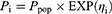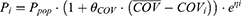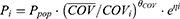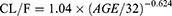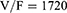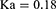Back to Journals » Drug Design, Development and Therapy » Volume 15
Modeling and Simulation for Individualized Therapy of Amisulpride in Chinese Patients with Schizophrenia: Focus on Interindividual Variability, Therapeutic Reference Range and the Laboratory Alert Level
Authors Huang S, Li L, Wang Z, Xiao T, Li X, Liu S, Zhang M, Lu H, Wen Y, Shang D
Received 12 July 2021
Accepted for publication 31 August 2021
Published 14 September 2021 Volume 2021:15 Pages 3903—3913
DOI https://doi.org/10.2147/DDDT.S327506
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Shanqing Huang,1,* Lu Li,1,2,* Zhanzhang Wang,1,2 Tao Xiao,1 Xiaolin Li,1 Shujing Liu,1 Ming Zhang,1,2 Haoyang Lu,1,2 Yuguan Wen,1,2 Dewei Shang1,2
1Department of Pharmacy, The Affiliated Brain Hospital of Guangzhou Medical University, Guangzhou, 510370, People’s Republic of China; 2Guangdong Engineering Technology Research Center for Translational Medicine of Mental Disorders, Guangzhou, 510370, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dewei Shang; Yuguan Wen
Department of Pharmacy, The Affiliated Brain Hospital of Guangzhou Medical University, 36 Mingxin Road, Guangzhou, 510370, People’s Republic of China
Tel +86-020-81268389
Fax +86-020-81891391
Email [email protected]; [email protected]
Purpose: To explain the high inter-individual variability (IIV) and the frequency of exceeding the therapeutic reference range and the laboratory alert level of amisulpride, a population pharmacokinetic (PPK) model in Chinese patients with schizophrenia was built based on therapeutic drug monitoring (TDM) data to guide individualized therapy.
Patients and Methods: Plasma concentration data (330 measurements from 121 patients) were analyzed using a nonlinear mixed-effects modeling (NONMEM) approach with first-order conditional estimation with interaction (FOCE I). The concentrations of amisulpride were detected by HPLC-MS/MS. Age, weight, sex, combination medication history and renal function status were evaluated as main covariates. The model was internally validated using goodness-of-fit, bootstrap and normalized prediction distribution error (NPDE). Recommended dosage regimens for patients with key covariates were estimated on the basis of Monte Carlo simulations and the established model.
Results: A one-compartment model with first-order absorption and elimination was found to adequately characterize amisulpride concentration in Chinese patients with schizophrenia. The population estimates of the apparent volume of distribution (V/F) and apparent clearance (CL/F) were 12.7 L and 1.12 L/h, respectively. Age significantly affected the clearance of amisulpride and the final model was as follows: CL/F=1.04×(AGE/32)− 0.624 (L/h). To avoid exceeding the laboratory alert level (640 ng/mL), the model-based simulation results showed that the recommended dose of amisulpride was no more than 600 mg/d for patients aged 60 years, 800 mg/d for those aged 40 years and 1200 mg/d for those aged 20 years, respectively.
Conclusion: Dosage optimization of amisulpride can be carried out according to age to reduce the risk of adverse reactions. The model can be used as a suitable tool for designing individualized therapy for Chinese patients with schizophrenia.
Keywords: amisulpride, population pharmacokinetics, therapeutic drug monitoring, modeling and simulation, individualized therapy
Introduction
Amisulpride, a substituted benzamide derivative, is a second-generation antipsychotic and a highly selective antagonist, which binds to dopamine D2/D3 receptors.1 Given its efficacy and tolerance, amisulpride has been suggested as a first-line treatment option in the management of schizophrenia.2 However, high inter-individual variability (IIV) of amisulpride pharmacokinetics has been observed in patients, and overdosing may lead to serious adverse reaction.3 Therapeutic drug monitoring (TDM) is the use of whole-blood plasma or serum drug (or metabolite) concentration measurements to assess adherence, guide dosage, and the highest possible probability of response and a minimized risk of adverse drug reactions/toxicity. The Arbeitsgemeinschaft für Neuropsychopharmacology und Pharmakopsychiatrie (AGNP) consensus guidelines considered TDM for amisulpride to be strongly recommended (level 1). The therapeutic reference range for amisulpride is 100–320 ng/mL, with a laboratory alert level of 640 ng/mL.4,5
The apparent distribution volume, protein binding rate and absolute bioavailability of amisulpride were 5.8 L/kg, 16% and 48%, respectively. After repeated administration, amisulpride did not accumulate in vivo and the pharmacokinetic parameters did not change with dosage. The elimination half-life was approximately 12–16 h. After intravenous administration, 50% of the drug was excreted as prototypes, most of them were excreted within 24 h (90% of urine excretion).6 Amisulpride was mainly metabolized into two inactive metabolites, accounting for 4% of the excreta.
Amisulpride is almost entirely renally eliminated, without hepatic metabolism or known interactions. Thus, CYP450 enzyme activities or metabolic enzyme gene polymorphisms are unlikely to occur with amisulpride.7 In addition, amisulpride is a substrate of SLC22 organic ion transporters in the kidneys, which suggests that active renal secretion is likely to be the major elimination pathway.8
Population pharmacokinetic (PPK) analysis is a robust tool for obtaining pharmacokinetic (PK) parameters from both sparsely and intensively sampled data. The influence of covariates on PK parameters can be quantified.9 PPK analysis has previously been applied to drugs with high IIV.10 One study in Caucasian adult patients investigated the effects of concomitant medications, genetic polymorphisms (such as the OCT1, OCT2 transporters, P-glycoprotein and some nuclear factors), age and lean body weight (LBW) on amisulpride concentration and included age and LBW in the final model.11 Another study in older people and Alzheimer’s disease found that age and body weight significantly affected amisulpride clearance at low dose (25–75 mg daily).12 In addition, two studies reported a higher dose-adjusted concentrations (C/D, the ratio of drug concentration to dose under steady-state and trough conditions) in elderly patients,13,14 which showed that age is most likely to be the major influence on amisulpride.
In the present study, a population PK (PPK) model for analyzing PK characteristics of amisulpride based on routine TDM data collected from Chinese patients with schizophrenia was built without a fixed absorption rate constant. After building the model and evaluating the correlation of covariates, the PK characteristics of amisulpride under different dosage regimens were simulated to clarify the relationship between steady-state concentration and the therapeutic reference range/laboratory alert level of amisulpride. The aim of this study was to make the best use of retrospective TDM data to guide individualized treatment of amisulpride in Chinese patients with schizophrenia.
Materials and Methods
Data Collection
A retrospective study of TDM data from psychiatric inpatients treated with amisulpride in the Affiliated Brain Hospital of Guangzhou Medical University was performed from 2019 to 2020.
Patients who met the following criteria were included in the analysis: 1) those who took oral amisulpride with serum drug concentration monitoring; 2) multiple serum concentration data were obtained under different dosage schemes; 3) patients who had hospital information recorded in medical records. The exclusion criteria included 1) problems with patient compliance; 2) wrong sampling time; and 3) concentrations of amisulpride were zero or below the lower limit of quantification.
Demographic data (age, sex, body weight, height), dosage regimen, serum amisulpride concentration, renal function (creatinine, creatinine clearance rate, uric acid, retinol-binding protein, urea, β2-microglobulin and cystatin C), glucolipid metabolism (cholinesterase, glucose, glycated serum protein, glycosylated hemoglobin, triglyceride, total cholesterol, low-density lipoprotein and high-density lipoprotein), and combination medication history (valproic acid, omeprazole, clozapine, lithium) were collected.
Bioanalysis
Serum amisulpride concentration was determined using a Shimadzu 20A HPLC system consisting of two LC-20AD pumps, a DGU-20A3R degassing unit, a SIL-20A autosampler, and a CTO-20A column oven (Shimadzu Corporation, Kyoto, Japan). MS detection was carried out using a Shimadzu LCMS-8040 triple-quad mass spectrometer (Shimadzu Corporation) equipped with an electrospray ionization source operating in the positive mode (ESI+). Quantification was performed using the multiple-reaction monitoring mode. Amisulpride (purity 99.9%) and amisulpride-d5 isotope (purity 98.0%) were produced by Toronto Research Chemicals (Toronto, Canada). Analytes were extracted using the acetonitrile protein precipitation method.
The separation was operated on an Agilent Eclipse XDB C18 column (4.6×50 mm, 1.8 µm) at 35°C. Using an electrospray ion source and multiple reaction monitoring (MRM) mode, the ion pairs used for quantitative analysis were m/z 370.2→m/z 242.1 (amisulpride), m/z 375.2→m/z 242.1 (amisulpride-d5). The mobile phase consisted of methanol-water (90:10, v/v, 2 mmol·L−1 ammonium formate), and the flow rate was 0.7 mL·min−1. The standard curve equation for amisulpride was y = 1.36x+3.20×10−3 (R2 > 0.99), and the calibration curve was 20, 40, 400, 800, 1200, 1600, and 2000 ng·mL−1. The linear range of amisulpride was from 20 to 2000 ng·mL−1, the relative standard deviation (RSD) of intra-day and inter-day precisions ranged from 1.92% to 13.77%, the recovery rates ranged from 92.95% to 103.84%, and the stability was good.
Software
We performed the PPK analysis through nonlinear mixed-effects modeling with NONMEM® (version 7, Level 2, Icon Development Solutions, Ellicott City, MD, USA). Subroutine ADVAN 2 and first-order conditional estimation with interaction option (FOCE-I) method were used in modeling. PsN (version 3.4.2, Uppsala University, Sweden) was applied for model construction and validation. The diagnostic plots of NONMEM® outputs were conducted using R version 4.0.5 (http://www.r-project.org).
Population Pharmacokinetics Model and Simulations
Population Pharmacokinetics Model
A one-compartment model with first-order absorption and elimination was evaluated to fit the concentration versus time data. The IIV was assessed using exponential random effects models as shown in Eq. 1.
where Pi is the estimated parameter for the ith individual; Ppop is the population typical value of the parameter; and ηi represents the difference in the estimated parameter for the ith subject from the population typical value, which was identically distributed with a mean of zero and a variance of ω2.
In this study, both continuous covariates and binary covariates were investigated with a fractional model. The relationship between the population typical value and a continuous covariate (such as age and weight) was evaluated using Eq.2- Eq.3.
where Pi is the parameter for individual i; COVi and 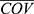 is the covariate value for individual i and the mean (or median) of covariate, respectively.
is the covariate value for individual i and the mean (or median) of covariate, respectively.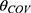 is the factor for adjusting the ith PK parameter.
is the factor for adjusting the ith PK parameter.
The binary covariates (such as sex and comedications) were evaluated using Eq.4. For the sex covariate: COVi = 0 represented a female, COVi = 1 represented a male.
The identification of significant covariates was performed through forward inclusion in which an objective function value (OFV) decreased of more than 6.63 from the base model was significant (chi-square P < 0.01, df = 1) and backward exclusion in which a ΔOFV increased by more than 10.83 was significant (chi-square, P < 0.001, df = 1). Each covariate was incorporated stepwise into the base model to develop the full models. For the final model, a backward elimination process was employed to identify significant covariates. The covariates in the full model were excluded one by one.
Model Validation
The OFV value, goodness-of-fit plots, plausibility and precision of parameter estimates, random and residual variances for the parameters and physiologically plausible were used to evaluate and validate the different models. The 95% confidence intervals of the parameters were evaluated through nonparametric bootstrapping without stratification (n = 1000). The normalized prediction distribution error (NPDE) method, which is suitable for evaluating models developed from disordered data, can be used for internal or external evaluation. The NPDE for each observation was calculated using R software with an attached package, NPDE (version 2.0), based on 1000 simulations. R software was also used to plot the NPDE diagrams.
Model-Based Simulations
In the model-based simulations, the final PPK model was used to simulate the time course of amisulpride serum concentrations at steady state by typical parameter estimates under regular oral administration of commonly used dosage regimens [BID (100 mg, 200 mg), BID (200 mg, 200 mg), BID (200 mg, 400 mg), BID (400 mg, 400 mg), BID (400 mg, 600 mg) or BID (600 mg, 600 mg)]. The simulated concentrations, represented by population predicted values, were compared with the upper boundary of the recommended therapeutic range (320 ng/mL) and the laboratory alert level (640 ng/mL) to serve as a tool for treatment regimen selection for different clinical populations.
Results
Patient Characteristics
A total of 330 amisulpride serum concentration data sets were collected from 121 patients (63 females, 58 males). The demographic (such as age and gender) and clinical characteristics (such as concomitant medications and renal function) of the patients were summarized in Table 1.
 |
Table 1 Clinical and Demographic Characteristics of Patients in TDM Analysis of Amisulpride 2019.1–2020.12 |
Population Pharmacokinetic Model
The PPK model of amisulpride was best characterized using a one-compartment model with first-order elimination. Residual variabilities were best described by a proportional error model. The final PPK parameters were summarized in Table 2. In the final model, age was identified as a significant covariate for the clearance of amisulpride which caused a significant decrease in OFV (ΔOFV = 44.65, P < 0.001). The estimated typical values of CL/F, θCL-AGE, V/F and Ka were 61.1 L/h, −0.624, 1720 L and 0.18 h-1, respectively. The relationship between clearance and age was shown in Figure 1 (the scatter plots of all covariates versus CL or V, both in base and final model were shown in Supplemental Figure 1).
 |
Table 2 Population PK Parameters Estimates and Bootstrap Results of the Final PK Model for TDM Data Set |
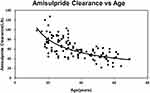 |
Figure 1 Age effect on amisulpride: clearance decreases exponentially with increasing age (y=488.63x−0.605, R2=0.4614). |
The final model can be described by the following equations:
Model Evaluation
The goodness-of-fit plots for the final PPK model for the TDM data set were shown in Figure 2. No significant deviation was found between observations and population predictions or individual predictions, indicating a good fit for the final model. In addition, there were no obvious trends in conditional weighted residuals vs time and conditional weighted residuals vs population predictions. And the QQ plots of CWRES for base and final model were displayed in Supplemental Figure 2. There was a deviation in the QQ diagram of CWRES between the basic model and the final model. Compared with the basic model, the QQ diagram of CWRES in the final model was improved.
The final PPK parameters, the standard errors, and the 95% CI on the basis of 1000 bootstraps were summarized in Table 2. All the parameters were relatively stable (RSE <30%) and the estimated mean values were close to the bootstrap results. The typical values of parameters from the final model all fell into the 95% confidence interval.
The PPK model of amisulpride was evaluated using the NPDE method to validate the predictions and the results of NPDE were shown in Figure 3. The cumulative distribution of amisulpride was obtained for each observation with 1000 simulations. The NPDE was distributed around a mean of 0.088 with a variance of 1.18. There was no trend in NPDE vs time and NPDE vs predicted amisulpride concentration. These results indicated that the PPK model of amisulpride was relatively accurate and reliable.
Model-Based Simulations for Conventional Amisulpride Doses
The typical time courses of steady state amisulpride concentrations were simulated in psychotic patients in their 20s, 40s, and 60s after different dosage regimens according to the final model (Figure 4). According to the Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology, the therapeutic reference range of amisulpride is 100–320 ng/mL. In order to maintain the concentration in the therapeutic reference range and not exceed the upper limit of the reference range (320 ng/mL), the model-based simulation results showed that the recommended dose of amisulpride was no more than 300 mg/d for those aged 60 years, 400 mg/d for those aged 40 years and 600 mg/d for those aged 20 years. In order to avoid exceeding the laboratory alert level (640 ng/mL), the model-based simulation results showed that the recommended dose of amisulpride was no more than 600 mg/d for those aged 60 years, 800 mg/d for those aged 40 years and 1200 mg/d for those aged 20 years, respectively. The model-based simulation results also showed that the serum concentration of amisulpride in patients aged 60 years was twice as high as that in those aged 20 years under the same administration scheme, which indicated that the serum concentration of amisulpride is more likely to exceed the therapeutic reference range or even exceed the laboratory alert level under the same routine administration scheme in elderly people.
Discussion
To our knowledge, this is the first report of the PPK of amisulpride in Chinese patients with schizophrenia to evaluate the therapeutic reference range (100–320 ng/mL) and laboratory alert level (640 ng/mL) for individualized medication recommendations simultaneously. Based on our previous exploration and research,5,13 the mean dosage-adjusted serum concentration (concentration/daily dose) of amisulpride was consistent with the AGNP guidelines,4 suggesting little difference in pharmacokinetics between races. The PPK model was used to quantify the effects of age on the clearance of amisulpride and to predict the concentrations of the compound at different dosage levels in Chinese patients with schizophrenia.
In a previous study, Reeves, S et al12,15 explored the use of amisulpride in the treatment of Alzheimer’s disease with a low dose (25–75 mg) for elderly patients, which is not suitable for Chinese psychotic patients as it is usually indicated for schizophrenia, which requires a wide range of recommended daily doses (50–1200 mg) for all age groups. In our study, there were only two patients who were younger than 18 years old. The sample size was insufficient to build the model for children. In addition, amisulpride is almost entirely renally eliminated. The youngest patient was 13 years old, in which kidney function has matured. Therefore, the research did not initially exclude patients aged from 13 to 18 years. Our study screened covariates with high standards (P < 0.001) and found that the most significant was the θCL-AGE as increasing age decreased amisulpride elimination. In agreement with this, Glatard, A et al11 reported that LBW was also a significant covariate for the CL/F of amisulpride. LBW with an allometric exponent of ~2/3 may be most suitable for describing an increase in CL with body size as it accounts for both body composition and allometric scaling principles concerning differences in metabolic rates across size.16 However, in our study, the effect of weight was attenuated after including age as a covariate since both age and body weight were needed to calculate the creatinine clearance.17 When the simulation data were compared, we found that the simulation results in our study were higher than those in their study under the same dosage regimen. According to a meta-analysis,5 pooled concentration levels of amisulpride were higher than the recommendation with wide individual variation, especially in older patients, female patients and patients taking amisulpride combined with lithium. In this study, combined lithium did not have a significant impact for the limited number of patients with combination medications. The mean concentration of amisulpride was 430.64 ± 263.39 ng/mL, which markedly exceeded the reference concentration range (100–320 ng/mL). Amisulpride showed large IIV and a considerable percentage of patients had concentration levels outside the reference range.18 Approximately 36% of the patients had amisulpride plasma levels higher than 320 ng/mL in the UK,19 which was 54.4% in a study conducted in China.13 A retrospective analysis of 253 samples was carried out to investigate the conformance of serum levels of olanzapine, aripiprazole, paliperidone, ziprasidone and amisulpride with the AGNP therapeutic reference ranges in Chinese patients. Good consistency was observed for the other four antipsychotics, while the plasma concentration of amisulpride was 445.2 ± 231.5 ng/mL, higher than the recommended range, which comparable with our research.20
The elimination of amisulpride was not affected by CYP enzymes as renal excretion was the main elimination pathway for this drug.21 Transporters in proximal renal tubules contribute to the disposition of numerous drugs. Amisulpride is a substrate of SLC22 transporters (organic cation transporters, such as OCT1, OCT2, OCT3, OCTN1, OCTN2) expressed in organs.8 Only a study using an animal model suggested that kidney transporter expression is affected by ontogeny and aging.22 No in vivo data on the implications of these transporters on amisulpride PK are available.23 Amisulpride is also a substrate of the P-glycoprotein (P-gp) transporter.24 Moreover, Glatard, A et al11 proved that OCT1 and OCT2 transporters, the P-glycoprotein, and some nuclear factors had no significant influence on the clearance of amisulpride. Therefore, amisulpride did not need to be adjusted according to genotype and transporters.
To date, only one study has provided evidence that amisulpride plasma levels of 100–320 ng/mL could be regarded as a useful therapeutic range to avoid both clinical nonresponse and EPS by receiver operating characteristic analysis.25 However, the dosage regimen was once daily in this study, which is significantly different from the various dosage regimens in clinical application. Furthermore, inherent to statistical methods and to the variability of clinical response, some patients are non-responders with plasma concentrations in this range (9% in the study population of Muller et al) and require amisulpride concentrations higher than 320 ng/mL to obtain a therapeutic response. Therefore, the concentrations simulated at high doses of amisulpride are very useful, especially in elderly individuals.
In clinical use, the starting dose for treatment of positive symptoms of schizophrenia is 400–800 mg/d, and is 50–300 mg/d for those experiencing predominantly negative symptoms.26 Individualized treatment is not provided by the guidelines. For this, we propose that the treatment strategy should simulate the distribution of serum concentration in patients of different ages under a conventional dose of amisulpride. Using this method, we found that patients in their 20s take twice as much as patients in their 60s to achieve the same trough concentration. It is not recommended that adolescents with schizophrenia take amisulpride in high doses due to high clearance. Patients in their 60s who take more than 600 mg/d are prone to exceeding the laboratory alert level, which will increase the risk of adverse reactions. Attention should be paid to the elderly to control the dose of amisulpride to prevent high serum concentrations resulting in drug poisoning. These predictions could prove valuable for dose adjustment during clinical maintenance in these patient groups.
In this study, age was the only covariate contributing to inter-individual variations in Chinese psychiatric patients aged 13–69 years. Other factors including gender, body weight, concomitant medications, renal function and glucolipid metabolism were not significant. Amisulpride concentrations could be well predicted by a concise PPK model with dosage regimens and age as influencing factors. Furthermore, the wide range of recommended daily doses (50–1200 mg) of amisulpride would inevitably lead to broad plasma concentrations, especially in patients with acute schizophrenic episodes or with severe positive symptoms who generally require higher doses. High concentrations of amisulpride were correlated with certain adverse events in specific groups of patients, eg, hyperprolactinemia in females or hypotension in elderly patients. In this case, whether to change the prescribing drug or adjust its dosage regimen should be carefully evaluated by its benefits and risks. Medical decisions should not be restricted to the reference concentration range given by AGNP, but under comprehensive considerations including, but not limited to, patient age, therapy response, physical disorders and adverse events.
There were some limitations in this study. Firstly, the current study included a relatively small proportion of older patients, which limited the prediction precision of the final model for Chinese elderly patients. Although the population model for amisulpride CL/F and V/F adequately illustrates the observed data, we suggest that it may be more suitable for patients aged 13–69 years. Secondly, the association of effectiveness and drug safety with serum concentration has not been explained. Thirdly, the sample size in this study was small. In spite of the above limitations, this study still provides a guide on the relationship between amisulpride dosage and serum concentration, a partial reason for the variation among individuals and the design of individualized treatment.
Conclusion
In conclusion, this one-compartment model was validated to have good predictability for amisulpride concentrations in Chinese patients with schizophrenia. Age was identified as a significant covariate affecting the clearance of amisulpride. The study predicted a reasonable concentration range of amisulpride in different age groups under conventional therapy. These findings may account for part of the variability in amisulpride exposure, and the dosage regimen in Chinese populations may need to be individualized for patients with schizophrenia based on age.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of the Affiliated Brain Hospital of Guangzhou Medical University (approval number: 2021-027). All patients in the study gave written informed consent after admission. We confirm that this study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
This work was supported by Bureau of Guangdong Province Traditional Chinese Medicine Bureau Research Project (No.20201272), Bureau of Health and Family Planning Science and Technology Project of Guangzhou City (No.20211A011043), Guangzhou Science and technology project (202002030399), Science and Technology Plan Project of Guangdong Province (2019B030316001), and Guangzhou Municipal Key Discipline in Medicine (2021–2023). Shanqing Huang and Lu Li are co-first authors of this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Curran MP, Perry CM. Spotlight on amisulpride in schizophrenia. CNS Drugs. 2002;16(3):207–211. doi:10.2165/00023210-200216030-00007
2. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. doi:10.1016/S0140-6736(13)60733-3
3. Mauri MC, Volonteri LS, Colasanti A, et al. Clinical pharmacokinetics of atypical antipsychotics: a critical review of the relationship between plasma concentrations and clinical response. Clin Pharmacokinet. 2007;46(5):359–388. doi:10.2165/00003088-200746050-00001
4. Hiemke C, Bergemann N, Clement HW, et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1–2):e1. doi:10.1055/s-0037-1600991
5. Li L, Li L, Shang DW, et al. A systematic review and combined meta-analysis of concentration of oral amisulpride. Br J Clin Pharmacol. 2020;86(4):668–678. doi:10.1111/bcp.14246
6. Rosenzweig P, Canal M, Patat A, et al. A review of the pharmacokinetics, tolerability and pharmacodynamics of amisulpride in healthy volunteers. Hum Psychopharmacol. 2002;17(1):1–13. doi:10.1002/hup.320
7. Bergemann N, Kopitz J, Kress KR, et al. Plasma amisulpride levels in schizophrenia or schizoaffective disorder. Eur Neuropsychopharmacol. 2004;14(3):245–250. doi:10.1016/j.euroneuro.2003.09.001
8. Dos Santos Pereira JN, Tadjerpisheh S, Abu Abed M, et al. The poorly membrane permeable antipsychotic drugs amisulpride and sulpiride are substrates of the organic cation transporters from the SLC22 family. AAPS J. 2014;16(6):1247–1258. doi:10.1208/s12248-014-9649-9
9. Kiang TK, Sherwin CM, Spigarelli MG, et al. Fundamentals of population pharmacokinetic modelling: modelling and software. Clin Pharmacokinet. 2012;51(8):515–525. doi:10.1007/BF03261928
10. Sheng L, Qu Y, Yan J, et al. Population pharmacokinetic modeling and simulation of huperzine A in elderly Chinese subjects. Acta Pharmacol Sin. 2016;37(7):994–1001. doi:10.1038/aps.2016.24
11. Glatard A, Guidi M, Delacrétaz A, et al. Amisulpride: real-world evidence of dose adaptation and effect on prolactin concentrations and body weight gain by pharmacokinetic/pharmacodynamic analyses. Clin Pharmacokinet. 2020;59(3):371–382. doi:10.1007/s40262-019-00821-w
12. Reeves S, Bertrand J, D’Antonio F, et al. A population approach to characterise amisulpride pharmacokinetics in older people and Alzheimer’s disease. Psychopharmacology(Berl). 2016;233(18):3371–3381. doi:10.1007/s00213-016-4379-6
13. Wang ZZ, Jia NX, Yang LH, et al. Research on therapeutic drug monitoring and clinical application of amisulpride tablets. Chinese J Clin Pharmacol. 2018;34:2704–2706.
14. Müller MJ, Eich FX, Regenbogen B, et al. Amisulpride doses and plasma levels in different age groups of patients with schizophrenia or schizoaffective disorder. J Psychopharmacol. 2009;23(3):278–286. doi:10.1177/0269881108089806
15. Reeves S, Bertrand J, McLachlan E, et al. A population approach to guide amisulpride dose adjustments in older patients with Alzheimer’s disease. J Clin Psychiatry. 2017;78(7):e844–e851. doi:10.4088/JCP.16m11216
16. McLeay SC, Morrish GA, Kirkpatrick CM, et al. The relationship between drug clearance and body size: systematic review and meta-analysis of the literature published from 2000 to 2007. Clin Pharmacokinet. 2012;51(5):319–330. doi:10.2165/11598930-000000000-00000
17. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi:10.1159/000180580
18. Jönsson AK, Spigset O, Reis M. A compilation of serum concentrations of 12 antipsychotic drugs in a therapeutic drug monitoring setting. Ther Drug Monit. 2019;41(3):348–356. doi:10.1097/FTD.0000000000000585
19. Bowskill SV, Patel MX, Handley SA, et al. Plasma amisulpride in relation to prescribed dose, clozapine augmentation, and other factors: data from a therapeutic drug monitoring service, 2002–2010. Hum Psychopharmacol. 2012;27(5):507–513. doi:10.1002/hup.2256
20. Wang ST, Li Y. Development of a UPLC-MS/MS method for routine therapeutic drug monitoring of aripiprazole, amisulpride, olanzapine, paliperidone and ziprasidone with a discussion of their therapeutic reference ranges for Chinese patients. Biomed Chromatogr. 2017;31:8. doi:10.1002/bmc.3928
21. Schoretsanitis G, Paulzen M, Unterecker S, et al. TDM in psychiatry and neurology: a comprehensive summary of the consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology, update 2017; a tool for clinicians. World J Biol Psychiatry. 2018;19(3):162–174. doi:10.1080/15622975.2018.1439595
22. Xu YJ, Wang Y, Lu YF, et al. Age-associated differences in transporter gene expression in kidneys of male rats. Mol Med Rep. 2017;15(1):474–482. doi:10.3892/mmr.2016.5970
23. Ivanyuk A, Livio F, Biollaz J, et al. Renal drug transporters and drug interactions. Clin Pharmacokinet. 2017;56(8):825–892.
24. Schmitt U, Abou El-Ela A, Guo LJ, et al. Cyclosporine A (CsA) affects the pharmacodynamics and pharmacokinetics of the atypical antipsychotic amisulpride probably via inhibition of P-glycoprotein (P-gp). J Neural Transm. 2006;113(7):787–801. doi:10.1007/s00702-005-0367-4
25. Müller MJ, Regenbogen B, Härtter S, et al. Therapeutic drug monitoring for optimizing amisulpride therapy in patients with schizophrenia. J Psychiatr Res. 2007;41(8):673–679. doi:10.1016/j.jpsychires.2005.10.003
26. Linden M, Scheel T, Xaver Eich F. Dosage finding and outcome in the treatment of schizophrenic inpatients with amisulpride. Results of a drug utilization observation study. Hum Psychopharmacol. 2004;19(2):111–119. doi:10.1002/hup.574
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.