the enrolled 12 articles, 3 are conference abstracts23–25 and 3 studies are prospective studies.31–33 A total of 2,391 patients were enrolled from Japan, the United Kingdom, Korea, the United States, and China. Treatments include nephrectomy, immunotherapy, and target therapy. The median age of patients was not more than 70 years. The sample size ranges from 32 to 406. Most of the studies followed up for a long time, and median follow-up ranged from 12.6 months to 98 months. Nine studies evaluated the association between mGPS and OS. Four studies reported the association between mGPS and CSS. Three studies revealed the outcome of RFS and 2 studies described the PFS. The characteristics of all eligible studies are shown in Table 1. As indicated in Table 1, all studies presented in our meta-analysis were regarded as high quality except for conference abstracts.
 |
Table 1 Characteristics of include studies |
Overall survival
A total of 9 studies involving 1,795 patients evaluated the association between mGPS and OS. The pooled results, as presented in Figure 2, revealed that higher mGPS was associated with poorer OS, the HR is 4.31(95%CI, 2.78–6.68; P<0.001). There was evidence for moderate heterogeneity among studies, I2=60.6% and P=0.005, so random-model was applied.
 |
Figure 2 Association between mGPS and OS in patients with renal cell carcinoma.Abbreviations: HR, hazard ratio; CI, confidence interval; mGPS, modified Glasgow prognostic score; OS, overall survival. |
Cancer-specific survival
Regarding 4 studies comprising 855 patients reported the HRs of CSS. There was a significant association between higher mGPS and worse CSS, the pooled HR is 5.88(95%CI, 3.93–8.78; P<0.001). There was no significant heterogeneity observed (I2=0%; P=0.521; Figure 3).
Recurrence-free survival and progression-free survival
In terms of 3 studies incorporating 765 patients and 2 studies including 103 patients, we found that higher mGPS had a significantly increased risk of recurrence and progression compared with lower mGPS. The pooled HRs for RFS and PFS were 3.15(95%CI, 2.07–4.79; P<0.001; Figure 4) and 1.91 (95%CI, 1.27–2.89; P=0.002; Figure 5), respectively. There was also no heterogeneity among studies.
Sensitivity analysis
To further evaluate the robustness of final results, we conducted sensitivity analyses by sequentially excluding enrolled each study. From Figure 6, we could observe that the adjusted results are consistent with previous results, which indicated the stableness of our results.
Publication bias
As described in “Methods”, we used Begg’s test and Egger’s test to identify publication bias. We did not detect publication bias for OS according to Egger’s test (P=0.140), while the P-value of Begg’s test is 0.029. Therefore, we used trim and fill method and did not observe publication bias. Furthermore, there was no evidence for publication bias of CSS, RFS and PFS in accordance with Egger’s test (CSS: P=0.787; RFS: P=0.464) and Begg’s test (CSS: P=1.000; RFS: P=0.296; PFS: P=1.000), respectively.
Subgroup analysis
Due to obvious heterogeneity among studies, we accomplished subgroup analyses for OS stratified by pathological type, the cutoff value of mGPS, stage, and regions. While because of the small number of enrolled studies, we only conducted subgroup analyses for CSS based on pathology, stage, and regions. As shown in Table 2, we demonstrated that higher mGPS had a poorer OS (HR=3.54; 95%CI, 2.48–5.06) and CSS (HR=5.99; 95%CI, 3.94–9.09) in patients with clear cell RCC. In other pathological types, higher mGPS are also associated with lower OS and CSS. Studies which used 1 and 2 as the cutoff values revealed that mGPS could serve as a prognostic factor for OS. In patients with localized RCC, increased mGPS was correlated to inferior OS (HR=3.93; 95%CI, 2.13–7.27) and CSS (HR=3.91; 95%CI, 1.94–7.86). For advanced/metastatic or all stages of RCC, there was also a significant association between mGPS and survival. The subgroup analyses based on regions show that both Asian and Western patients with higher mGPS had a decline of OS and CSS. Besides, heterogeneity was reduced in several subgroups. Detailed results of subgroup analyses are summarized in Table 2.
 |
Table 2 Subgroup analyses for OS and CSS |
Discussion
Our studies pooled all eligible studies to evaluate the association between mGPS and survival in patients with RCC. The pooled results suggested that higher mGPS was significantly linked with decreased OS and CSS and increased risk of recurrence and progression. Since there was obvious heterogeneity among studies, we carried out subgroup analyses based on pathological type, the cutoff value of mGPS, stage, and regions. We found that higher mGPS results in poorer OS and CSS in patients with clear cell RCC and other RCC. In different cutoff values, the mGPS also could be considered as a predictive factor for survival. In patients with different stages of RCC, there was also a significant association between mGPS and survival. As for patients of different regions, the pooled results are consistent with previous results. Although heterogeneity existed after subgroup analyses, it decreased in several subgroups. We also performed sensitivity analyses, and the trend of adjusted results did not alter. Besides, we also did not detect the publication bias, which indicates the reliability of our study.
It is difficult to predict the prognosis of patients with RCC because of its heterogeneous biological nature.18 TNM stage and Fuhrman nuclear grade are important prognostic factors for RCC, but these factors cannot accurately predict the prognosis. An increasing number of studies searched for additional factors, suggesting cachexia, platelet count, performance status, CRP, and others, may be potential prognostic factors for patients with RCC.34–37
Currently, more evidence revealed that local immune response and systemic inflammation are associated with tumor progression, metastasis, and survival of cancer patients.38,39 The inflammation response is characterized by white blood cells, platelets, lymphocytes, neutrophils, CRP, and albumin.37 And kinds of combinations of these factors, such as NLR, PLR, GPS, and mGPS, were reported to be predictive for prognosis of cancers, including RCC.10–18 CRP is a typical acute-protein produced by hepatocyte, induced by cytokine especially IL-6.40 More studies suggested that CRP is associated with prognosis of various cancers.10–13 Wang performed a meta-analysis and found that elevated CRP is correlated with poor prognosis in patients with RCC.37 Some studies observed that RCC cells can produce IL-6, which is recognized as a promoter of tumor cells growth and functioned as an autocrine growth factor of RCC.41,42 According to these evidence, we found that systemic inflammation is associated with prognosis of RCC. Furthermore, Chen et al conducted a meta-analysis and demonstrated that decreased pretreatment serum albumin levels result in poor prognosis of patients with RCC.43 Albumin was reduced during chronic inflammation by immune response including CRP, increased vascular permeability for albumin, and decreased hepatic albumin synthesis.44 Albumin levels can reflect the nutritional status of patients, malnutrition is associated with poor prognosis. Reportedly, CRP/albumin ratio, a combination of CRP and albumin, could serve as a prognostic factor of OS for patients with RCC.30 GPS, originated from CRP and albumin and described by Forrest firstly, was used for predicting prognosis of non-small cell lung cancer.45 Patients with both CRP increase (>10 mg/L) and hypoalbuminemia (<35 g/L) were defined as a score of 2. Patients with normal CRP level and albumin level were given a score of 0. Patients with either increased CRP level or hypoalbuminemia receive a score of 1. However, Proctor et al found that a low albumin level is not associated with poor survival in some cancers including bladder, prostate, renal, colorectal cancers and others based on a large cohort study.46 Therefore, mGPS was modified by GPS and gave a score of 1 only for an elevated CRP, evaluating both systemic inflammation and nutrition status. As a result, the prognostic value of mGPS may be more accurate than GPS. The mGPS is easily measured, considered as a prognostic factor for various solid tumors.13,16–17 Based on our pooled results, we also found that higher mGPS was correlated to the poor prognosis of patients with different stages of RCC. And Tsujino et al observed that the predictive value of mGPS seems to be equivalent to those of Stage Size Grade Necrosis and University of California, Los Angeles Integrated Staging System.26 Lamb et al also suggested that mGPS is at least equivalent to and independent of other current validated prognostic scoring systems for patients with RCC.33 The mGPS, combining CRP and albumin, may provide more accurate prognostic information, and Cho revealed that mGPS is superior to CRP alone.18 To sum up, mGPS appears to be superior to other established prognostic scores and factors and could provide physicians with suggestions for patients’ management. Close follow-up after treatments and optimal adjuvant therapies could be emphasized on patients with higher mGPS. And individualized decision-making is needed with mGPS as a clinical tool to help guide therapy. Besides, further large-scale-based external validation is necessary.
However, this study is not devoid of limitations. Firstly, only 12 studies incorporating 2,391 patients were enrolled for pooled analysis, which is a small number and may limit the power of results. Further large-scale studies are necessary. Next, most studies are retrospective, increasing the risk of bias. Thirdly, the patients’ baseline varied from study to study and may affect the pooled results. Although we performed subgroup analyses based on available data, some other confounders may exist and result in heterogeneity. Lastly, the measurements of CRP and albumin of enrolled studies may be different, which may also affect the final results.
Conclusion
Our study demonstrated that mGPS could serve as a predictive tool for the survival of patients with RCC. In the different subgroups, the results are consistent with previous results. In conclusion, pretreatment higher mGPS is associated with poorer OS, CSS, RFS, and PFS. Further external validations are necessary to strengthen this concept.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387
3. Brookman-May SD, May M, Shariat SF, et al. Time to recurrence is a significant predictor of cancer-specific survival after recurrence in patients with recurrent renal cell carcinoma–results from a comprehensive multi-centre database (CORONA/SATURN-Project). BJU Int. 2013;112(7):909–916. doi:10.1111/bju.12246
4. You D, Jeong IG, Ahn JH, et al. The value of cytoreductive nephrectomy for metastatic renal cell carcinoma in the era of targeted therapy. J Urol. 2011;185(1):54–59. doi:10.1016/j.juro.2010.09.018
5. Chen DY, Uzzo RG, Viterbo R. Thinking beyond surgery in the management of renal cell carcinoma: the risk to die from renal cell carcinoma and competing risks of death. World J Urol. 2014;32(3):607–613.
6. Lang H, Lindner V, de Fromont M, et al. Multicenter determination of optimal interobserver agreement using the Fuhrman grading system for renal cell carcinoma: assessment of 241 patients with >15-year follow-up. Cancer. 2005;103(3):625–629.
7. Keegan KA, Schupp CW, Chamie K, Hellenthal NJ, Evans CP, Koppie TM. Histopathology of surgically treated renal cell carcinoma: survival differences by subtype and stage. J Urol. 2012;188(2):391–397.
8. de Vivar Chevez AR, Finke J, Bukowski R. The role of inflammation in kidney cancer. Adv Exp Med Biol. 2014;816:197–234.
9. Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nature Rev Immunol. 2004;4(8):641–648.
10. Hu H, Yao X, Xie X, et al. Prognostic value of preoperative NLR, dNLR, PLR and CRP in surgical renal cell carcinoma patients. World J Urol. 2017;35(2):261–270. doi:10.1007/s00345-016-1864-9
11. Semeniuk-Wojtas A, Lubas A, Stec R, Syrylo T, Niemczyk S, Szczylik C. Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and c-reactive protein as new and simple prognostic factors in patients with metastatic renal cell cancer treated with tyrosine kinase inhibitors: a systemic review and meta-analysis. Clin Genitourin Cancer. 2018;16(3):e685–e693. doi:10.1016/j.clgc.2018.01.010
12. Aurello P, Tierno SM, Berardi G, et al. Value of preoperative inflammation-based prognostic scores in predicting overall survival and disease-free survival in patients with gastric cancer. Ann Surg Oncol. 2014;21(6):1998–2004. doi:10.1245/s10434-014-3533-9
13. Fan H, Shao ZY, Xiao YY, et al. Comparison of the Glasgow Prognostic Score (GPS) and the modified Glasgow Prognostic Score (mGPS) in evaluating the prognosis of patients with operable and inoperable non-small cell lung cancer. J Cancer Res Clin Oncol. 2016;142(6):1285–1297. doi:10.1007/s00432-015-2113-0
14. Shim SR, Kim SJ, Kim SI, Cho DS. Prognostic value of the Glasgow Prognostic Score in renal cell carcinoma: a meta-analysis. World J Urol. 2017;35(5):771–780. doi:10.1007/s00345-016-1940-1
15. Driver RJ, Handforth C, Radhakrishna G, Bennett MI, Ford AC, Everett SM. The Glasgow prognostic score at the time of palliative esophageal stent insertion is a predictive factor of 30-day mortality and overall survival. J Clin Gastroenterol. 2018;52(3):223–228. doi:10.1097/MCG.0000000000000773
16. Walsh SM, Casey S, Kennedy R, Ravi N, Reynolds JV. Does the modified Glasgow Prognostic Score (mGPS) have a prognostic role in esophageal cancer?. J Surg Oncol. 2016;113(7):732–737. doi:10.1002/jso.24225
17. Chen H, Hu N, Chang P, et al. Modified Glasgow prognostic score might be a prognostic factor for hepatocellular carcinoma: a meta-analysis. Panminerva Med. 2017;59(4):302–307. doi:10.23736/S0031-0808.16.03236-5
18. Cho DS, Kim SI, Choo SH, Jang SH, Ahn HS, Kim SJ. Prognostic significance of modified Glasgow Prognostic Score in patients with non-metastatic clear cell renal cell carcinoma. Scand J Urol. 2016;50(3):186–191. doi:10.3109/21681805.2015.1136677
19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
20. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi:10.1186/1745-6215-8-16
21. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi:10.1136/bmj.327.7414.557
22. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463.
23. Lorentz A, Gupta M, Broggi M, Leung A, Patil D, Master V. Utility of inflammatory markers in prognosis for patients with renal cell carcinoma and vena cava tumor thrombus. J Urol. 2017;197(4 Supplement 1):e180. doi:10.1016/j.juro.2017.02.509
24. Baum Y, De La Calle C, Patil D, et al. Commonly-used cost-effective pre-operative inflammatory markers provide prognostic information in patients with localized clear cell renal cell carcinoma. J Urol. 2015;1:e428.
25. Cross B, Johnson T, Kucuk O, et al. External validation of the modified Glasgow Prognostic Score (mGPS) for renal cancer. J Urol. 2012;1:e680.
26. Tsujino T, Komura K, Matsunaga T, et al. Preoperative measurement of the modified glasgow prognostic score predicts patient survival in non-metastatic renal cell carcinoma prior to nephrectomy. Ann Surg Oncol. 2017;24(9):2787–2793. doi:10.1245/s10434-017-5948-6
27. Ohmura H, Uchino K, Kajitani T, Sakamoto N, Baba E. Predictive value of the modified Glasgow Prognostic Score for the therapeutic effects of molecular-targeted drugs on advanced renal cell carcinoma. Mol Clin Oncol. 2017;6(5):669–675. doi:10.3892/mco.2017.1205
28. Harris WB, Zhang C, Liu Y, et al. Time-dependent effects of prognostic biomarkers of systemic inflammation in patients with metastatic renal cell carcinoma. Tumour Biol. 2017;39(6):1010428317705514. doi:10.1177/1010428317705514
29. Ishihara H, Kondo T, Omae K, et al. Sarcopenia and the modified Glasgow prognostic score are significant predictors of survival among patients with metastatic renal cell carcinoma who are receiving first-line sunitinib treatment. Target Oncol. 2016;11(5):605–617. doi:10.1007/s11523-016-0430-0
30. Chen Z, Shao Y, Fan M, et al. Prognostic significance of preoperative C-reactive protein: albumin ratio in patients with clear cell renal cell carcinoma. Int J Clin Exp Pathol. 2015;8(11):14893–14900.
31. Tai CG, Johnson TV, Abbasi A, et al. External validation of the modified Glasgow prognostic score for renal cancer. Indian J Urol. 2014;30(1):33–37. doi:10.4103/0970-1591.124203
32. Qayyum T, McArdle PA, Lamb GW, et al. Prospective study of the role of inflammation in renal cancer. Urol Int. 2012;88(3):277–281. doi:10.1159/000334971
33. Lamb GW, Aitchison M, Ramsey S, Housley SL, McMillan DC. Clinical utility of the Glasgow Prognostic Score in patients undergoing curative nephrectomy for renal clear cell cancer: basis of new prognostic scoring systems. Br J Cancer. 2012;106(2):279–283. doi:10.1038/bjc.2011.556
34. Bensalah K, Leray E, Fergelot P, et al. Prognostic value of thrombocytosis in renal cell carcinoma. J Urol. 2006;175(3 Pt 1):859–863. doi:10.1016/S0022-5347(05)00526-4
35. Kim HL, Belldegrun AS, Freitas DG, et al. Paraneoplastic signs and symptoms of renal cell carcinoma: implications for prognosis. J Urol. 2003;170(5):1742–1746. doi:10.1097/01.ju.0000092764.81308.6a
36. Patard JJ, Leray E, Cindolo L, et al. Multi-institutional validation of a symptom based classification for renal cell carcinoma. J Urol. 2004;172(3):858–862. doi:10.1097/01.ju.0000135837.64840.55
37. Wang Z, Peng S, Wang A, et al. C-reactive protein is a predictor of prognosis in renal cell carcinoma patients receiving tyrosine kinase inhibitors: A meta-analysis. Clin Chim Acta. 2017;475:178–187. doi:10.1016/j.cca.2017.10.021
38. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–e503. doi:10.1016/S1470-2045(14)70263-3
39. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi:10.1016/j.cell.2010.01.025
40. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805–1812. doi:10.1172/JCI18921
41. Alberti L, Thomachot MC, Bachelot T, Menetrier-Caux C, Puisieux I, Blay JY. IL-6 as an intracrine growth factor for renal carcinoma cell lines. Int J Cancer. 2004;111(5):653–661. doi:10.1002/ijc.20287
42. Cuadros T, Trilla E, Sarro E, et al. HAVCR/KIM-1 activates the IL-6/STAT-3 pathway in clear cell renal cell carcinoma and determines tumor progression and patient outcome. Cancer Res. 2014;74(5):1416–1428.
43. Chen Z, Shao Y, Wang K, et al. Prognostic role of pretreatment serum albumin in renal cell carcinoma: a systematic review and meta-analysis. Onco Targets Ther. 2016;9:6701–6710.
44. Ballmer PE. Causes and mechanisms of hypoalbuminaemia. Clin Nutr. 2001;20(3):271–273.
45. Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br J Cancer. 2004;90(9):1704–1706.
46. Proctor MJ, Morrison DS, Talwar D, et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow inflammation outcome study. Br J Cancer. 2011;104(4):726–734.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




