Back to Journals » OncoTargets and Therapy » Volume 12
MLL3 promotes the senescence of esophageal squamous cell carcinoma
Authors Xia M, Ling F, Gao F, Tao C
Received 14 September 2018
Accepted for publication 17 January 2019
Published 1 March 2019 Volume 2019:12 Pages 1575—1582
DOI https://doi.org/10.2147/OTT.S187540
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Manhui Xia,1,* Feng Ling,2,* Feng Gao,1 Chunmu Tao2
1Department of Thoracic Surgery, Jingjiang People’s Hospital of Jiangsu Province, Jingjiang City 214500, Jiangsu Province, China; 2Department of Gastroenterology, Jingjiang People’s Hospital of Jiangsu Province, Jingjiang City 214500, Jiangsu Province, China
*These authors contributed equally to this work
Background: Senescence has been recognized as a mechanism for the suppression of tumorigenesis. However, how the senescence is regulated is not fully understood.
Aims: The present study aims to elucidate MLL3-mediated regulation of senescence.
Materials and methods: MLL3 protein levels in esophageal squamous cell carcinoma (ESCC) tissues were examined by Western blotting and immunohistochemistry. The effects of MLL3 on the growth and senescence of ESCC cells were examined using MTT assay, soft agar assay, and β-gal staining. The interaction between MLL3 and P16 was evaluated by immunoprecipitation and GST pull-down assay.
Results: In this study, we found that MLL3 promoted the senescence of ESCC cells. MLL3 was downregulated in ESCC. MLL3 inhibited the growth and colony formation of ESCC cells. Mechanistically, MLL3 interacted with P16 and impaired the interaction between P16 and UHRF1 (the E3 ligase for P16), thus upregulating the protein levels of several senescence regulators.
Conclusion: Collectively, this study demonstrated the regulation of senescence by MLL3.
Keywords: ESCC, MLL3, senescence, P16
Introduction
Esophageal cancer is one of the most common and aggressive malignancies in the world.1 Two pathology subtypes (esophageal squamous cell carcinoma [ESCC] and esophageal adenocarcinoma) have been observed.2 The most prevalent subtype in China is ESCC.3 Current nonspecific cytotoxic therapies cure only a fraction of patients. Further understanding the pathogenesis of esophageal cancer would advance treatment.
Cellular senescence is the phenomenon by which normal diploid cells cease to divide.4 DNA damage response, DNA damage, elevated ROS, and activation of oncogenes induce senescence.5 Molecular mechanistic studies demonstrated that P53, P16, and Rb are key regulators of senescence.6 Recently, a panel of effectors was identified as regulators of senescence by modulating P53, P16, and Rb protein levels.7
UHRF1 is a RING-finger-type E3 ubiquitin ligase.8 The protein binds to specific DNA sequences and recruits a histone deacetylase to regulate gene expression.9 Several studies have demonstrated that UHRF1 is a modulator of senescence. Decreased UHRF1 expression is a key initial event in the suppression of DNMT1-mediated DNA methylation and the consequent induction of senescence via increasing WNT5A expression.10 Consistently, destabilization of UHRF1 is necessary for PIM1-induced cellular senescence.11 Moreover, UHRF1 represses P16 expression.12 These observations suggest that UHRF1 is a negative regulator of senescence.
MLL3 is a lysine methyltransferase, possesses histone methylation activity and is involved in transcriptional coactivation.13 The role of MLL3 in senescence remains unknown. We previously demonstrated MLL3 dysfunction in ESCC.14 In this study, we examined the roles of MLL3 in ESCC cell senescence.
Materials and methods
Cell culture
Normal esophageal epithelial cell line SHEE and ESCC cell lines (Caes17, KYSE180, and Eca109) were gifts from Professor Xu (Shantou, Guangdong Province, China). KYSE180 and Eca109 were highly differentiated ESCC cell lines, and Caes17 was poorly differentiated cell line. These cell lines were maintained in DMEM containing 10% FBS and antibiotics. Cells were maintained in an atmosphere at 37°C with 5% CO2. Cells were transfected using Lipofectamine 2000 according to the instructions of the manufacturer. The use of the cell lines has been approved by the ethics committee of Jingjiang People’s Hospital.
Plasmid construction
The MLL3 (NM170606.2) coding sequence was inserted into the pLVX lentivirus vector or pcDNA3.1 vector to obtain the Flag tag or myc tag. The P16 (NM000077.4) coding sequence was inserted into the pGEX4T-1, pCMVTag2B, or pCMV-HA vector to obtain the GST tag, Flag tag, or HA tag. The UHRF1 coding sequence was inserted into the pCMVTag2B vector to obtain the Flag tag.
Western blot
Proteins were extracted from the cell cultures and tissues using RIPA buffer. After centrifugation and quantification via Bradford assay, the proteins were loaded onto a polyacrylamide gel, and the SDS-PAGE was performed. Then, the proteins were transferred to PDVF membrane, blocked with 5% milk and incubated with the primary antibody overnight at 4°C. On the next day, the membranes were washed with TBST solution and incubated with the secondary antibody for 1 hour at room temperature. After three washes, the membranes were examined using an enhanced chemiluminescence kit. Anti-MLL3 (PLA0028, 1:1,000) and Anti-GST (SAB4200692, 1:1,000) were obtained from Sigma; anti-GAPDH (#5174, 1:1,000), anti-Flag (#14793, 1:1,000), anti-Myc (#2276, 1:1,000), anti-P16 (#80772, 1:1,000), anti-P21 (#2947, 1:1,000), anti-P27 (#3686, 1:1,000), and anti-P53 (#2524, 1:1,000) were obtained from Cell Signaling Technology. Mouse IgG (#63630, 1:2,000) and Rabbit IgG (#3900, 1:2,000) were obtained from Cell Signaling Technology.
Immunohistochemistry (IHC)
Fresh clinical samples were deparaffinized using terebinth and ethanol. After hydration, antigen recovery was performed using EDTA solution at 100°C for 3 minutes. The tissues were incubated with the primary antibody solution overnight at 4°C after inactivating endogenous peroxidase activity. On the next day, the tissues were washed and incubated with the secondary antibody for 1 hour at room temperature. After three washes, the tissues were developed using DAB.
MTT assay
MTT assay was performed to examine ESCC cell proliferation. On day 0, cells were seeded in 96-well plates (1,000 cells/well) with 200 μL DMEM/well. On days 1, 3, 5, and 7, 20 μL MTT solution was added to each well and incubated with the cells for 4 hours. Then, the supernatant was removed, and MTT was dissolved using 200 μL DMSO. The experiments were performed in triplicate. The OD 540 nm was measured.
Soft agar assay
The soft agar assay was performed to examine the anchorage-independent growth of ESCC cells. Details about the soft agar assay were described in a previous study.15
β-gal staining
Cells were washed twice with PBS and fixed with 4% formaldehyde for 5 minutes. Then, cells were incubated at 37°C with β-gal staining solution overnight. Stained cells were evaluated using a microscope. Percentages of positively stained cells were calculated.
GST pull down
The coding sequence of P16 was inserted into the pGEX-4T-1 vector. GST protein and the fusion protein were purified using glutathione Sepharose 4B beads according to the manufacturer’s instructions. Briefly, 5 μg GST or GST-P16 proteins were incubated with 500 μg cell lysates overnight at 4°C. On the next day, glutathione Sepharose 4B beads were added and incubated with the cell lysates for an additional 4 hours. The beads were washed thrice, and immunoprecipitated proteins were eluted using loading buffer.
Immunoprecipitation
For immunoprecipitation to detect the interaction between the exogenously expressed proteins, ESCC cells were transfected with the indicated plasmids. Forty-eight hours later, the cells were harvested using RIPA buffer. After centrifugation and determination of the concentration, the beads coupled with an anti-Flag antibody were added to the supernatant and incubated for additional 2 hours. After an extensive wash, the beads were boiled with loading buffer, and the immunoprecipitated protein was examined using Western blotting.
For immunoprecipitation to detect the interaction between the endogenously expressed proteins, the indicated primary antibody was added to the supernatant and incubated overnight at 4°C. On the next day, Protein A beads were added to the supernatant and incubated for additional 4 hours. After extensive washes, the beads were boiled with loading buffer, and the immunoprecipitated protein was examined via Western blotting.
Statistical analysis
Statistical analysis was performed by Student’s t-test (two-tailed) using Prism GraphPad software. P<0.05 was considered statistically significant. Data were presented as the mean ± standard error of the mean.
Results
MLL3 protein levels are reduced in ESCC
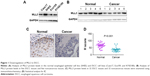
Our previous study demonstrated the downregulation of MLL3 mRNA levels in ESCC tissues.14 To further confirm these results, we first examined MLL3 protein levels in the normal esophageal cell line SHEE and ESCC cell line Caes17, KYSE180, and Eca109 (Figure 1A). Obviously, MLL3 protein levels were increased in the normal esophageal cell line SHEE. Then, we examined MLL3 protein levels in ESCC tissues using Western blotting and IHC. MLL3 protein levels were reduced in ESCC tissues as demonstrated by Western blot analysis (Figure 1B). Based on IHC staining, MLL3 protein was mainly localized in the nuclei of normal esophageal epithelia, and its expression was lost in cancerous tissues (Figure 1C and D). In summary, these observations suggested the downregulation of MLL3 protein in ESCC.
MLL3 inhibited growth and colony formation and promoted the senescence of the ESCC cells
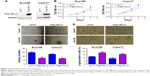
We first forced the expression of MLL3 in Eca109 and Caes17 cells (Figure 2A) and examined the effects of MLL3 on the growth, colony formation, and senescence of ESCC cells. As shown in Figure 2B and C, MLL3 significantly inhibited plate-based growth and colony formation of ESCC cells on soft agar. In addition, MLL3 overexpression increased Eca109 and Caes17 cell senescence (Figure 2D).
Next, we knocked down MLL3 expression in Eca109 and Caes17 cells (Figure 3A). Downregulation of MLL3 promoted ESCC cell growth as revealed by MTT assay (Figure 3B). Moreover, reduced MLL3 expression enhanced anchorage-independent growth of ESCC cell growth (Figure 3C). Consistently, MLL3 downregulation reduced ESCC cell senescence based on β-gal staining (Figure 3D). Taken together, MLL3 inhibited the growth and colony formation and promoted the senescence of ESCC cells.
MLL3 inhibited the expression of senescence regulators
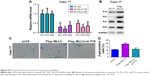
To further explore the mechanism through which MLL3 induced senescence, we examined the mRNA levels of P21, P27, and P53 in the Caes17 cells with reduced MLL3 expression. As shown in Figure 4A, knocking down MLL3 expression reduced P21, P27, and P53 mRNA levels. Consistently, overexpression of MLL3 upregulated P16, P21, P27, and P53 protein levels (Figure 4B). In the functional study, knocking down the expression of P16 abolished the senescence-promoting effects of MLL3 (Figure 4C and D). Collectively, these results suggested that MLL3 promoted senescence by upregulating senescence regulators.
MLL3 interacted with P16
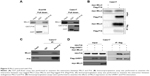
To further elucidate the molecular mechanisms, the interactions between MLL3 and senescence regulators were examined. As demonstrated by GST pull-down assay (Figure 5A), the interaction between MLL3 and P16 was detected in both Eca109 and Caes17 cells. In the immunoprecipitation assay, exogenously expressed MLL3 (myc-MLL3) and P16 (Flag-P16) interacted with each other (Figure 5B). Similarly, endogenous MLL3 and P16 formed a complex (Figure 5C). UHRF1 interacts with P16 and downregulates P16 protein levels. We next examined whether the interaction between P16 and UHRF1 was affected by MLL3 expression. Indeed, MLL3 disrupted the interaction between UHRF1 and P16 (Figure 5D), indicating that MLL3 blocked the degradation of P16 induced by UHRF1.
Discussion
MLL3 is a histone demethylase widely expressed, and we demonstrate that its expression is downregulated in human ESCC tissues, the most common malignancy in China. We demonstrate a role for MLL3 in preventing the malignant progression of ESCC. Our findings suggest that MLL3 is a tumor suppressor in ESCC, define it as an upstream protector of p16, and thereby reveal that alteration in MLL3 represents a point of vulnerability in cancer.
In our functional studies, we reveal significant senescence in the cell population that overexpresses MLL3. In addition, knocking down MLL3 expression reduced senescence. Consistent with these observations, MLL3 positively regulated the expression of senescence-associated regulators, providing a good explanation for the biological functions of MLL3 in ESCC.
Importantly, we identified a physiological function for MLL3 as a protector of p16 by weakening the interaction between p16 and its E3 ligase UHRF1. This finding is consistent with previous findings demonstrating that loss of p16 expression was associated with poor differentiation of ESCC.16 p16 is one of the key regulators of senescence. MLL3 interacts with p53 (another regulator for senescence) and forms a tumor suppressive coactivator complex.17 Collectively, these observations in combination with the present study suggested that MLL3 might be a regulator of senescence.
Another interesting finding of this study is the modification of the p16–UHRF1 interaction by MLL3. Several studies have demonstrated the downregulation of p16 protein levels by UHRF1.12 Our study further demonstrated that MLL3 protected p16 by disrupting the association between UHRF1 and p16.
In the present study, we demonstrate the tumor suppressive roles of MLL3 in ESCC and suggest that restoration of MLL3 expression might be a promising strategy for treatment.
Compliance with ethical standards
Ethical approval for research involving animals: The studies were approved by the ethics committee of Jingjiang People’s Hospital of Jiangsu Province. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Research involving human participants: The studies were approved by the ethics committee of Jingjiang People’s Hospital of Jiangsu Province. All procedures in studies involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Written informed consent was obtained from the patients.
Acknowledgment
This study was supported by a grant from the Health and Family Planning of Jiangsu Municipal Commission (grant No YG201511).
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. | ||
Zeng H, Zheng R, Zhang S, et al. Esophageal cancer statistics in China, 2011: estimates based on 177 cancer registries. Thorac Cancer. 2016;7(2):232–237. | ||
Rice TW, Blackstone EH. pT2N0M0 esophageal squamous cell cancer: location, grade, and statistics. J Thorac Cardiovasc Surg. 2013;145(6):1426–1427. | ||
Strzyz P. Controlling the senescence-associated secretory phenotype. Nat Rev Mol Cell Biol. 2016;17(12):740. | ||
Duarte JH. SIRT6 prevents chondrocyte senescence and DNA damage. Nat Rev Rheumatol. 2015;11(5):260. | ||
Pérez-Mancera PA, Young AR, Narita M. Inside and out: the activities of senescence in cancer. Nat Rev Cancer. 2014;14(8):547–558. | ||
Ohashi M, Korsakova E, Allen D, et al. Loss of MeCP2 leads to activation of p53 and neuronal senescence. Stem Cell Reports. 2018;10(5):1453–1463. | ||
Vaughan RM, Dickson BM, Whelihan MF, et al. Chromatin structure and its chemical modifications regulate the ubiquitin ligase substrate selectivity of UHRF1. Proc Natl Acad Sci U S A. 2018;115(35):8775–8780. | ||
Hashimoto H, Horton JR, Zhang X, Cheng X. UHRF1, a modular multi-domain protein, regulates replication-coupled crosstalk between DNA methylation and histone modifications. Epigenetics. 2009;4(1):8–14. | ||
Jung HJ, Byun HO, Jee BA, et al. The ubiquitin-like with PHD and ring finger domains 1 (UHRF1)/DNA methyltransferase 1 (DNMT1) axis is a primary regulator of cell senescence. J Biol Chem. 2017;292(9):3729–3739. | ||
Yang J, Liu K, Yang J, et al. PIM1 induces cellular senescence through phosphorylation of UHRF1 at Ser311. Oncogene. 2017;36(34):4828–4842. | ||
Wang F, Yang YZ, Shi CZ, et al. UHRF1 promotes cell growth and metastasis through repression of p16(ink4a) in colorectal cancer. Ann Surg Oncol. 2012;19(8):2753–2762. | ||
Rabello DDA, Ferreira V, Berzoti-Coelho MG, et al. MLL2/KMT2D and MLL3/KMT2C expression correlates with disease progression and response to imatinib mesylate in chronic myeloid leukemia. Cancer Cell Int. 2018;18:26. | ||
Xia M, Xu L, Leng Y, et al. Downregulation of MLL3 in esophageal squamous cell carcinoma is required for the growth and metastasis of cancer cells. Tumor Biol. 2015;36(2):605–613. | ||
Ji XD, Li G, Feng YX, et al. EphB3 is overexpressed in non-small-cell lung cancer and promotes tumor metastasis by enhancing cell survival and migration. Cancer Res. 2011;71(3):1156–1166. | ||
Dey B, Raphael V, Khonglah Y, Girilynrah K. Expression of cyclin D1 and p16 in esophageal squamous cell carcinoma. Middle East J Dig Dis. 2015;7(4):220–225. | ||
Lee J, Kim D-H, Lee S, et al. A tumor suppressive coactivator complex of p53 containing ASC-2 and histone H3-lysine-4 methyltransferase MLL3 or its paralogue MLL4. Proc Natl Acad Sci U S A. 2009;106(21):8513–8518. | ||
Baumann K. Cell signalling: limiting the side effects of senescence. Nat Rev Mol Cell Biol. 2015;16(8):451. | ||
Sharpless NE, Sherr CJ. Forging a signature of in vivo senescence. Nat Rev Cancer. 2015;15(7):397–408. | ||
Sulli G, di Micco R, D’Adda di Fagagna F. Crosstalk between chromatin state and DNA damage response in cellular senescence and cancer. Nat Rev Cancer. 2012;12(10):709–720. | ||
Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15(7):482–496. | ||
Bourgeois B, Madl T. Regulation of cellular senescence via the FOXO4-p53 axis. FEBS Lett. 2018;592(12):2083–2097. | ||
Yang F, Yi M, Liu Y, Wang Q, Hu Y, Deng H. Glutaredoxin-1 silencing induces cell senescence via p53/p21/p16 signaling axis. J Proteome Res. 2018;17(3):1091–1100. | ||
Patnaik D, Estève PO, Pradhan S. Targeting the SET and RING-associated (SRA) domain of ubiquitin-like, PHD and RING finger-containing 1 (UHRF1) for anti-cancer drug development. Oncotarget. 2018;9(40):26243–26258. | ||
Elia L, Kunderfranco P, Carullo P, et al. UHRF1 epigenetically orchestrates smooth muscle cell plasticity in arterial disease. J Clin Invest. 2018;128(6):2473–2486. | ||
Zhao JX, Zhang HX, Li B, et al. Role of MLL3 in regulating cardiac stem cells following cardiac cachexia. Eur Rev Med Pharmacol Sci. 2017;21(21):4924–4929. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.