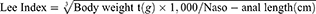Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Moderate Treadmill Exercise Modulates Gut Microbiota and Improves Intestinal Barrier in High-Fat-Diet-Induced Obese Mice via the AMPK/CDX2 Signaling Pathway
Authors Wang J, Zhang Q, Xia J, Sun H
Received 6 November 2021
Accepted for publication 24 December 2021
Published 20 January 2022 Volume 2022:15 Pages 209—223
DOI https://doi.org/10.2147/DMSO.S346007
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Jing Wang,1,2,* Qiang Zhang,1,2,* Jie Xia,1,2 Haiji Sun3
1Key Laboratory of Adolescent Health Assessment and Exercise Intervention of Ministry of Education, East China Normal University, Shanghai, 200241, People’s Republic of China; 2School of Physical Education & Health, East China Normal University, Shanghai, 200241, People’s Republic of China; 3Key Laboratory of Animal Resistance Biology of Shandong Province, School of Life Science, Shandong Normal University, Jinan, 250014, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Haiji Sun Tel +86 13064094818
Email [email protected]
Objective: The aim of this study was to investigate the effects of moderate treadmill exercise on gut microbiota, expression of proteins associated with gut barrier and to elucidate the mechanisms underlying their role in high-fat-diet-induced obese mice.
Methods: Six-week-old male C57BL/6 mice were randomly divided into standard chow diet control group (SD + Sed, n=6), chow diet exercise group (SD + Exe, n=6), high-fat diet control group (HFD + Sed, n=6) and high-fat diet exercise group (HFD + Exe, n=6). Exercise groups were trained on a motorized treadmill for 45 min/d at running speeds of 12 m/min, 5 days/week, for 12 consecutive weeks. The body weight and fasting blood glucose of the mice were recorded before euthanasia. Thereafter, the mice were sacrificed and the alteration of adipose mass, colonic histopathology, gut microbiome and gut barrier-related molecules were tested.
Results: It was found that the moderate treadmill exercise prevented the development of adiposity and hyperglycemia and effectively improved the loss of diversity and the relative abundance of intestinal microflora induced by high-fat diet. Moreover, regular exercise reversed the intestinal pathology and elevated the number of goblet cells in obesity. Besides, compared with the sedentary obese mice, the protein expression levels of colonic ZO-1 and occludin were enhanced and AMPK/CDX2 signaling pathway was significantly upregulated in obese mice that underwent exercise.
Conclusion: Long-term moderate treadmill exercise can markedly reduce the degree of obesity, modulate the colonic gut microbiota, and effectively activating AMPK/CDX2 signaling pathway to improve intestinal barrier in obese mice induced by high-fat diet.
Keywords: obesity, high-fat diet, moderate treadmill exercise, gut microbiota, gut barrier, AMPK/CDX2 signal
Introduction
Obesity can lead to metabolic syndromes such as metabolic-associated fatty liver disease (MAFLD), diabetes, hypertension, atherosclerosis, etc1 and has become a great public-health concern. Obesity has been found to be primarily mediated by an imbalance between energy intake and expenditure. Therefore, high-fat diet (HFD) is the one of main causes of obesity. It has been established that HFD can significantly impact the gut microbiome and increase the possibility that the changed composition of the gut microflora might directly influence the amount of energy extracted from the diet. For instance, previous study has shown that the germ-free mice administered HFD displayed a significantly lower body mass index when compared with the conventional mice.2 Furthermore, compared with lean donors, adiposity occurred with a significantly greater increase while transplanting the microbiota from diet-induced obese mice to lean germ-free recipients,3 which suggests that gut microbiota can influence markedly the development of metabolic syndrome.4 Thus, the host gut microbiota plays a key role in mediating the interactions between obesity and HFD.5,6
Additionally, by using multivariate analysis to investigate a statistical link between the phenotype and microbiota, the research showed that the various changes induced by HFD in intestinal barrier function and gut microbiota have been found to be dynamic and region dependent, indicating that intestinal barrier function could significantly change with the corresponding composition of the gut microbiota.7 Mouries et al8 reported that microbiota transplantation from HFD-fed mice into germ-free recipients could induce the gut vascular barrier damage and bacterial translocation into the liver and further accelerate the development of non-alcoholic steatohepatitis (NASH). It has also been reported that increased intestinal permeability is associated with different risk factors for metabolic disease.9 Hence, the gut barrier structural disruption and the loss of function may be the early events leading to obesity. Likewise, obesity-associated complications, such as hyperglycemia, can aggravate intestinal barrier integrity by causing transcriptional reprogramming of intestinal epithelial cells and alteration of tight and adherence junction integrity.10 Human studies also have shown that higher levels of circulating zonulin (marker of intestinal permeability), microbial product influx and inflammation markers were found in the obese subjects, whereas increased intestinal permeability was reduced to within the normal range after weight reduction in patients with obesity.10–12 Therefore, effectively modulating the gut microbiota composition and intestinal permeability can serve as an important strategy for both the prevention and treatment of obesity induced by HFD.
Exercise is beneficial for obesity and its associated complications,13 as well as gut microbiota diversity and intestinal health.14 It has been found that the long-term exercise can significantly improve intestinal permeability in type 2 diabetes patients,15 thereby clearly indicating that moderate intensity exercise can substantially increase the expression of the various intestinal tight junction proteins, but the underlying mechanisms are not completely known. Adenosine monophosphate-activated protein kinase (AMPK) is one of the most important target molecules activated by exercise. Meanwhile, its phosphorylation also plays an important role to improve gut barrier function and intestinal epithelial cells (IECs) differentiation through increasing caudal-type homeobox 2 (CDX2) expression and thus effectively promoting the assembly of TJs complexes.16 However, there have not been prior research about the possible role of exercise in regulating the AMPK/CDX2 signaling pathway in the intestinal tract of mice with diet-induced obesity (DIO). This study aimed to observe the effects of moderate exercise on the gut microbiota, intestinal integrity and AMPK/CDX2 signaling pathway of obese mice induced by HFD, so as to reveal the biological mechanisms of exercise that could be potentially involved in the regulation of intestinal permeability in obesity.
Materials and Methods
Experimental Animals
Twenty-four C57BL/6J mice (male, 5 weeks old) were purchased from the Laboratory AnimaI Centre of East China Normal University (Shanghai, China). All mice were housed in the specific pathogen-free (SPF) class animal facility with controlled environment and following conditions: temperature fluctuated slightly at 23±2°C; relative humidity was maintained at 40–70%; 12/12 light–dark cycle was set for circadian conditions. Mice were libitum administered with chow diet or HFD containing 60% kcal derived from fat (Research Diets, NJ, USA). The ingredient composition of standard chow and HFD has been tabulated in Supplementary Tables 1 and 2. All the mice received human care as per the standards set by the National Academy of Sciences and the Laboratory Animal Care and Use Guide (NIH Publication, 8th Edition, 2011). The in vivo studies were approved by the Animal Experiment Committee of East China Normal University (m20201006).
Animal Experiments
After acclimating for 1 week, the mice were randomly divided into four different groups: Standard chow diet control (SD + Sed), standard chow diet exercise (SD + Exe), high-fat diet control (HFD + Sed) and high-Fat diet exercise (HFD + Exe), containing six mice in each group. The mice in the obesity model group were administered with HFD as previously described.17 We first approximately evaluated the degree of obesity by comparing body weight of the HFD group with chow diet animals. The mice with the values that were 25% or 40% greater body weight than age-matched control mice fed chow were regarded as moderate or severe obesity separately. Thereafter, we verified the degree of obesity again according to the related indexes18 after tissue collection. Treadmill exercise training protocol included two distinct phases: adaptation and training. During the adaptation period, the mice in the exercise group underwent exercise preconditioning for 1 week as described in Supplementary Table 3. Thereafter, the training was performed at an intensity of 12 m/min (producing a VO2 equivalent to 76% of the VO2max),19 45 min/day, 5 days/week for 12 weeks20 at the same time every day (between 17:30 and 19:30).
Sample Preparation
At the end of the experiment, the mice were subjected to overnight fasting (about 12 hours) and then sacrificed by cervical dislocation after being anesthetized with isoflurane. The colons and epididymal adipose tissue (eWAT) were quickly isolated. The fecal samples were then collected from the colon with sterile medical devices and stored at −80 °C until analysis. Thereafter, upper part and lower part of colons were respectively placed in 4% paraformaldehyde and liquid nitrogen for further analysis.
Determination of the Body Mass Indexes, eWAT Mass and Fasting Blood Glucose
The mice were weighed prior to exercise weekly, and the weight change trend of the four groups was regularly monitored. The length from head to caudal region after the last exercise session was evaluated to estimate the Lee Index, which was calculated using the following formula:
The tips of the mice tails were clipped and fasting blood glucose (FBG) levels were measured by using a glucose meter (Accu-Chek Aviva; Roche Diagnostics) before the euthanasia. The eWAT was weighed to calculate the relative weight.
16S rRNA Gene Sequencing and Analyses
Fecal microbial DNA was extracted by using phenol-chloroform-isoamyl alcohol (25:24:1 by volume) and purified by ethanol precipitation. The DNA was then amplified by using barcoded universal bacterial primers targeting variable 3–4 (V3–V4) region of the 16S rRNA gene: 343F (primer 5’-TACGGRAGGCAGCAG-3’) and 798R (primer 5’-AGGGTATCTAATCCT-3’). Thereafter, sequencing was completed on Illumina MiSeq platform.
Trimmomatic (Version 0.35) was used to eliminate impurities and filter quality of original FASTQ files. The quality of the original sequences obtained from MiSeq were filtered by QIIME and operational taxonomic units (OTUs) were clustered by Vsearch (version 2.4.2). Subsequently, OTUs were classified by using RDP classifier Naive Bayesian to calculate the relative abundance of microbiota at different levels. Then, linear discriminant effect size (LEfSe) analysis was conducted to identify key OTUs that were differentially represented among the each group, with linear discriminant analysis (LDA) significance threshold ≥3.
Histological Analysis and Goblet Cell Staining
The three upper colons in each group were collected immediately after death, fixed overnight in 4% paraformaldehyde and then embedded in paraffin. Thereafter, the tissue blocks were cut into 5-μm sections and stained with hematoxylin and eosin (H&E) for histological examination. Goblet cells (GCs) were then stained with periodic acid-Schiff (PAS). The number of GCs in villi per view was counted, and the average number of GCs per villus was estimated.
Western Blotting
The tissue samples were homogenized with RIPA buffer containing phosphatase inhibitors and protease inhibitors (#sc-364162, Santa Cruz, CA, USA) to extract the total protein. All protein samples were assigned to two pieces of 6% or 10% SDS-PAGE gel (#P0012AC, Beyotime Biotechnology, Jiangsu, China) separately and then transferred to PVDF membranes (#IPVH00010, Millipore, MA, USA). The processes were performed in same tank simultaneously. After blocking and subsequent washing, primary and second antibody incubation was carried out successively, and the target proteins on the membrane were observed by the FluorChem FC2 system (Alpha, Germany).
The primary antibodies used were as follows: ZO-1 (#AF5145, Affinity, OH, USA), occludin (#DF7504, Affinity), AMPK (#AF6423, Affinity), p-AMPK (#AF3423, Affinity), CDX2 (#DF7606, Affinity), β-actin (#AF7018, Affinity). The secondary antibodies, goat anti-rabbit IgG (H+L) HRP (#S0001, Affinity,), were also used.
Statistical Analysis
Statistical analysis was performed using IBM SPSS 23.0 and GraphPad Prism version 7.0. According to the data distribution and the homogeneity of variance, ANOVA and Student’s t-test or Kruskal–Wallis test with Wilcoxon signed-rank test were employed to analyze the significant differences of gut microbiota among groups. Besides, two-way ANOVA with LSD post hoc test was performed to analyze other data whether there were significant differences at the level of P-value <0.05. The results have been represented as mean± SEM.
Results
Effects of Exercise on Body Weight, eWAT Mass and Blood Glucose of Obese Mice
To assess the effects of an HFD and exercise on adiposity, we measured body weight, eWAT mass and fasting blood-glucose (Figure 1). The body weight of the mice in SD + Sed group and SD + Exe group remained at lower levels and increased slowly during the experiment. However, the weight of the mice in the HFD + Sed group continued to increase rapidly, and the body weight as well as increased percentage at the end of experimental exercise was 1.5 times (P < 0.0001) and 2.4 times (P < 0.0001) of the mice in SD + Sed group, respectively. It was found that exercise intervention effectively inhibited the body mass (P < 0.001), and significantly decreased the percentage gain (P < 0.001) and Lee index (P < 0.001) in obese mice. Meanwhile, as expected, HFD produced significant increase in both eWAT mass (P < 0.0001) and FBG (P < 0.0001) in sedentary mice that did not have access to a treadmill. Exercise decreased visceral adiposity (P < 0.01) and improved the blood glucose level (P < 0.05) in HFD + Exe group.
Effects of Exercise on the Gut Microbiota in Obese Mice
Effects of Exercise on the Diversities of Gut Microbiota in Obese Mice
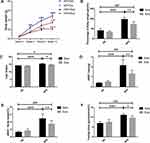
Alpha diversity refers to the diversity of an ecosystem in a specific region. It is also a summation index reflecting the richness and evenness, including Chao1 index, Shannon index, etc. As shown in Figure 2A and B, compared with SD + Sed group, HFD significantly reduced Chao1 (P<0.0001) and Shannon (P<0.01) index that indicated a marked decrease in the microbial species richness, while exercise intervention increased the Alpha diversity of gut bacteria that was obtained from Chao1 (P<0.01) and Shannon index. β diversity measures the similarity of microbial composition among different samples based on the structure of microbial community. Principal Component Analysis (PCA) is the common method for measurement of β diversity. The PCA revealed that every group exhibited distinct microbial structure and was completely separate (Figure 2C). The HFD + Sed group shared an obvious different structure of the colonic microflora as compared to that of the SD + Sed group. However, both the exercise treatment groups altered the microbial structure in comparison with the sedentary mice. Overall, these findings clearly suggested that HFD significantly reduced the diversity of gut microbiota in mice, while moderate intensity treadmill exercise exhibited positive effects on the upregulation and recovery of it.
Effects of Exercise on Relative Abundance of Gut Microbiota in Obese Mice
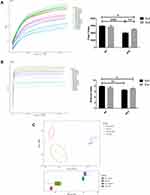
As shown in Figure 3A–E, at the phylum level, the predominant species in the colon of the mice were Bacteroidetes, Firmicutes and Proteobacteria, which accounted for more than 90% of the total relative abundance of bacteria. The relative abundance of Bacteroidetes and Firmicutes in each group displayed no statistical difference. The ratio of Firmicutes to Bacteroidetes (F/B ratio) in HFD + Sed group was increased by 58% compared with that in SD + Sed group while in HFD + Exe group was 20% lower than that in HFD + Sed group. In addition, it was observed that exercise could effectively improve the relative abundance reduction of Verrucomicrobia (P<0.05) which was induced by HFD.
At the family level, Muribaculaceae, Bacteroidaceae, Rikenellaceae, Lachnospiraceae and Ruminococcaceae were found to be the dominant species in each group (Figure 4A). Bifidobacteriaceae, Akkermansia and Ruminococcaceae_UCG-014 were analyzed at the family and genus levels respectively (Figure 4B–E), and compared with the SD + Sed group, the relative abundances of Bifidobacteriaceae (P<0.05), Akkermansia (P<0.0001) as well as Ruminococcaceae_UCG-014(P<0.0001) in HFD + Sed group were significantly decreased, while that of Akkermansia and Ruminococcaceae_UCG-014 in HFD + Exe group were 4 times and 3.59 times higher than those in HFD + Sed group (P < 0.01). Although the relative abundance of Bifidobacteriaceae was reduced by 69% in obese mice induced by HFD and exercise failed to reverse this trend, it increased by 91% in mice with standard chow diet underwent exercise.
LDA effect size (LEfSe) analysis can emphasize statistical significance and biological correlation. LDA can be used to identify specific fecal microbial species of obesity induced by HFD to recognize biomarkers with appropriate statistical differences among groups. As shown in Figure 5, there were four distinct microbial groups with significant different abundance in the SD + Sed group, including Muribaculaceae, uncultured_bacterium, Alloprevotella and Muribaculum. However, in the SD + Exe group, four microbial groups were significantly different, including Ambiguous_taxa, Proteobacteria, Gammaproteobacteria and Parasutterella. Additionally, in the HFD + Sed group, Bacteroidaceae, Bacteroides, Parabacteroides, Tannerellaceae and Blautia were significantly different. Furthermore, HFD combined exercise significantly changed the Rikenellaceae, Rikenellaceae_RC9_gut_group, Alistipes, Marinifilaceae and Odoribacter.
 |
Figure 5 LDA effect size analysis. The composition of distinct species in the communities (SD + Sed, SD + Exe,HFD + Sed and HFD + Exe). |
The above results indicated that both diet and exercise might exhibit specific effects on the structure of the gut microbiota.
Effects of Exercise on Colonic Barrier Integrity in Obese Mice
Effects of Exercise on the Colonic Histopathology in Obese Mice
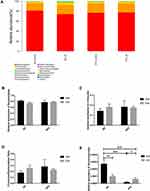
As the largest barrier between the body and the environment, the gut barrier organization consists of the mucus layer, TJs, IECs, immune cells and gut microbiota.21 “Leaky gut” refers to the damage of intestinal barrier that predominantly arises a result of changes of intestinal permeability, which results in the translocation of intestinal gram-negative bacteria and their composition such as lipopolysaccharide (LPS) into systemic circulation and peripheral tissues to generate a series of immune activation.22 The major components of the mucus layer, such as mucin, water and inorganic salts, are primarily secreted by GCs, and can form a mucous barrier on the surface of the intestinal mucosa. This plays an important role in resisting the invasion of foreign bacteria and intestinal microbes, thereby maintaining the dynamic balance of intestinal mucosa and regulating the microbial-host immune response.23 So, we performed H&E and PAS of the colonic sections to analyze the effects of exercise on histopathology and to evaluate the number of colonic GCs in mice (Figure 6). It was found that compared with the SD + Sed group, colonic epithelial cells in HFD + Sed group were arranged disorderly and villi were injured that was accompanied by increased infiltration with a number of inflammatory cells. Obese mice that underwent exercise showed significant improvement in the colon morphology, such as orderly cell arrangement, reduced villi injury, higher microstructure integrity, and lesser infiltration with the inflammatory cells. The PAS results also showed that the average number of the positive cells distribution in the HFD + Sed group (P < 0.001) and SD + Exe group (P < 0.05) was significantly lower and uneven than that of the SD + Sed group, and interestingly, alterations in HFD mice was markedly improved by exercise (P < 0.05), thus indicating that exercise might display differential effects on GCs in the different diets.
Effects of Exercise on the Colonic TJs in Obese Mice
TJs consist of several transmembrane proteins, such as occludin, and cytosolic proteins, such as ZO-1. Occludin is the one of the main TJs that can interact with ZO1 to anchor the cytoskeleton and can bind to the lateral-apical epithelial surface with adjacent IECs harboring TJs of the same type.21 Thus, occludin and ZO1 serve as an important representative markers to identify intestinal barrier integrity. A number of previous in vivo experiments and clinical trials have demonstrated that the increased permeability of the mucosal barrier and significantly reduced expression of occludin and ZO-1 in the intestine could be observed in both the rats and patients with MAFLD.24 These results indicated that the impaired gut barrier function in HFD may be related to the decreased expression of TJs. In the present study, the protein levels of zonula occludens 1(ZO-1) and occludin in the colon were also detected (Figure 7). It was found that compared with SD + Sed group, the expression levels of the colonic ZO-1 (P < 0.05) and Occludin in the HFD + Sed group were significantly decreased, and exercise effectively reversed the decrease of colonic TJs level (P < 0.05) which were effectively induced by HFD.
Effects of Exercise on Colonic AMPK/CDX2 Signaling Pathway in Obese Mice
The change of energy metabolism is one of the core manifestations of exercise. AMPK regulation on energy metabolism plays an important role both at the cellular level and in the whole organisms. Moreover, AMPK has been also related to functions of the gut barrier. So, we evaluated the potential role of AMPK in effects of exercise on colonic barrier (Figure 8). It was found that HFD reduced the protein expression level and phosphorylation level of AMPK in colon of mice (P < 0.05), thereby reducing the protein level of CDX2 (P < 0.05). On the contrary, long-term moderate intensity treadmill exercise can effectively promote the phosphorylation of AMPK (P < 0.05) in the colon of obese mice and effectively reverse the reduction of CDX2 (P < 0.01) protein in the colon induced by HFD.
Discussion
The rapidly increasing incidence of obesity has become a serious global medical and social problem. However, there are still no effective weight-loss medicines that do not exhibit serious side effects. Hence, besides strict diet, scientific physical exercise is the most effective measure to prevent and treat obesity though balancing the calories intake with output for modulating the metabolic disturbance. In this study, according to the statistical analysis of body mass and its increments, Lee’s index and eWAT mass, as well as the FBG, we found that regular exercise can significantly attenuate obesity and hyperglycemia induced by HFD, which was in agreement with the various previously published studies.25
The constitution of gut microbiota has been found to be closely connected with diet while HFD is one of the main factors inducing obesity, so the relationships between the three have been reported to be both intimate and complicated. DIO is accompanied by gut microbial imbalance or dysbiosis that has been generally found to display reduced biodiversity and marked alterations in the relative abundance among different varieties of microorganisms. For example, changes in F/B ratio (the increased F/B ratio arising from an increased Firmicutes and reduced Bacteroidetes is generally perceived as one of the critical markers to HFD) have been reported earlier.6,26 Exercise exhibits significant effects on obesity induced by HFD, concurrent with the prevention of the relative balance of the major bacterial phyla. For example, the F/B ratio was found to be proportional to the distance covered by athletes.27 Moreover, consistent with the previously published studies, our results also demonstrated that HFD significantly decreased in microbial diversity and clearly differentiated in the community structure from the control groups. On the contrary, exercise exhibited a reverse effect. Exercise reduced the F/B ratio of HFD administered, though the ratio between HFD + Sed group and HFD + Exe group appeared to have no significant statistical difference, thereby suggesting that exercise may inhibit the microbiome of DIO from boosting the capacity to harvest energy from HFD.28 Akkermansia is a mucin-degrading bacteria that belongs to the phylum of Verrucomicrobia. It is essential for maintaining intestinal homeostasis and its abundance has been negatively correlated with obesity.29 Ruminococcaceae is a member of phylum of Firmicutes and its abundance has been positively correlated with the body weight loss and causing an increase in energy metabolism. Ruminococcaceae can also contribute significantly to the fiber metabolism.30,31 In this study, we observed that exercise can indeed significantly increase the relative abundance of Verrucomicrobia, Akkermansia and Ruminococcaceae_UCG-014, while HFD can decrease their relative abundance. Surprisingly and interestingly, as a common probiotics, Bifidobacteriaceae decreased significantly in mice that were administered HFD and exercise markedly enhanced it with normal diet, but there was no athletic effect noticed on obese mice. Moreover, the findings in the SD + Sed, HFD + Sed and HFD + Exe groups were consistent with the hypotheses while SD + Exe group demonstrated a marked opposite result contrary to what we assumed previously, whether in α diversity or in the relative abundance among Verrucomicrobia, Akkermansia and others, suggesting that the effects of exercise on gut microbiota are distinct under different diets. We believe that the above unexpected results may be explained by fluctuating energy metabolism of ecosystem established in gut. Colon, as the most densely populated microbial habitat in host, can be potentially viewed as an anaerobic bioreactor containing trillions of bacteria and archaea, which can effectively modulate the host’s energy balance through programing to perform the various metabolic functions in numbers of intertwined pathways. Moreover, the comparative metagenomic studies have exhibited that the gut microflora of ob/ob was enriched for the genes that were able to harvest calories from complex plant-derived polysaccharides than their lean littermates with both fed with chow diet.28 It was found that under the treatment of chow diet, exercise can expend energy thereby inducing compensatory energy intake and consequently resulting in lower concentration of energy substrates in intestinal lumen. Some microbes with high energy requirement are better able to adapt to the nutrient-poor habitat because of their greater ability to harvest nutrients. On the contrary, HFD can enhance the relative abundance of the species of high energy requirement as a selection, whereas exercise can significantly weaken this effect. These might be the reasons to generate the similar trend in both the SD + Exe group and HFD + Sed group. Additionally, human studies have shown that the different diets with different exercise load can have differential effects on the gut microbiota, thereby indicating that the relationship between them was complex.14 However, due to the lack of more relevant research, we still need metagenomics in combination with larger sample size to explore the detailed molecular mechanisms.
In recent years, the effects of physical exercise on the various intestinal diseases have been widely analyzed. Studies have confirmed that exercise can attenuate colonic inflammation and exhibit beneficial effects in preventing the obesity-associated colon cancer in the high-risk populations.32 Moreover, 4-week treadmill exercise can also promote the levels of ZO-1 and occludin in the elderly.33 Our findings indicate that 12-weeks’ moderate exercise can significantly alleviated colonic pathology and inhibit inflammatory cell infiltrate as well as the barrier damage. Meanwhile, exercise enhanced the expression of both colonic occludin and ZO-1 in obese mice induced by giving HFD. It is remarkable, however, that exercise significantly mitigated the reduction in the number of colonic GCs induced by HFD while decreasing the number of GCs conversely in chow diet mice that were subjected to exercise. The differential effects on the SD + Exe group and HFD + Exe group maybe synergistically caused by the dietary pattern, energy homeostasis and microbial-host immune response. These two diets are heterogenetic in constituent. Compared with the pellets offered to the HFD, chow diet are characterized as being rough and consists of higher fiber content; hence, colon needs to generate more GCs secreting mucus to assist in the propulsion for feces.34 Moreover, it was observed that in the chow diet condition, exercise can markedly increase the energy intake and then substantially strengthen the requirement of better digestion for food and rapid absorption of more nutrients. So, it can considerably weaken the response of proliferation of GCs that are caused by the stimulation of the fibers. HFD not only contains less fibers but also can effectively reduce glycosylated residuals in the GCs,35 elevate the enteral and circulating bile acid levels, such as deoxycholic acid, which are cytotoxic,36 thereby significantly decreasing the number of GCs. Thus, it is feasible that exercise may ameliorate these undesirable physiological and biochemical processes by balancing energy metabolism.37 In addition, the changes observed in the number of GCs also corresponded with the relative abundance of Akkermansia in the present study.
According to epidemiological studies, about 20–40% of the patients with IBD are obese. In addition, obesity might increase the risk of developing IBD because of its perpetual state of chronic low-grade inflammation. Furthermore, it also might increase the risk of complication and result in suboptimal response to therapy in patients with IBD.38,39 Meanwhile, another point to be noted is that unlike inflammatory disease with obvious symptoms and/or lesions, inflammation induced by damage of intestinal barrier integrity is a premorbid chronic process that occurs at microscopic level and is difficult to diagnose and treat efficiently. Therefore, given its excellent anti-inflammatory and anti-obesity effect, exercise not only can serve as an adjunct therapeutic approach to IBD patients40 but also can be effectively used to prevent IBD resulted from early inflammatory events induced by intestinal barrier damage in obesity.41,42 Nevertheless, still little is known about the biological mechanisms underlying these processes, so the study of its mechanisms may provide other possible ideas for developing novel strategies for both clinical diagnosis and treatment. AMPK is one of the most direct and sensitive effector molecules affected by exercise. A number of recent studies have revealed that both intestinal epithelial differentiation and barrier function are substantially damaged in mice with AMPKα1 gene specifically knocked out in IECs where villin is expressed.16 Activated AMPK and its effectors have been shown to possess the ability to promote assembly of TJs, accelerate reorganization of occludin and ZO-1, and improve the development of paracellular permeability. In addition, enhanced AMPK activation can substantially upregulate CDX2 expression and promote the differentiation of intestinal epithelial. In contrast, CDX2 deficiency can also effectively impair apical-basolateral polarity and morphogenesis in colonic epithelial cells to inhibit the enterocyte differentiation. Furthermore, 5-aminoimidazole-4-carboxamide riboside (AICAR) can promote CDX2 activity, while compound C can inhibit it, which can effectively function as AMPK activator and inhibitor, respectively.16 Additionally, a recently published study further presented an interesting link between gut microbiota structure and mitochondrial biological process mediated by AMPK activity.43 Li et al44 have reported that the higher fiber diet was ineffectual to the loss in higher proportions of Akkermansia and Bifidobacterium when compound C was added in the drinking water. Therefore, we investigated the effect of exercise on the colonic AMPK/CDX2 signaling pathway in obese mice induced by HFD. Similar to previous studies, we noticed that although there were no statistically significant differences of AMPK among different groups, exercise can significantly upregulate the phosphorylation of AMPK induced by HFD. Meanwhile, 12-weeks’ moderate exercise was found to markedly reverse the expression level of CDX2 protein. Although our hypotheses have been supported by the findings from this study, it still should be acknowledged that we need individually targeted methods like gene manipulation to further validate the major outcomes of this study.
Conclusion
In summary, we provide evidence that long-term moderate treadmill exercise not only can effectively modulate the colonic gut microbiota via increasing their variety and abundance but also can restore integrity of intestinal barrier by activating AMPK/CDX2 signaling pathway to enhance the expression of TJs proteins such as ZO-1 and occludin while significantly inhibiting weight accumulation in obese mice model induced by HFD.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (grant number 32170496).
Disclosure
Dr Haiji Sun report's grants from the National Natural Science Foundation of China, during the conduct of the study. The authors report no other potential conflicts of interest for this work.
References
1. Greenway FL, Smith SR. The future of obesity research. Nutrition. 2000;16(10):976–982. doi:10.1016/S0899-9007(00)00406-8
2. Rabot S, Membrez M, Bruneau A, et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010;24(12):4948–4959. doi:10.1096/fj.10-164921
3. Turnbaugh PJ, Bäckhed F, Fulton L, et al. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3(4):213–223. doi:10.1016/j.chom.2008.02.015
4. Ussar S, Griffin N, Bezy O, et al. Interactions between gut microbiota, host genetics and diet modulate the predisposition to obesity and metabolic syndrome. Cell Metab. 2015;22(3):516–530. doi:10.1016/j.cmet.2015.07.007
5. Ley RE, Turnbaugh PJ, Klein S, et al. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi:10.1038/4441022a
6. Bisanz JE, Upadhyay V, Turnbaugh JA, et al. Meta-analysis reveals reproducible gut microbiome alterations in response to a high-fat diet. Cell Host Microbe. 2019;26(2):265–272.e4. doi:10.1016/j.chom.2019.06.013
7. Hamilton MK, Boudry G, Lemay DG, et al. Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region dependent. Am J Physiol Gastrointest Liver Physiol. 2015;308(10):G840–G851. doi:10.1152/ajpgi.00029.2015
8. Mouries J, Brescia P, Silvestri A, et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J Hepatol. 2019;71(6):1216–1228. doi:10.1016/j.jhep.2019.08.005
9. Leech B, McIntyre E, Steel A, et al. Risk factors associated with intestinal permeability in an adult population: a systematic review. Int J Clin Pract. 2019;73(10):10. doi:10.1111/ijcp.13385
10. Thaiss CA, Levy M, Grosheva I, et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359(6382):1376–1383. doi:10.1126/science.aar3318
11. Żak-gołąb A, Kocełak P, Aptekorz M, et al. Gut microbiota, microinflammation, metabolic profile, and zonulin concentration in obese and normal weight subjects. Int J Endocrinol. 2013;2013:1–9. doi:10.1155/2013/674106
12. Damms-Machado A, Louis S, Schnitzer A, et al. Gut permeability is related to body weight, fatty liver disease, and insulin resistance in obese individuals undergoing weight reduction. Am J Clin Nutr. 2017;105(1):127–135. doi:10.3945/ajcn.116.131110
13. Dankel SJ, Loenneke JP, Loprinzi PD. Health outcomes in relation to physical activity status, overweight/ obesity, and history of overweight/obesity: a review of the WATCH paradigm. Sports Med. 2017;47(6):1029–1034. doi:10.1007/s40279-016-0641-7
14. Clarke SF, Murphy EF, O’Sullivan O, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63(12):1913–1920. doi:10.1136/gutjnl-2013-306541
15. Pasini E, Corsetti G, Assanelli D, et al. Effects of chronic exercise on gut microbiota and intestinal barrier in human with type 2 diabetes. Minerva Med. 2019;110(1):3–11. doi:10.23736/S0026-4806.18.05589-1
16. Sun X, Yang Q, Rogers CJ, et al. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ. 2017;24(5):819–831. doi:10.1038/cdd.2017.14
17. Ma X, Xu L, Alberobello A, et al. Celastrol protects against obesity and metabolic dysfunction through activation of a HSF1-PGC1α transcriptional axis. Cell Metab. 2015;22(4):695–708. doi:10.1016/j.cmet.2015.08.005
18. Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev. 2010;23(2):270–299. doi:10.1017/S0954422410000168
19. Schefer V, Talan MI. Oxygen consumption in adult and aged C57BL/6J mice during acute treadmill exercise of different intensity. Exp Gerontol. 1996;31(3):387–392. doi:10.1016/0531-5565(95)02032-2
20. Baker EJ, Gleeson TT. The effects of intensity on the energetics of brief locomotor activity. J Exp Biol. 1999;202(22):3081–3087. doi:10.1242/jeb.202.22.3081
21. Rohr MW, Narasimhulu CA, Rudeski-Rohr TA, et al. Negative effects of a high-fat diet on intestinal permeability: a review. Adv Nutr. 2019. doi:10.1093/advances/nmz061
22. Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi:10.2337/db06-1491
23. Kurashima Y, Kiyono H. Mucosal ecological network of epithelium and immune cells for gut homeostasis and tissue healing. Annu Rev Immunol. 2017;35(1):119–147. doi:10.1146/annurev-immunol-051116-052424
24. Liu B, Zhang J, Sun P, et al. Raw bowl tea (Tuocha) polyphenol prevention of nonalcoholic fatty liver disease by regulating intestinal function in mice. Biomolecules. 2019;9(9):435. doi:10.3390/biom9090435
25. Stoner L, Rowlands D, Morrison A, et al. Efficacy of exercise intervention for weight loss in overweight and obese adolescents: meta-analysis and implications. Sports Med. 2016;46(11):1737–1751. doi:10.1007/s40279-016-0537-6
26. Ley RE, Backhed F, Turnbaugh P, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–11075. doi:10.1073/pnas.0504978102
27. Evans CC, LePard KJ, Kwak JW, et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS One. 2014;9(3):e92193. doi:10.1371/journal.pone.0092193
28. Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi:10.1038/nature05414
29. Li D, Wang P, Wang P, et al. The gut microbiota: a treasure for human health. Biotechnol Adv. 2016;34(7):1210–1224. doi:10.1016/j.biotechadv.2016.08.003
30. Menni C, Jackson MA, Pallister T, et al. Gut microbiome diversity and high-fibre intake are related to lower long-term weight gain. Int J Obes. 2017;41(7):1099–1105. doi:10.1038/ijo.2017.66
31. Patra AK, Yu Z. Essential oils affect populations of some rumen bacteria in vitro as revealed by microarray (RumenBactArray) analysis. Front Microbiol. 2015;6. doi:10.3389/fmicb.2015.00297
32. Liu W, Wang T, Zhou F, et al. Voluntary exercise prevents colonic inflammation in high-fat diet-induced obese mice by up-regulating PPAR-γ activity. Biochem Biophys Res Commun. 2015;459(3):475–480. doi:10.1016/j.bbrc.2015.02.047
33. Shin HE, Kwak SE, Zhang DD, et al. Effects of treadmill exercise on the regulation of tight junction proteins in aged mice. Exp Gerontol. 2020;141:111077. doi:10.1016/j.exger.2020.111077
34. Scoaris CR, Rizo GV, Roldi LP, et al. Effects of cafeteria diet on the jejunum in sedentary and physically trained rats. Nutrition. 2010;26(3):312–320. doi:10.1016/j.nut.2009.04.012
35. Mastrodonato M, Calamita G, Mentino D, et al. High-fat diet alters the glycosylation patterns of duodenal mucins in a murine model. J Histochem Cytochem. 2020;68(4):279–294. doi:10.1369/0022155420911930
36. Huang D, Xiong M, Xu X, et al. Bile acids elevated by high-fat feeding induce endoplasmic reticulum stress in intestinal stem cells and contribute to mucosal barrier damage. Biochem Biophys Res Commun. 2020;529(2):289–295. doi:10.1016/j.bbrc.2020.05.226
37. Carbajo-Pescador S, Porras D, García-Mediavilla MV, et al. Beneficial effects of exercise on gut microbiota functionality and barrier integrity, and gut-liver crosstalk in an in vivo model of early obesity and non-alcoholic fatty liver disease. Dis Model Mech. 2019;12(5). doi:10.1242/dmm.039206
38. Singh S, Dulai PS, Zarrinpar A, et al. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017;14(2):110–121. doi:10.1038/nrgastro.2016.181
39. Harper JW, Zisman TL. Interaction of obesity and inflammatory bowel disease. World J Gastroenterol. 2016;22(35):7868. doi:10.3748/wjg.v22.i35.7868
40. Hashash JG, Binion DG. Exercise and Inflammatory Bowel Disease. Gastroenterol Clin North Am. 2017;46(4):895–905. doi:10.1016/j.gtc.2017.08.010
41. Bilski J, Mazur-Bialy A, Brzozowski B, et al. Can exercise affect the course of inflammatory bowel disease? Experimental and clinical evidence. Pharmacol Rep. 2016;68(4):827–836. doi:10.1016/j.pharep.2016.04.009
42. Bilski J, Brzozowski B, Mazur-Bialy A, et al. The role of physical exercise in inflammatory bowel disease. Biomed Res Int. 2014;2014:1–14.
43. Clark A, Mach N. The crosstalk between the gut microbiota and mitochondria during exercise. Front Physiol. 2017;8. doi:10.3389/fphys.2017.00319
44. Li Q, Zhang M, Wu T, et al. Potential correlation between carbohydrate-active enzyme family 48 expressed by gut microbiota and the expression of intestinal epithelial AMP-activated protein kinase β. J Food Biochem. 2020;44(2). doi:10.1111/jfbc.13123
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.