Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
MMSE Subscale Scores as Useful Predictors of AD Conversion in Mild Cognitive Impairment
Authors Choe YM , Lee BC , Choi IG , Suh GH , Lee DY , Kim JW
Received 20 May 2020
Accepted for publication 26 June 2020
Published 24 July 2020 Volume 2020:16 Pages 1767—1775
DOI https://doi.org/10.2147/NDT.S263702
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Taro Kishi
Young Min Choe,1,2 Boung Chul Lee,2,3 Ihn-Geun Choi,4 Guk-Hee Suh,1,2 Dong Young Lee,5 Jee Wook Kim1,2 On behalf of the Alzheimer’s Disease Neuroimaging Initiative
1Department of Neuropsychiatry, Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Gyeonggi, Republic of Korea; 2Department of Psychiatry, Hallym University College of Medicine, Chuncheon, Republic of Korea; 3Department of Neuropsychiatry, Hallym University Hangang Sacred Heart Hospital, Seoul, Republic of Korea; 4Department of Psychiatry, Seoul W Psychiatric Office, Seoul, Republic of Korea; 5Department of Neuropsychiatry, Seoul National University Hospital, Seoul, Republic of Korea
Correspondence: Jee Wook Kim
Department of Neuropsychiatry, Hallym University Dongtan Sacred Heart Hospital, 7 Keunjaebong-gil, Hwaseong-si, Gyeonggi-do 18450, Republic of Korea
Tel +82 31 8086 2340
Fax +82 31 8086 2029
Email [email protected]
Purpose: This study was performed to examine the usefulness of subscores on the Mini-Mental State Examination (MMSE) for predicting the progression of Alzheimer’s disease (AD) dementia in individuals with mild cognitive impairment (MCI).
Patients and Methods: A total of 306 MCI individuals in the Alzheimer’s Disease Neuroimaging Initiative database were included in the study. Standardized clinical and neuropsychological tests were performed at baseline and at 2-year follow-up. Logistic regression analysis was conducted to examine the MMSE total and subscale scores to predict progression to AD dementia in MCI individuals.
Results: The MMSE total score and the MMSE memory, orientation, and construction subscores were inversely associated with AD progression after controlling for all potential confounders; MMSE attention and language subscores were not correlated with AD progression. The MMSE delayed recall score among the MMSE memory subscores and the MMSE time score among the orientation subscores, especially week and day, were inversely associated with AD progression; the MMSE immediate recall and place scores were not correlated with progression.
Conclusion: Our findings suggest that the MMSE memory, orientation, and construction subscores, which are simple and readily available clinical measures, could provide useful information to predict AD dementia progression in MCI individuals in practical clinical settings.
Keywords: mini-mental state examination, MMSE, mild cognitive impairment, MCI, Alzheimer’s disease, AD, memory, orientation, construction
Introduction
Mild cognitive impairment (MCI) is classified as a transitional state between normal aging and mild dementia.1 Longitudinal studies have found that the annual risk of conversion from MCI to probable Alzheimer’s disease (AD) was 10–15%.2,3 Due to the high risk of conversion to AD, MCI has become a major concern for early detection of AD to initiate preventive measures. However, it is difficult to predict progression from MCI to AD, and a significant portion of MCI individuals remain stable or return to a normal cognitive state.4,5
A number of studies have attempted to identify useful predictors of conversion from MCI to AD, including a number of neuropsychological markers,6–8 neuroimaging biomarkers,9–11 genetic markers,12–14 and biochemical markers15–18 alone and in various combinations.19,20 Especially, neuroimaging biomarkers, including magnetic resonance imaging (MRI) and positron emission tomography (PET) with fluorodeoxyglucose and beta amyloid tracers, showed high sensitivity and specificity.21 However, they are expensive, are feasible only in specialized medical centers,22 and are not appropriate for use in primary care settings, routine bedside check-ups, preventive health care settings, and large community-based studies.
It is important to identify a relatively simple, time-saving, and cost-effective predictor of AD conversion that can be easily used in a practical clinical setting. Previous studies suggested that the Mini-Mental State Examination (MMSE),23 Clinical Dementia Rating (CDR) Sum of Boxes (CDR-SB),24 and CDR orientation score25 were good candidates for such a simple clinical predictor of AD progression. In this study, we examined the use of specific domains of the MMSE as potential markers of AD conversion. The MMSE has long been widely used as a tool to screen for cognitive impairment.26 The MMSE total score comprises subscores representing each cognitive domain: memory, orientation, attention, language, and construction. Several studies have reported the usefulness of MMSE subscores. More rapid decline in the MMSE language subscore was observed in both language and behavioral variants of frontotemporal degeneration,27 and MMSE subscores were helpful in differentiating between dementia with Lewy bodies and AD.28
Not all domains of cognitive function deteriorate at the same time during the period of early cognitive changes in AD. In general, the decline of non-memory areas and related functions follow decline of episodic memory.29 Therefore, specific MMSE domains other than memory may be useful for predicting conversion to AD in MCI individuals. However, little is known about this issue. This study was performed to investigate the usefulness of MMSE total and subscale scores for predicting AD dementia progression within a 2-year follow-up period in elderly individuals with MCI.
Materials and Methods
Study Design and Participants
Demographic information and clinical data used in this study were obtained from the Alzheimer’s Disease Neuroimaging Initiative 1 (ADNI-1) database (http://adni.loni.usc.edu) on 2 February 2015. The ADNI was launched in 2003 as a public–private partnership led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial MRI, PET, and other biological markers along with clinical and neuropsychological assessments can be combined to measure the progression of MCI and early AD. For up-to-date information, see http://www.adni-info.org. The study protocol was approved by the institutional review board of each participating site, and written informed consent was obtained from all participants. The complete list of ADNI sites’ IRBs can be found at:
http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
From the ADNI-1 cohort, this study included 306 participants who were MCI at baseline evaluation and had at least one 2-year follow-up visit. All individuals with MCI met the current consensus criteria for amnestic MCI:30 a memory complaint by the subject or their representative, objective memory loss measured by education-adjusted scores on the Wechsler Memory Scale Logical Memory II, absence of significant levels of impairment in other cognitive domains, essentially preserved activities of daily living, and an absence of dementia. All of the MCI subjects had total MMSE scores of 24–30 and a CDR of 0.5. Details of inclusion and exclusion criteria for participants can be found at http://www.adni-info.org.
Baseline and Follow-Up Assessments
All participants underwent a standardized clinical evaluation based on the study protocol. Neurological assessments included the Alzheimer Disease Assessment Scale–Cognitive (ADAS-Cog),31 MMSE, and CDR-SB. The MMSE scores were divided into subscores for orientation, memory, attention, language, and construction. The data at baseline and 24 months were used to determine AD conversion, and subjects were considered to have progressed to AD if they met the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders (NINCDS-ADRDA) criteria for AD.32
Statistical Analysis
The subjects were divided into two groups according to the clinical state at the 2-year follow-up evaluation: those who progressed to AD dementia (MCIp group) and those who did not (MCInp group). Between-group comparisons for baseline continuous data, including demographic and clinical data, were performed using two-tailed t-tests. Baseline categorical data were analyzed by chi-square test or Fisher’s exact test. Logistic regression analysis was performed to examine the ability of MMSE total or subscale scores to predict progression to AD dementia in MCI individuals. For each analysis of the association between MMSE and progression to AD dementia, three models were tested for stepwise control of potential confounders. The first model did not include any covariates; the second model included age, gender, and education as covariates; and the third model included all potential covariates: age, gender, education, and CDR-SR. In all analyses, two-tailed p-values <0.05 were taken to indicate statistical significance.
Results
Presence of AD Progression Within 2-Year Follow-Up Period
All subjects (n = 306) were diagnosed with MCI at baseline assessments. After a 2-year follow-up, 111 (36.3%) had progressed to AD dementia (MCIp group), whereas the remaining 195 (63.7%) had not (MCInp group) (Table 1).
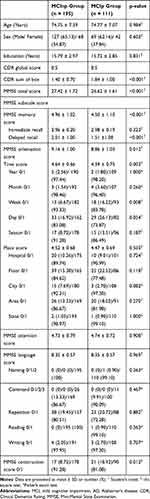 |
Table 1 Baseline Characteristics of the Amnestic MCI Group That Did Not Progress to AD Dementia (MCInp Group) and the Group That Did (MCIp Group) at 2-Year Follow-Up (n = 306) |
Baseline Characteristics of AD Progression and Non-Progression Groups
The baseline demographics and clinical characteristics of the MCIp and MCInp groups are shown in Table 1. There were no significant differences between the two groups with regard to age, sex, education, and CDR global score. The MCIp group had significantly higher CDR-SB scores and lower MMSE total and some MMSE subscores (memory, orientation, and construction) than the MCInp group. In terms of the MMSE memory subscore, the MCIp group showed significantly lower MMSE delayed recall scores than the MCInp group, but there was no significant difference in the MMSE immediate recall score between the two groups. With regard to the MMSE orientation subscore, there was a significant difference between the two groups in time subscore, but not in the place subscore. Among the MMSE time subscores, the week score of the MCIp group was significantly lower (p = 0.008) compared to those of the MCInp group, and the time score was almost significantly lower (p = 0.054). However, there were no significant differences in other orientation subscale scores between the two groups.
Association of MMSE Total and Orientation Score with AD Progression
As shown in Table 2, MMSE total score and memory, orientation, and construction subscale scores showed significant negative associations with AD progression after controlling for potential confounding variables, whereas attention and language score showed no such relations. Table 3 shows that the delayed recall score in the memory subscale scores and the time score in the orientation subscale scores were negatively associated with AD progression, whereas the immediate recall and place scores showed no such relations.
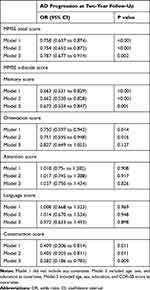 |
Table 2 Results of Multiple Logistic Regression Analysis to Assess the Relationships of MMSE Total and Subscale Scores with AD Progression at 2-Year Follow-Up in Individuals with MCI |
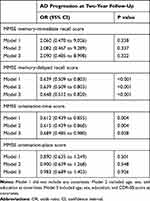 |
Table 3 Results of Multiple Logistic Regression Analysis to Assess the Relationships of MMSE Memory and Orientation Subscale Scores with AD Progression at 2-Year Follow-Up in Individuals with MCI |
To assess the relationships of time subscale scores with AD progression, a series of logistic regression analyses were conducted in four steps (Table 4). In the first step, we tested one-item models: among the five models, the MMSE time–week (MMSE-W) model was statistically significant. In the second step, we tested two-item models, which included the MMSE-W model, and found that only the MMSE time–week and day (MMSE-WD) model was significant. In the third step, MMSE time–season, week, and day (MMSE-SWD) and MMSE time–month, week, and day (MMSE-MWD) were significant among the three-item models that included MMSE-WD. In the fourth step, all four-item models that included MMSE time-SWD or -MWD were significant.
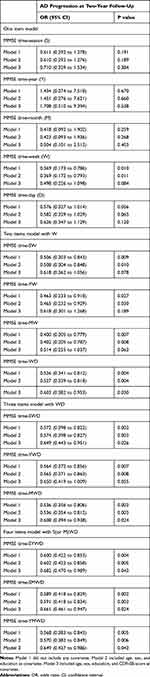 |
Table 4 Results of Multiple Logistic Regression Analysis to Assess the Relationship of MMSE Time Subscale Scores with AD Progression at 2-Year Follow-Up in Individuals with MCI |
Discussion
This study was performed to investigate whether specific MMSE domains are useful predictors of AD conversion in MCI individuals through a 2-year follow-up. This is the first longitudinal study using MMSE subscores to determine AD conversion among individuals with MCI. Our results showed that MMSE subscores for orientation and construction, as well as for memory, are useful predictors of conversion from MCI to AD.
As AD is characterized by a long preclinical period in which defects in episodic memory can be detected,33 episodic memory decline is a well-known predictor of AD progression.34 In accordance with these results, we showed that the MMSE delayed recall subscore predicted conversion to AD. Furthermore, our results showed that the MMSE time orientation subscore could be useful for predicting AD conversion in MCI. These results were consistent with a previous study showing that memory and temporal orientation were initial MMSE items that were lost during the course of AD.35 Another study also demonstrated that the MMSE orientation for time predicted cognitive decline in elderly people.36
In terms of the usefulness of the MMSE orientation score, one possible interpretation is that orientation consists of multiple cognitive domains, including attention and visuospatial perception as well as memory.25 Episodic memory impairment is the earliest symptom of AD, followed by attention and visuospatial dysfunction.37 Individuals with MCI already have reduced memory performance,38 so examination of orientation, which contains more information in addition to memory, may be more effective compared to examination of memory alone. In terms of orientation-related neural substrates, a clinicopathological study reported that both temporal and spatial disorientation in AD were related to neurofibrillary tangle densities in the hippocampus, superior parietal, and posterior cingulate cortex.39 In contrast to this result, we found that place orientation could not predict AD conversion, whereas time orientation showed predictive capability. A previous PET study showed differences in the biological underpinnings of time and place orientation: time orientation was associated with the rate of glucose metabolism in the posterior cingulate gyri and right middle temporal gyrus, whereas place orientation was correlated with glucose metabolism in the right posterior cingulate gyrus.40 Our results also suggested that time orientation may have specific neural substrates that are distinct from those for place orientation.
We found that the MMSE construction score was a valid predictor of AD conversion. The construction subscore was obtained using an interlocking pentagon copying item, which is given a maximum score of 1 point. The pentagon copying test is known to be an effective method for distinguishing between patients with dementia with Lewy bodies from those with AD.41 Our results demonstrated the possibility of using the pentagon copying test as a screening tool for AD conversion. To complement the crude scoring method (0/1 score) and increase the ability to identify subtle differences, further studies using a wider range of scoring methods, eg, Bender–Gestalt test (0–4-point scores) or the Qualitative Scoring MMSE Pentagon Test (0–13-point scores),42 should be performed in the future.
The use of the MMSE subscores has a number of merits, including simplicity of administration and ease of use and interpretation of the results. In addition, it is possible to obtain information about AD conversion through existing routine tests without additional tests. However, this study also has several limitations. The MMSE score is known to be highly affected by age, sex, and education.43,44 In general assessments, the normality of the MMSE total score was determined by using age-, sex-, and education-adjusted normative data; unfortunately, no such norms are available for the subscores of MMSE. However, these factors are unlikely to have affected the results of this study because we found no significant differences in age, sex, or education level between the MCIp and MCInp groups, and the results remained significant after controlling for age, sex, and education. Another issue was that the NINCDS-ADRDA criteria were used to determine AD conversion, instead of using biomarkers of AD. The objective of the present study was to investigate predictors of AD conversion in a practical clinical setting, so we excluded these biomarkers. The importance of biomarkers in AD research is growing, and the ADNI study included comprehensive measures of biomarkers, such as MRI, FDG, and amyloid PET. Outside the practical clinical setting, further research using biomarker changes as outcome variables in addition to clinical AD conversion is required. Finally, we used only 2-year follow-up data to investigate the earliest predictors of AD conversion within a short period. However, further studies with a longer follow-up period are required.
Conclusion
Our findings emphasize the importance of assessing orientation and construction domains to identify subjects at high risk of AD conversion among elderly people whose memory function is already impaired. In terms of simplicity, rapid administration, and ease of interpretation, MMSE subscales of memory, orientation, and construction could be useful screening tools for predicting conversion to AD from MCI in practical clinical settings.
Acknowledgments
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. This research was also supported by Hallym University Research Fund (HURF-2019-62).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi:10.1001/archneur.56.3.303
2. Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63(4):494–506. doi:10.1002/ana.21326
3. Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66(12):1447–1455. doi:10.1001/archneurol.2009.266
4. Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63(1):115–121. doi:10.1212/01.WNL.0000132523.27540.81
5. Spasov S, Passamonti L, Duggento A, Lio P, Toschi N. Alzheimer’s disease neuroimaging I. A parameter-efficient deep learning approach to predict conversion from mild cognitive impairment to alzheimer’s disease. NeuroImage. 2019;189:276–287. doi:10.1016/j.neuroimage.2019.01.031
6. Gainotti G, Quaranta D, Vita MG, Marra C. Neuropsychological predictors of conversion from mild cognitive impairment to Alzheimer’s disease. J Alzheimers Dis. 2014;38(3):481–495. doi:10.3233/JAD-130881
7. Pereira T, Ferreira FL, Cardoso S, et al. Neuropsychological predictors of conversion from mild cognitive impairment to Alzheimer’s disease: a feature selection ensemble combining stability and predictability. BMC Med Inform Decis Mak. 2018;18(1):137. doi:10.1186/s12911-018-0710-y
8. Tabert MH, Manly JJ, Liu X, et al. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry. 2006;63(8):916–924. doi:10.1001/archpsyc.63.8.916
9. Liu Y, Paajanen T, Zhang Y, et al. Analysis of regional MRI volumes and thicknesses as predictors of conversion from mild cognitive impairment to Alzheimer’s disease. Neurobiol Aging. 2010;31(8):1375–1385. doi:10.1016/j.neurobiolaging.2010.01.022
10. Chetelat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60(8):1374–1377. doi:10.1212/01.WNL.0000055847.17752.E6
11. Jack CR
12. Farlow MR, He Y, Tekin S, Xu J, Lane R, Charles HC. Impact of APOE in mild cognitive impairment. Neurology. 2004;63(10):1898–1901. doi:10.1212/01.WNL.0000144279.21502.B7
13. Blom ES, Giedraitis V, Zetterberg H, et al. Rapid progression from mild cognitive impairment to Alzheimer’s disease in subjects with elevated levels of tau in cerebrospinal fluid and the APOE epsilon4/epsilon4 genotype. Dement Geriatr Cogn Disord. 2009;27(5):458–464. doi:10.1159/000216841
14. Petersen RC, Smith GE, Ivnik RJ, et al. Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. JAMA. 1995;273(16):1274–1278. doi:10.1001/jama.1995.03520400044042
15. Samtani MN, Raghavan N, Shi Y, et al. Disease progression model in subjects with mild cognitive impairment from the Alzheimer’s disease neuroimaging initiative: CSF biomarkers predict population subtypes. Br J Clin Pharmacol. 2013;75(1):146–161. doi:10.1111/j.1365-2125.2012.04308.x
16. De Meyer G, Shapiro F, Vanderstichele H, et al. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol. 2010;67(8):949–956. doi:10.1001/archneurol.2010.179
17. Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64(3):343–349. doi:10.1001/archneur.64.3.noc60123
18. Smach MA, Charfeddine B, Ben Othman L, et al. Evaluation of cerebrospinal fluid tau/beta-amyloid(42) ratio as diagnostic markers for Alzheimer disease. Eur Neurol. 2009;62(6):349–355. doi:10.1159/000241881
19. Gomar JJ, Bobes-Bascaran MT, Conejero-Goldberg C, Davies P, Goldberg TE. Alzheimer’s disease neuroimaging I. Utility of combinations of biomarkers, cognitive markers, and risk factors to predict conversion from mild cognitive impairment to alzheimer disease in patients in the Alzheimer’s disease neuroimaging initiative. Arch Gen Psychiatry. 2011;68(9):961–969. doi:10.1001/archgenpsychiatry.2011.96
20. Davatzikos C, Bhatt P, Shaw LM, Batmanghelich KN, Trojanowski JQ. Prediction of MCI to AD conversion, via MRI, CSF biomarkers, and pattern classification. Neurobiol Aging. 2011;32(12):2322 e2319–e2327. doi:10.1016/j.neurobiolaging.2010.05.023
21. Risacher SL, Saykin AJ. Neuroimaging and other biomarkers for Alzheimer’s disease: the changing landscape of early detection. Annu Rev Clin Psychol. 2013;9:621–648. doi:10.1146/annurev-clinpsy-050212-185535
22. Qin Y, Tian Y, Han H, et al. Risk classification for conversion from mild cognitive impairment to Alzheimer’s disease in primary care. Psychiatry Res. 2019;278:19–26. doi:10.1016/j.psychres.2019.05.027
23. Nakata E, Kasai M, Kasuya M, et al. Combined memory and executive function tests can screen mild cognitive impairment and converters to dementia in a community: the Osaki-Tajiri project. Neuroepidemiology. 2009;33(2):103–110. doi:10.1159/000222092
24. Daly E, Zaitchik D, Copeland M, Schmahmann J, Gunther J, Albert M. Predicting conversion to Alzheimer disease using standardized clinical information. Arch Neurol. 2000;57(5):675–680. doi:10.1001/archneur.57.5.675
25. Kim JW, Byun MS, Sohn BK, et al. Clinical dementia rating orientation score as an excellent predictor of the progression to Alzheimer’s disease in mild cognitive impairment. Psychiatry Investig. 2017;14(4):420–426. doi:10.4306/pi.2017.14.4.420
26. Mossello E, Boncinelli M. Mini-mental state examination: a 30-year story. Aging Clin Exp Res. 2006;18:271–273. doi:10.1007/BF03324660
27. Chow TW, Hynan LS, Lipton AM. MMSE scores decline at a greater rate in frontotemporal degeneration than in AD. Dement Geriatr Cogn Disord. 2006;22(3):194–199. doi:10.1159/000094870
28. Ala TA, Hughes LF, Kyrouac GA, Ghobrial MW, Elble RJ. The mini-mental state exam may help in the differentiation of dementia with Lewy bodies and Alzheimer’s disease. Int J Geriatr Psychiatry. 2002;17(6):503–509. doi:10.1002/gps.550
29. Chehrehnegar N, Nejati V, Shati M, et al. Early detection of cognitive disturbances in mild cognitive impairment: a systematic review of observational studies. Psychogeriatrics. 2020;20(2):212–228. doi:10.1111/psyg.12484
30. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi:10.1111/j.1365-2796.2004.01388.x
31. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141(11):1356–1364.
32. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34(7):939–944. doi:10.1212/WNL.34.7.939
33. Backman L, Small BJ, Fratiglioni L. Stability of the preclinical episodic memory deficit in Alzheimer’s disease. Brain. 2001;124(Pt 1):96–102. doi:10.1093/brain/124.1.96
34. Tierney MC, Szalai JP, Snow WG, et al. Prediction of probable Alzheimer’s disease in memory-impaired patients: a prospective longitudinal study. Neurology. 1996;46(3):661–665. doi:10.1212/WNL.46.3.661
35. Ashford JW, Kolm P, Colliver JA, Bekian C, Hsu LN. Alzheimer patient evaluation and the mini-mental state: item characteristic curve analysis. J Gerontol. 1989;44(5):P139–P146. doi:10.1093/geronj/44.5.P139
36. Guerrero-Berroa E, Luo X, Schmeidler J, et al. The MMSE orientation for time domain is a strong predictor of subsequent cognitive decline in the elderly. Int J Geriatr Psychiatry. 2009;24(12):1429–1437. doi:10.1002/gps.2282
37. Lee DY, Youn JC, Choo IH, et al. Combination of clinical and neuropsychologic information as a better predictor of the progression to alzheimer disease in questionable dementia individuals. Am J Geriatr Psychiatry. 2006;14(2):130–138. Doi:10.1097/01.JGP.0000192487.58426.d2
38. Tounsi H, Deweer B, Ergis AM, et al. Sensitivity to semantic cuing: an index of episodic memory dysfunction in early Alzheimer disease. Alzheimer Dis Assoc Disord. 1999;13(1):38–46. doi:10.1097/00002093-199903000-00006
39. Giannakopoulos P, Gold G, Duc M, Michel JP, Hof PR, Bouras C. Neural substrates of spatial and temporal disorientation in Alzheimer’s disease. Acta Neuropathol. 2000;100(2):189–195. doi:10.1007/s004019900166
40. Hirono N, Mori E, Ishii K, et al. Hypofunction in the posterior cingulate gyrus correlates with disorientation for time and place in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1998;64(4):552–554. doi:10.1136/jnnp.64.4.552
41. Ala TA, Hughes LF, Kyrouac GA, Ghobrial MW, Elble RJ. Pentagon copying is more impaired in dementia with Lewy bodies than in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2001;70(4):483–488. doi:10.1136/jnnp.70.4.483
42. Mitolo M, Salmon DP, Gardini S, Galasko D, Grossi E, Caffarra P. The new Qualitative Scoring MMSE Pentagon Test (QSPT) as a valid screening tool between autopsy-confirmed dementia with Lewy bodies and Alzheimer’s disease. J Alzheimers Dis. 2014;39(4):823–832. doi:10.3233/JAD-131403
43. Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the mini-mental state examination by age and educational level. JAMA. 1993;269(18):2386–2391. doi:10.1001/jama.1993.03500180078038
44. Monsch AU, Foldi NS, Ermini-Funfschilling DE, et al. Improving the diagnostic accuracy of the mini-mental state examination. Acta Neurol Scand. 1995;92(2):145–150. doi:10.1111/j.1600-0404.1995.tb01029.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
