Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Mitochondrial Damage of Lymphocytes in Patients with Acute Relapse of Schizophrenia: A Correlational Study with Efficacy and Clinical Symptoms
Authors Hu A, Li F, Guo L, Zhao X, Xiang X
Received 20 July 2022
Accepted for publication 12 October 2022
Published 27 October 2022 Volume 2022:18 Pages 2455—2466
DOI https://doi.org/10.2147/NDT.S380353
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Richard J Porter
Aqian Hu,1,* Faping Li,2,* Lei Guo,1 Xiaoxi Zhao,1 Xiaojun Xiang1
1Department of Psychiatry and National Clinical Research Center for Mental Disorders, The Second Xiangya Hospital of Central South University, Changsha, 410011, People’s Republic of China; 2Department of Psychiatry, The Second People’s Hospital of Guizhou Province, Guiyang, 550004, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaojun Xiang, Department of Psychiatry and National Clinical Research Center for Mental Disorders, The Second Xiangya Hospital of Central South University, Changsha, 410011, People’s Republic of China, Tel +86-731-85295814, Email [email protected]
Objective: Accumulating evidence has demonstrated that schizophrenia is associated with mitochondrial and immune abnormalities. In this pilot case–control study, we investigated the level of mitochondrial impairment in lymphocytes in patients with acute relapse of schizophrenia and explored the correlation between the level of mitochondrial damage and symptoms or treatment response.
Methods: Lymphocytic mitochondrial damage was detected using mitochondrial fluorescence staining and flow cytometry in 37 patients (at admission and discharge) and 24 controls. Clinical symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS) and Clinical Global Impression Scale (CGI-S).
Results: The levels of mitochondrial damage in CD3+ T, CD4+ T, and CD8+ T lymphocytes of the patients with schizophrenia at admission were significantly higher than those of the controls (p< 0.05) and did not return to normal at discharge (p> 0.05). The mitochondrial damage of T cells significantly improved at discharge for responsive patients only, as compared with that at admission (P< 0.05). However, no significant difference was found in mitochondrial damage in CD19+ B cells between patients and healthy controls, or between admission and discharge (p> 0.05). Furthermore, the reduction in mitochondrial damage of CD3, CD4, and CD8 lymphocytes was positively correlated with the reduction of the score of the PANSS positive scale at discharge (p< 0.05), while no significant correlation was found between the level of mitochondrial damage in lymphocytes and the scores of PANSS and CGI-S.
Conclusion: Acute relapse of schizophrenia might be associated with higher levels of mitochondrial damage in peripheral blood T lymphocytes. The degree of recovery of mitochondrial impairment in the T cells may be used as a predictor of treatment response in schizophrenia. As this is a pilot study, the conclusion still needs further verification in large-scale studies.
Keywords: immune metabolism, mitochondrial impairment, lymphocyte, schizophrenia, biological marker
Introduction
Schizophrenia is a serious, complex, chronic, and disabling psychiatric disorder that causes severe cognitive, emotional, and social impairment.1 Its typical clinical symptoms mainly include paranoid delusions, auditory hallucinations, disorganized behaviors, and disordered thoughts.1,2 According to statistics in 2016, approximately 21 million people were living with schizophrenia globally, which brought about an extremely high disease burden and accounted for 13.4 million years lived with disability (YLDS); the figures have been rising continuously as the population ages.3
The precise physiopathology of complicated psychiatric disorders has not been elucidated despite the extensive studies on neurotransmitters, neural connectivity, and synaptic plasticity in this field. A growing body of evidence has shown the role of immune-inflammatory responses or defective mitochondria in psychiatric illnesses.4–8
Studies have consistently demonstrated that schizophrenia is linked to chronic inflammation. Some studies using animal models of schizophrenia indicated immune-inflammatory reactions in the brain of animals.9,10 Postmortem and positron emission tomography (PET) studies also found infiltration of T cells and B cells, as well as microglial activation in several brain regions, suggesting blood–brain barrier impairment and neuroinflammation, which may be involved in the pathophysiology of schizophrenia.11,12 Notably, cells of the nervous system and lymphocytes have similar receptors and signal transduction mechanisms, and in psychiatric disorders, the disturbances of transmitters in the central nervous system co-occur with changes in lymphocyte function and metabolism in peripheral blood. Thus, lymphocytes could be a peripheral model for metabolic alterations in the central nervous system of schizophrenia.13
Recent studies have linked mitochondrial dysfunction in schizophrenia to the activation of immune inflammation via the release of reactive oxygen species (ROS) and damage-related molecular markers.9,14 Mitochondria are complex organelles that respond to external and internal signals by regulating core functions of the cell, such as redox, energy metabolism, apoptosis, and calcium homeostasis. Perturbations such as oxidative stress can lead to misfolding of mitochondrial permeability transition (MPT) pores,15,16 which results in non-selective permeability of the inner mitochondrial membrane, membrane potential collapse, mitochondrial swelling, calcium overload, and cytochrome c release, thereby triggering the activation of caspase and apoptosis.17–19 MPT plays a key role in inducing ROS, Ca2+ toxicity, cell necrosis, and apoptosis.15,20 Mitochondria damage can also promote the release of mitochondrial danger-associated molecules (DAMPs) and ROS, which plays a key role in activating and maintaining inflammatory immune responses, and such inflammatory responses can further aggravate the mitochondrial damage.15,21,22
Recent studies have found elevated levels of serum inflammatory factors and oxidative stress markers in patients with schizophrenia, which have important implications for cognitive impairment in patients with psychiatric disorders.23,24 In addition to its effects on the brain, inflammation is also believed to enhance the permeability of the blood–brain barrier and accelerate the entry of immune factors into the brain.25 Microglia in the brain can be activated by the peripheral inflammatory immunological response, which causes them to release cytokines that affect synaptic plasticity and neurotransmitters, eventually resulting in cognitive, emotional, and behavioral alterations.26 Other studies have also indicated that mitochondrial dysfunction plays an important role in immune response, and the dysfunction might be one of the causes of schizophrenia,27 which is consistent with the role of mitochondria in neuronal growth, activity, and plasticity.28
Circumstantial evidence also indicates that schizophrenia is associated with mitochondrial and immune abnormalities; however, most of these studies are focused on brain areas. For instance, some postmortem studies revealed that the number of mitochondria and volume of oligodendrocytes in brain regions such as the prefrontal cortex of schizophrenia is significantly reduced.29,30 Due to the limited availability of samples of brain areas, the discovery of peripheral blood biomarkers is crucial for the diagnosis and prognosis evaluation of schizophrenia. Thus, the present study aimed to do so by investigating the association between the level of mitochondrial damage in peripheral lymphocytes and schizophrenia.
Recently, a new immunofluorescence technology has emerged to reflect mitochondrial function by measuring single-cell mitochondrial mass (SCMM).31,32 Mitochondria are highly dynamic organelles with mitophagy mechanisms, and damaged mitochondria caused by mild stress can be eliminated by mitophagy. Intense stress leads to more MPT in mitochondria, which impairs mitophagy15 and results in abnormal mitochondria that cannot be eliminated, leading to increased mitochondrial mass.33 Therefore, the parameter of mitochondrial mass may reflect mitochondrial disturbance.31,32 Flow cytometry assay in this study relies on a mitochondrial special dye, which is a green fluorescence that binds specifically to the mitochondria of living cells. The median fluorescence intensity (MFI) reflects the mitochondria mass (MM).31,34,35 At the same time, lymphocyte subsets were labeled with specific antibodies of CD3, CD4, CD8, and CD19 for the cell count of each subset. In the present study, the single-cell mitochondrial fluorescence intensity of each cell population, reflecting SCMM, was obtained by using the built-in algorithm based on the MFI and the counts of cell subsets, which was recorded as mitochondrial damage index (MDI).
Materials and Methods
Subjects
Patients who met the following criteria were included: meeting the diagnostic criteria for schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV); in an acute episode of recurrent schizophrenia; being 18–60 years of age; having a Clinical Global Impression Severity score (CGI-S) of ≥4; having complete clinical and biological data; having signed the informed consent form. Participants who met any of the following criteria were excluded: with current infections, allergies, autoimmune disorders, or use of anti-inflammatory, antioxidants and antiviral drugs; with a history of substance abuse; with other mental illnesses; with significant cognitive dysfunction; with cancer, hypertension, coronary heart disease, diabetes, or nervous system diseases. Healthy controls were recruited among healthy volunteers with no personal or family history of psychiatric disorders.
This study was conducted in accordance with the Declaration of Helsinki. It was approved by the Medical Research Ethics Committee of the Second Xiangya Hospital of Central South University, China (dated 02.01.2019, approval No. [2019] 2019–004). All the subjects provided written informed consent upon full explanation of the study procedure.
Measurements
All the recruited patients underwent a demographic interview, a systematic assessment of medical history, and an interview based on the Structured Clinical Interview for DSM-IV Axis I Disorders. The severity of psychotic symptoms was evaluated using PANSS and CGI-S. The test of lymphocyte mitochondria was performed only once for the control group, while performed at admission and discharge for the case group.
Venipuncture was performed between 6:00 a.m. and 7:00 a.m.; 3–5 mL of venous blood from each participant was collected using sterile EDTA anticoagulant tubes and stored at room temperature for 24–36 hours. Cells were labeled with a 96-well plate containing a mitochondria-specific dye (UB1024, UBBiotechnology Co., Ltd., Hangzhou, China) that can be activated by a 633-nm laser. The plate was wrapped with aluminum foil to keep it from light; the content was incubated at room temperature for 3–5 minutes and centrifuged for 1 minute at 250×g. Then, immunophenotypic antibodies were used to prepare the “mixed reagent” to label cell populations, including CD3-FITC (UB104411, UBBiotechnology Co., Ltd., Hangzhou, China), CD4-PE-Cy7 (UB105441), CD8-PE (UB106421), CD45-PerCP-Cy5.5 (UB109481), and CD19 (RUO: UBR26-200). Contents in the plate were added with 20 ul of “mixed reagent” and incubated for 15 minutes. Then, 100 µl of anticoagulated human peripheral blood was added and incubated for 15 minutes at room temperature, away from light. Hemolysin (NH Lysis Solution, 10×) 400 µl was processed in the same way.
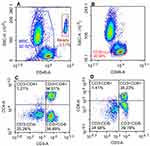
Finally, flow cytometry (NovoCyte, Agilent Technologies, US) was used to detect the counts of T and B cell subsets (CD3, CD4, CD8, and CD19) in the peripheral blood (shown in Figures 1 and 2) and the fluorescence intensity of mitochondrial staining of each cell population (shown in Figure 3).
Statistical Analysis
The SPSS 25.0 software (IBM Corp) was used for statistical analyses. The results were tested for normality using the Shapiro–Wilk test. Student t-test (for normally distributed data) or Mann–Whitney U-test (for non-normally distributed data) were used to analyze the differences in continuous variables between patients and controls. A paired t-test (for normally distributed data) or Wilcoxon signed-rank test (for non-normally distributed data) was used to compare the mitochondrial damage before and after treatment. The Chi-square test was used to analyze categorical variables. Spearman-Rho correlation test was used to evaluate the possible relationship between the level of lymphocyte damage and the severity of the acute episode of relapsed schizophrenia (reflected by the score of PANSS). For all the tests, P≤0.05 was considered statistically significant.
Results
Demographic and Clinical Characteristics
The present case–control pilot study was conducted in the Second Xiangya Hospital, Hunan Province, China. A total of 61 participants were enrolled, including 37 patients and 24 healthy controls. The average age of onset in the case group was 20.41±6.68 years, with a disease duration of 80.38±85.95 months. The average length of hospital stay was 24.6 days (±6.99 days), which was equivalent to the follow-up period. After treatment, the PANSS score of the patients with schizophrenia decreased significantly at discharge (p<0.001). The demographic and clinical characteristics of the patients and controls as well as the psychopathology features of the patients are presented in Table 1.
 |
Table 1 Demographic and Clinical Characteristics of Patients and Controls |
Differences in Mitochondrial Impairment in Lymphocytes Between Patients and Controls
MDI was used to reflect the degree of mitochondrial impairment in lymphocytes, as shown in Table 2. The mitochondrial damage in CD3, CD4, and CD8 T lymphocytes was significantly higher in the case group than in the control group (4.34 [2.37–7.4] vs 1.76 [0.60–3.60], p<0.001; 8.78 [4.35–17.07] vs 4.48 [1.47–11.08], p=0.016; 11.16 [7.40–18.24] vs 3.43 [1.42–7.82], p<0.001) (Table 2). No significant difference in mitochondrial damage in CD19 B cells was found between patients and healthy controls (70.96 [26.94–122.63] vs 60.61 [18.72–136.40], p=0.626) (Table 2).
 |
Table 2 Comparison of Parameters Between Patients and Controls |
The Level of Mitochondrial Damage Was Mitigated in Responsive Patients
There were no significant differences in the level of mitochondrial damage between admission and discharge for the overall patient group, and both levels were higher than that of the controls (Table 2). However, when patients were divided into a responsive group (PANSS score reduction rate ≥50%) and an unresponsive group (PANSS score reduction rate <50%), which were matched for medication use based on the chlorpromazine-equivalent doses of their antipsychotics.36 The result showed that the levels of mitochondrial damage in T cells, including CD3, CD4, and CD8 cells, were considerably lower at discharge than that at admission for the responsive group only (p<0.05) (Table 3). Furthermore, no significant change in the MDI level in CD19+ cells was noted before and after treatment, and no significant difference was found regarding CD19+ between patients and controls (Tables 2 and 3).
 |
Table 3 Indicators of Responsive and Unresponsive Patients with Schizophrenia at Admission and Discharge |
Associations Between the Severity of Symptoms and the Degree of Lymphocyte Mitochondrial Impairments
Spearman’s rank correlation coefficient was used to analyze the correlation between the PANSS score and the level of mitochondrial damage, as the data were not normally distributed. No correlation of the degree of mitochondrial impairment was found with the total, positive, negative, and general scores of PANSS, or the GGI-S score (Table 4). However, the result showed that, after treatment, the improvement of mitochondrial damage in CD3, CD4, and CD8 lymphocytes were positively correlated with the reduction of PANSS scores, especially the positive score (Table 5). In addition, no significant association was found between the age of onset or disease duration and the mitochondrial damage (all P>0.05).
 |
Table 4 Correlations Between PANSS Scores and Mitochondrial Damage in Lymphocytes |
 |
Table 5 Correlation Between the Reduction of PANSS Scores and Improvement of Lymphocyte Mitochondrial Impairment After Treatment |
Discussion
To our knowledge, this pilot study was the first to explore the association of the severity of psychotic symptoms and patient response with the level of mitochondrial damage in lymphocytes among patients in an acute episode of recurrent schizophrenia. The results demonstrated that (1) mitochondrial damage in CD3+, CD4+, and CD8+ T lymphocytes was significantly higher in patients in an acute episode of recurrent schizophrenia, as compared to controls; (2) the levels of mitochondrial damage in CD3+, CD4+, and CD8+ T cells substantially improved in responsive patients only, which may partly reflect the therapeutic effect of schizophrenia; (3) the level of mitochondrial damage in CD19+ B cells did not change significantly through admission and discharge, as compared with the control group; (4) after treatment, alterations in the level of mitochondrial impairment in CD3, CD4, and CD8 lymphocytes were associated with the reduction of the PANSS positive score.
The most notable finding of this study was that the level of mitochondrial impairment in T lymphocytes was significantly higher in patients in an acute episode of relapsed schizophrenia, as compared to controls, which was in line with the results of many prior studies. Mitochondrial dysfunction can result in decreased energy generation, which substantially impacts neuronal differentiation and may be involved in abnormal cognitive and behavioral processes.37,38 It has been reported that mitochondrial dysfunction might be associated with many neuropsychiatric disorders,39 such as autism,40 Parkinson’s disease, and Alzheimer’s disease.41 According to prior studies, mitochondrial dysfunction was involved in the majority of mental diseases, and patients with mitochondrial diseases were more likely to present with psychiatric symptoms.42,43 Previous studies have suggested that the upstream abnormalities of genes might be associated with damaged mitochondria, leading to manifestations of diseases.8 For instance, the abnormality of genes associated with the risk of schizophrenia, such as the nuclear-encoded D-amino-acid oxidase activator (DAOA) and its splice variant LG72 that encodes mitochondrial proteins, might lead to mitochondrial damage.44 Consistent with these findings, evidence from patients, cell lines, animal models, and postmortem studies also suggested that mitochondrial dysfunction might be involved in the pathogenesis of schizophrenia.38,45,46 A study found that oxygen consumption was reduced in the brains of schizophrenic patients,47 and some other studies have shown decreased mitochondrial volume in the oligodendrocytes of the prefrontal lobe as well as a decreased number of mitochondria in the striatum.29,48,49
Similar results have been observed in the peripheral blood of patients with psychiatric disorders.46 Patients with schizophrenia were found to have fewer mitochondria in lymphocytes and lower mitochondrial density in the large activated lymphocytes, as compared with healthy controls.50,51 It was speculated that the reduction of mitochondria in lymphocytes might be an ultrastructural basis of immune dysfunction in patients with schizophrenia.4,45,52 Moreover, several recent studies found that the lymphocytes had lower oxygen consumption (mitochondrial respiration) and generated more mitochondrial oxidative stress-related products in patients with schizophrenia, as compared with healthy controls,38,53,54 implying that patients with schizophrenia might have mitochondrial dysfunction in lymphocytes. Animal models of schizophrenia also showed mitochondrial impairment and up-regulation of immune-related genes, and the mitochondrial dysfunction was alleviated after treatment with clozapine.55 The above findings are in line with the results of the present study, which showed mitochondrial damage in T lymphocytes, indicating that mitochondrial damage might be involved in the pathogenesis of schizophrenia. The present study found no mitochondrial damage in B lymphocytes, and a recent study also reported mitochondrial damage in T cells only in patients with schizophrenia.56 A possible explanation is that cellular immunity may be more involved in the pathological process of schizophrenia than humoral immunity. To date, there is still insufficient evidence of mitochondrial damage in B lymphocytes in patients with schizophrenia. A meta-analysis suggested no significant difference in the number of B lymphocytes between patients with schizophrenia and healthy controls or before and after treatment, which was in line with the present study.57 Conversely, some studies indicate that B cells are involved in the pathological process of schizophrenia.58 The disparities might be attributed to the differences in medication use, disease duration, and other factors across different studies. Further studies on the role of B cells and their mitochondrial functions in patients with schizophrenia are warranted.
Mitochondrial dysfunction contributes to the development of inflammatory immunity, and inflammation and ROS can further promote mitochondrial damage.59 Drugs that inhibit immune inflammation or oxidative stress may help stabilize mitochondrial function and reduce mitochondrial damage; thus, mitochondria might also be a therapeutic target for schizophrenia. Previous studies have shown that some psychotropic drugs have antioxidant components, such as N-acetylcysteine and coenzyme Q10, which can improve mitochondrial activity.60 Several studies indicated that some antipsychotic drugs, such as olanzapine and clozapine, could protect mitochondrial activity through their anti-inflammatory and antioxidant effects or regulation of mitochondrial gene expression.53,60,61 However, some typical antipsychotics could negatively impact mitochondria.60 These findings suggested that mitochondria might be a predictor of patient response and a target of treatment for schizophrenia.
In this pilot study, we found that patients who were responsive to treatment showed a considerable drop in the level of mitochondrial damage in T cells. As indicated by Bar-Yosef et al, patient response to treatment was associated with mitochondrial parameters, approximately half of the mitochondrial parameters (respiratory parameters) were improved in patients with schizophrenia who were responsive, but these parameters returned to normal only in those who received long-term treatment.62 Our results also indicated that parameters related to mitochondrial impairment in lymphocytes might be predictors of patient response. Nevertheless, no significant improvement was found in the mitochondrial damage in CD3, CD4, and CD8 lymphocytes after treatment, which might be due to the short follow-up duration (about 24 days) in this study.
Mitochondria are a major source of ROS, and their damage causes the increase of free radicals, resulting in cell dysfunction and neuronal loss.63 Sultana et al demonstrated that the level of ROS produced by mitochondria in peripheral lymphocytes elevated in patients with Alzheimer’s Disease, and the level was inversely correlated with the patients’ cognitive level,63,64 indicating that the level of ROS might reflect the brain impairment in Alzheimer’s Disease and could be a potential biomarker for diagnosis and prognosis.63 Furthermore, many studies suggested that immunological dysfunction and inflammation might contribute to the negative, cognitive, and positive symptoms of schizophrenia.7 In the present study, we attempted to explore the relationship between psychiatric symptoms and mitochondrial damage, but no correlation was found between the degree of mitochondrial damage before and after treatment and the positive, negative, or general symptoms of schizophrenia. This result was inconsistent with the findings of some previous studies. A recent study showed a linear relationship between mitochondrial damage and PANSS scores,56 and Leirer et al found that the expression of mitochondrial genes in the peripheral blood was associated with negative symptoms of schizophrenia.65 Some other investigations have revealed that the degree of mitochondrial deficit in the blood was positively correlated with the severity of paranoid symptoms or PANSS scores.46,50,56 The above studies have suggested an association between mitochondrial dysfunction and psychiatric symptoms. The disparities between our results and previous findings might be attributed to the insufficient sample size to detect a relationship, short treatment duration, different effects of drugs on mitochondria, or different stages of the patient’s disease. Besides, abnormalities in proteins that regulate mitochondrial function were also found in patients with schizophrenia, and such abnormalities might be related to alterations in symptoms and treatment.66 To some extent, mitochondrial damage in lymphocytes can also be used as a biomarker of treatment response.56 This was supported by our findings, which suggested that the improvement in mitochondrial damage of CD3, CD4, and CD8 lymphocytes were positively correlated with the level of improvement of positive symptoms, but not the negative symptoms. Thus, our results indicated that the improvement of positive symptoms might be more associated with mitochondrial improvement, as compared with negative symptoms, which is generally consistent with the findings that the positive and negative symptoms of schizophrenia have different pathways of development, and that the positive symptoms are more associated with immune pathways.65 It was also possible that the sample of our study was too small to detect a relationship between the improvement in negative symptoms and mitochondrial improvement. An open-label experiment showed that the improvement of mitochondrial function was conducive to the recovery of negative symptoms of schizophrenia.67 Thus, future works with larger sample sizes, longer follow-up periods, and controlling for the types of drugs are needed to investigate the connection between mitochondrial damage and schizophrenia, to identify potential therapeutic targets.
Limitations
Some limitations in this study should be considered. The relatively small sample size might have reduced the statistical power to detect significant relationships. Thus, the negative findings might be interpreted as preliminary results, which need to be validated using larger cohorts. Furthermore, without an extended follow-up period and dynamic monitoring of mitochondrial damage in lymphocytes, our study focused primarily on the changes in the indicators before and after treatment. In addition, different antipsychotics might have varied effects on mitochondria, thus not controlling for the medication types might have led to certain inaccuracies. Future studies are warranted to explore the effect of different types of antipsychotic medications on mitochondria. Despite the above limitations, our pilot study is of great value, as it has provided preliminary data on mitochondrial impairment in lymphocytes in patients with schizophrenia. As the mitochondrial data in this study were obtained using peripheral blood, the mitochondrial damage in lymphocytes might only be an indirect indicator of the pathological changes in the central nervous system. Further studies are needed to identify more representative indicators by using more direct methods, such as in vivo quantitative neuroimaging.
Conclusion
In conclusion, this pilot study indicated that the level of mitochondrial damage in CD3, CD4, and CD8 T lymphocytes in the peripheral blood was significantly more severe in patients in an acute episode of relapsed schizophrenia, as compared to healthy controls. After treatment, the improvement of mitochondrial impairment was significant in responsive patients only and was positively associated with the improvement in positive symptoms. These findings suggested that mitochondrial impairment in lymphocytes might be present in patients with schizophrenia, and the degree of its improvement might be used as a predictor for patient response. However, the findings still need further verification in large-scale studies. Despite the limitations, this pilot study helped improve our understanding of the pathophysiology of schizophrenia as well as the role of mitochondria in the treatment of this disease.
Acknowledgments
We thank the patients and providers who participated in the study.
Funding
This work was supported by the Natural Science Foundation of Hunan (Grant No. 2021JJ30962), the National Natural Science Foundation of China (Grant No. 81571306), and the National Key R&D Program of China (Grant No. 2017YFC0907800).
Disclosure
All authors have no conflicts of interest to declare in this work.
References
1. Insel TR. Rethinking schizophrenia. Nature. 2010;468(7321):187–193. doi:10.1038/nature09552
2. McGlashan TH. A selective review of recent North American long-term followup studies of schizophrenia. Schizophr Bull. 1988;14(4):515–542. doi:10.1093/schbul/14.4.515
3. Charlson FJ, Ferrari AJ, Santomauro DF, et al. Global epidemiology and burden of schizophrenia: findings from the global burden of disease study 2016. Schizophr Bull. 2018;44(6):1195–1203. doi:10.1093/schbul/sby058
4. Müller N, Riedel M, Gruber R, et al. The immune system and schizophrenia. An integrative view. Ann N Y Acad Sci. 2000;917(1):456–467. doi:10.1111/j.1749-6632.2000.tb05410.x
5. Müller N. Immunological aspects of the treatment of depression and schizophrenia. Dialogues Clin Neurosci. 2017;19(1):55–63. doi:10.31887/DCNS.2017.19.1/nmueller
6. Ganguli R, Brar JS, Rabin BS. Immune abnormalities in schizophrenia: evidence for the autoimmune hypothesis. Harv Rev Psychiatry. 1994;2(2):70–83. doi:10.3109/10673229409017120
7. Khandaker GM, Cousins L, Deakin J, et al. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry. 2015;2(3):258–270. doi:10.1016/S2215-0366(14)00122-9
8. Manji H, Kato T, Di Prospero NA, et al. Impaired mitochondrial function in psychiatric disorders. Nat Rev Neurosci. 2012;13(5):293–307. doi:10.1038/nrn3229
9. Anderson G, Berk M, Dodd S, et al. Immuno-inflammatory, oxidative and nitrosative stress, and neuroprogressive pathways in the etiology, course and treatment of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:1–4. doi:10.1016/j.pnpbp.2012.10.008
10. Nawa H, Takei N. Recent progress in animal modeling of immune inflammatory processes in schizophrenia: implication of specific cytokines. Neurosci Res. 2006;56(1):2–13. doi:10.1016/j.neures.2006.06.002
11. Busse S, Busse M, Schiltz K, et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun. 2012;26(8):1273–1279. doi:10.1016/j.bbi.2012.08.005
12. Schlaaff K, Dobrowolny H, Frodl T, et al. Increased densities of T and B lymphocytes indicate neuroinflammation in subgroups of schizophrenia and mood disorder patients. Brain Behav Immun. 2020;88:497–506. doi:10.1016/j.bbi.2020.04.021
13. Gladkevich A, Kauffman HF, Korf J. Lymphocytes as a neural probe: potential for studying psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):559–576. doi:10.1016/j.pnpbp.2004.01.009
14. Kim Y, Vadodaria KC, Lenkei Z, et al. Mitochondria, metabolism, and redox mechanisms in psychiatric disorders. Antioxid Redox Signal. 2019;31(4):275–317. doi:10.1089/ars.2018.7606
15. Lemasters JJ. Modulation of mitochondrial membrane permeability in pathogenesis, autophagy and control of metabolism. J Gastroenterol Hepatol. 2007;22(Suppl 1):S31–37. doi:10.1111/j.1440-1746.2006.04643.x
16. Kowaltowski AJ, Castilho RF, Vercesi AE. Mitochondrial permeability transition and oxidative stress. FEBS Lett. 2001;495(1–2):12–15. doi:10.1016/s0014-5793(01)02316-x
17. Zamzami N, Susin SA, Marchetti P, et al. Mitochondrial control of nuclear apoptosis. J Exp Med. 1996;183(4):1533–1544. doi:10.1084/jem.183.4.1533
18. Kantrow SP, Tatro LG, Piantadosi CA. Oxidative stress and adenine nucleotide control of mitochondrial permeability transition. Free Radic Biol Med. 2000;28(2):251–260. doi:10.1016/s0891-5849(99)00238-5
19. Yarana C, Sripetchwandee J, Sanit J, et al. Calcium-induced cardiac mitochondrial dysfunction is predominantly mediated by cyclosporine A-dependent mitochondrial permeability transition pore. Arch Med Res. 2012;43(5):333–338. doi:10.1016/j.arcmed.2012.06.010
20. Kim JS, He L, Lemasters JJ. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun. 2003;304(3):463–470. doi:10.1016/s0006-291x(03)00618-1
21. Kabelitz D. Expression and function of Toll-like receptors in T lymphocytes. Curr Opin Immunol. 2007;19(1):39–45. doi:10.1016/j.coi.2006.11.007
22. Rajasekaran A, Venkatasubramanian G, Berk M, et al. Mitochondrial dysfunction in schizophrenia: pathways, mechanisms and implications. Neurosci Biobehav Rev. 2015;48:10–21. doi:10.1016/j.neubiorev.2014.11.005
23. Pedrini M, Massuda R, Fries GR, et al. Similarities in serum oxidative stress markers and inflammatory cytokines in patients with overt schizophrenia at early and late stages of chronicity. J Psychiatr Res. 2012;46(6):819–824. doi:10.1016/j.jpsychires.2012.03.019
24. Martínez-Cengotitabengoa M, Mac-Dowell KS, Leza JC, et al. Cognitive impairment is related to oxidative stress and chemokine levels in first psychotic episodes. Schizophr Res. 2012;137(1–3):66–72. doi:10.1016/j.schres.2012.03.004
25. Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol. 2009;31(4):497–511. doi:10.1007/s00281-009-0177-0
26. Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi:10.1038/nrn2297
27. Wood SJ, Yücel M, Pantelis C, et al. Neurobiology of schizophrenia spectrum disorders: the role of oxidative stress. Ann Acad Med Singap. 2009;38(5):396.
28. Ben-Shachar D, Ene HM. Mitochondrial targeted therapies: where do we stand in mental disorders? Biol Psychiatry. 2018;83:770–779. doi:10.1016/j.biopsych.2017.08.007
29. Kung L, Roberts RC. Mitochondrial pathology in human schizophrenic striatum: a postmortem ultrastructural study. Synapse. 1999;31:67–75. doi:10.1002/(sici)1098-2396(199901)31:1<67::Aid-syn9>3.0.Co;2-#
30. Uranova N, Orlovskaya D, Vikhreva O, et al. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001;55:597–610. doi:10.1016/s0361-9230(01)00528-7
31. Yu F, Hao Y, Zhao H, et al. Distinct mitochondrial disturbance in CD4+T and CD8+T cells from HIV-infected patients. J Acquir Immune Defic Syndr. 2017;74:206–212. doi:10.1097/qai.0000000000001175
32. Pang LX, Cai WW, Chen L, et al. The diagnostic value of mitochondrial mass of peripheral T lymphocytes in early sepsis. Front Public Health. 2022;10:928306. doi:10.3389/fpubh.2022.928306
33. Scaini G, Mason BL, Diaz AP, et al. Dysregulation of mitochondrial dynamics, mitophagy and apoptosis in major depressive disorder: does inflammation play a role? Mol Psychiatry. 2022;27:1095–1102. doi:10.1038/s41380-021-01312-w
34. Puleston D. Detection of mitochondrial mass, damage, and reactive oxygen species by flow cytometry. Cold Spring Harb Protoc. 2015;2015:
35. Clutton G. Corrigendum: a reproducible, objective method using MitoTracker® fluorescent dyes to assess mitochondrial mass in T cells by flow cytometry. Cytometry A. 2021;99:753. doi:10.1002/cyto.a.24346
36. Wright J, Gray AH, Bruce L, et al. Clinical Pharmacy Pocket Companion. Pharmaceutical Press; 2015.
37. Robicsek O, Karry R, Petit I, et al. Abnormal neuronal differentiation and mitochondrial dysfunction in hair follicle-derived induced pluripotent stem cells of schizophrenia patients. Mol Psychiatry. 2013;18:1067–1076. doi:10.1038/mp.2013.67
38. Ben-Shachar D. Mitochondrial multifaceted dysfunction in schizophrenia; complex I as a possible pathological target. Schizophr Res. 2017;187:3–10. doi:10.1016/j.schres.2016.10.022
39. Cooper JM, Schapira AH. Mitochondrial dysfunction in neurodegeneration. J Bioenerg Biomembr. 1997;29:175–183. doi:10.1023/a:1022642114734
40. Castora FJ. Mitochondrial function and abnormalities implicated in the pathogenesis of ASD. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:83–108. doi:10.1016/j.pnpbp.2018.12.015
41. Picca A, Calvani R, Coelho-Junior HJ, et al. Mitochondrial dysfunction, oxidative stress, and neuroinflammation: intertwined roads to neurodegeneration. Antioxidants. 2020;9. doi:10.3390/antiox9080647
42. Anglin RE, Tarnopolsky MA, Mazurek MF, et al. The psychiatric presentation of mitochondrial disorders in adults. J Neuropsychiatry Clin Neurosci. 2012;24:394–409. doi:10.1176/appi.neuropsych.11110345
43. Kramer P, Bressan P. Our (Mother’s) mitochondria and our mind. Perspect Psychol Sci. 2018;13:88–100. doi:10.1177/1745691617718356
44. Kvajo M, Dhilla A, Swor DE, et al. Evidence implicating the candidate schizophrenia/bipolar disorder susceptibility gene G72 in mitochondrial function. Mol Psychiatry. 2008;13:685–696. doi:10.1038/sj.mp.4002052
45. Karry R, Klein E, Ben Shachar D. Mitochondrial complex I subunits expression is altered in schizophrenia: a postmortem study. Biol Psychiatry. 2004;55:676–684. doi:10.1016/j.biopsych.2003.12.012
46. Roberts RC. Mitochondrial dysfunction in schizophrenia: with a focus on postmortem studies. Mitochondrion. 2021;56:91–101. doi:10.1016/j.mito.2020.11.009
47. Takahashi Y. An enzymological study on brain tissue of schizophrenic patients. carbohydrate metabolism. Part I. Glucose. Psychiatry Clin Neurosci. 1953;7:214–237.
48. Uranova NA, Vikhreva OV, Rakhmanova VI, et al. Ultrastructural pathology of oligodendrocytes adjacent to microglia in prefrontal white matter in schizophrenia. NPJ Schizophr. 2018;4:26. doi:10.1038/s41537-018-0068-2
49. Uranova NA, Vikhreva OV, Rakhmanova VI, et al. Dystrophy of oligodendrocytes and adjacent microglia in prefrontal gray matter in schizophrenia. Front Psychiatry. 2020;11:204. doi:10.3389/fpsyt.2020.00204
50. Uranova N, Bonartsev P, Brusov O, et al. The ultrastructure of lymphocytes in schizophrenia. World J Biol Psychiatry. 2007;8:30–37. doi:10.1080/15622970600960207
51. Inuwa IM, Peet M, Williams MA. QSAR modeling and transmission electron microscopy stereology of altered mitochondrial ultrastructure of white blood cells in patients diagnosed as schizophrenic and treated with antipsychotic drugs. Biotech Histochem. 2005;80:133–137. doi:10.1080/10520290500303349
52. Park C and Park S Ki. (2012). Molecular links between mitochondrial dysfunctions and schizophrenia. Mol Cells, 33(2), 105–110. doi:10.1007/s10059-012-2284-3
53. Chestkov IV, Jestkova EM, Ershova ES, et al. ROS-induced DNA damage associates with abundance of mitochondrial DNA in white blood cells of the untreated schizophrenic patients. Oxid Med Cell Longev. 2018;2018:8587475. doi:10.1155/2018/8587475
54. Rosenfeld M, Brenner-Lavie H, Ari SG, et al. Perturbation in mitochondrial network dynamics and in complex I dependent cellular respiration in schizophrenia. Biol Psychiatry. 2011;69:980–988. doi:10.1016/j.biopsych.2011.01.010
55. Amiri S, Dizaji R, Momeny M, et al. Clozapine attenuates mitochondrial dysfunction, inflammatory gene expression, and behavioral abnormalities in an animal model of schizophrenia. Neuropharmacology. 2021;187:108503. doi:10.1016/j.neuropharm.2021.108503
56. Tianyi Z, Shize L, Yichuan L, et al. Study on the mitochondrial mass of peripheral blood immune cells in patients with schizophrenia. Chin J Psychiatry. 2022:254–262. doi:10.3760/cma.j.cn113661-20220322-00068.
57. Miller BJ, Gassama B, Sebastian D, et al. Meta-analysis of lymphocytes in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2013;73:993–999. doi:10.1016/j.biopsych.2012.09.007
58. Maino K, Gruber R, Riedel M, et al. T- and B-lymphocytes in patients with schizophrenia in acute psychotic episode and the course of the treatment. Psychiatry Res. 2007;152:173–180. doi:10.1016/j.psychres.2006.06.004
59. Faas MM, de Vos P. Mitochondrial function in immune cells in health and disease. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165845. doi:10.1016/j.bbadis.2020.165845
60. Chan ST, McCarthy MJ, Vawter MP. Psychiatric drugs impact mitochondrial function in brain and other tissues. Schizophr Res. 2020;217:136–147. doi:10.1016/j.schres.2019.09.007
61. Möller M, Du Preez JL, Viljoen FP, et al. Social isolation rearing induces mitochondrial, immunological, neurochemical and behavioural deficits in rats, and is reversed by clozapine or N-acetyl cysteine. Brain Behav Immun. 2013;30:156–167. doi:10.1016/j.bbi.2012.12.011
62. Bar-Yosef T, Hussein W, Yitzhaki O, et al. Mitochondrial function parameters as a tool for tailored drug treatment of an individual with psychosis: a proof of concept study. Sci Rep. 2020;10:12258. doi:10.1038/s41598-020-69207-4
63. Sultana R, Baglioni M, Cecchetti R, et al. Lymphocyte mitochondria: toward identification of peripheral biomarkers in the progression of Alzheimer disease. Free Radic Biol Med. 2013;65:595–606. doi:10.1016/j.freeradbiomed.2013.08.001
64. Sultana R, Mecocci P, Mangialasche F, et al. Increased protein and lipid oxidative damage in mitochondria isolated from lymphocytes from patients with Alzheimer’s disease: insights into the role of oxidative stress in Alzheimer’s disease and initial investigations into a potential biomarker for this dementing disorder. J Alzheimers Dis. 2011;24:77–84. doi:10.3233/jad-2011-101425
65. Leirer DJ, Iyegbe CO, Di Forti M, et al. Differential gene expression analysis in blood of first episode psychosis patients. Schizophr Res. 2019;209:88–97. doi:10.1016/j.schres.2019.05.011
66. Goetzl EJ, Srihari VH, Guloksuz S, et al. Neural cell-derived plasma exosome protein abnormalities implicate mitochondrial impairment in first episodes of psychosis. FASEB j. 2021;35:e21339. doi:10.1096/fj.202002519R
67. Bruno A, Pandolfo G, Crucitti M, et al. Acetyl-L-carnitine augmentation of clozapine in partial-responder schizophrenia: a 12-week, open-label uncontrolled preliminary study. Clin Neuropharmacol. 2016;39:277–280. doi:10.1097/wnf.0000000000000170
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.