Back to Journals » OncoTargets and Therapy » Volume 12
Mitochondria P-glycoprotein confers paclitaxel resistance on ovarian cancer cells
Authors Guo W, Dong W, Li M, Shen Y
Received 4 November 2018
Accepted for publication 27 February 2019
Published 17 May 2019 Volume 2019:12 Pages 3881—3891
DOI https://doi.org/10.2147/OTT.S193433
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Federico Perche
Weina Guo,* Weihong Dong,* Min Li, Yi Shen
Department of Obstetrics and Gynecology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, China
*These authors contributed equally to this work
Background: Subcellular expression of P-glycoprotein (P-gp) may play an essential role in multidrug resistance (MDR) in many cancers. However, the mitochondria expression and functional activity of P-gp in ovarian cancer are still unclear. In this study, we isolated mitochondria from A2780 cell line and its paclitaxel-resistant subline A2780T and investigated the expression and function of mitochondria P-gp.
Methods: Immunocytochemistry was used to evaluate P-gp expression and subcellular localization in cancer cells. Immunofluorescence and laser confocal microscopy were used to detect the co-localization of P-gp and mitochondria both in ovarian cancer tissues and in cell lines. Western blotting (WB), transmission electron microscopy and JC-1 kit were used to evaluate the purity, integrity and activity of the isolated mitochondria. P-gp expression in the whole cell and the isolated mitochondria was evaluated by WB. Flow cytometry was used to evaluate the efflux function of mitochondria P-gp.
Results: P-gp expression was detected at the membrane, cytoplasm and nuclei of the A2780T cells, but not in the A2780 cells. Co-localization of P-gp and mitochondria was observed in the A2780T cell line and ovarian cancer tissues, but not in A2780 cells. The purity, integration and activity of the isolated mitochondria are high. P-gp was highly expressed in the A2780T cells and the isolated mitochondria, but was not found in A2780 cells. Rho123 efflux rate was significantly increased in isolated A2780T mitochondria compared to those in A2780 (43.2% vs 9.6%), but it was partly reversed by cyclosporin A (CsA, a P-gp inhibitor).
Conclusion: P-gp is highly expressed in mitochondria of taxol-resistant ovarian cancer cells and ovarian cancer tissues and mediates the drug efflux, which probably protect cancer cells from chemotherapy.
Keywords: ovarian carcinoma, mitochondria, P-glycoprotein, multidrug resistance
Introduction
Chemotherapy combined with cytoreductive surgery is the standard treatment for ovarian cancer. However, <50% of women with ovarian cancer can survive 5 years after primary treatment.1–3 Multidrug resistance (MDR) employed by cancer cells represents the bottleneck of cancer chemotherapy. MDR is a multifactorial phenomenon. In spite of considerable efforts being been made in this field over the last four decades, the molecular mechanisms involved in MDR have not yet been fully elucidated.4 The MDR phenotype is characterized by the overexpression of drug efflux pumps on cellular membrane.5 P-glycoprotein (P-gp, 170 kDa) encoded by the multi-drug resistance-1 (Mdr1) gene, namely ABCB1, belongs to the family of ATP-binding cassette (ABC) transporters that mediate intrinsic and acquired drug resistance in cancer cells.4–6 High-level P-gp expression is associated with shorter progression-free survival (PFS) in patients with epithelial ovarian cancer, including in suboptimally debulked patients.7
It is well known that the P-gp at plasma membrane functions by extruding drugs and other toxic agents by ATP binding and hydrolysis.8 As respected, P-gp is able to actively extrude more than 20 cytostatic drugs from cell,9 which resulted in MDR in many cancer.10 Interestingly, ABC transporters have been detected in cell nucleus, Golgi apparatus, and endoplasmic reticulum in colon adenocarcinoma cells and lung cancer cells,11,12 which suggested intracellular ABC transporters may be also involved in MDR in cancers. Mitochondria play a crucial role in cell homeostasis which determines the survival of cells.13 Mitochondrial dysfunction involved in tumor microenvironment has been associated with the selective response to certain chemotherapeutic drugs of malignant tumors.14 Previous study found that decreased mitochondrial priming determines chemoresistance of colon cancer stem cells.15 Moreover, mitochondrial location of P-gp is a controversial topic.16,17 Ho et al found that Mdr1 had an unexpected protective role for the mitochondria homeostasis in intestinal inflammation and Mdr1 deficiency increased mitochondrial ROS (mROS) resulting in mitochondrial dysfunction.18 However, the intracellular expression of P-gp and its function in ovarian cancer remains unexplored.
Most chemotherapies function by inducing a form of irreversible programmed cell death called apoptosis, including intrinsic and extrinsic pathways.19 In the intrinsic pathway, mitochondrial outer membrane permeabilization (MOMP) is the crucial event that drives initiator caspase activation and apoptosis.20,21 It is triggered clinically when a chemotherapeutic agent induces a sufficient amount of stress within a cancer cell by damaging critical cellular components such as microtubules, DNA, or key signaling pathways.22,23 Similar to other taxanes, paclitaxel binds to the β-subunit of tubulin, stabilizes microtubule dynamics, and causes mitotic arrest at G2/M phase, thus triggering apoptosis.24 Previous studies found that the activation of Bax/Bak, release of cytochrome c, and selective activation of caspase-9/Apaf-1 were detected in paclitaxel-treated breast cancer cells.20,25 The best-known mechanism involved in the cytotoxic effect by platinum is the generation of DNA adducts, the activation of the DNA damage response, and the induction of mitochondrial apoptosis pathway.26 These results suggest that the paclitaxel/platinum-induced cell death partly occurs through the mitochondrial apoptotic pathway. Both cisplatin and paclitaxel are the substrates of P-gp,9 and exposure to cisplatin could cause the upregulation of P-gp in porcine kidney epithelial LLC-PK1 cells and renal brush-border membranes.27,28 P-gp upregulation in the liver, kidney, and brain of oxycodone-treated rats significantly hindered the accumulation of paclitaxel in these tissues.29 For patients with ovarian cancer, Mdr1 gene expression is supposed to be a useful predictor for paclitaxel-based chemotherapy.30 Therefore, P-gp in mitochondria may play a protective role against the chemotherapies, thus resulting in chemoresistance.
The expression of P-gp in mitochondrial membrane has been controversial reported.16,31–33 It is still inconclusive whether the mitochondrial sequestration of drugs is associated with mitochondrial localization of P-gp or not and the potential role of P-gp in mitochondria apoptosis pathway. We used paclitaxel-resistant cell line A2780T and its parental line A2780, along with ovarian cancer biopsies to conduct our experiment. A2780 is an ovarian cancer cell line that was established from an ovarian endometroid adenocarcinoma tumor in an untreated patient. It has an epithelial morphology and was one of the most commonly used cell line model of ovarian cancer. A2780T is a paclitaxel-resistant cell line which was derived from A2780 cells.34 The present study was aimed to investigate the mitochondrial expression of P-gp and its role in subcellular distribution of paclitaxel and chemoresistance in epithelial ovarian cancer. The results may deepen our understanding of the mechanisms underlying the P-gp-associated chemoresistance and catalyze new ideas overcoming the resistance of ovarian cancer.
Materials and methods
Patients and ethical statement
Paraffin-embedded specimens were obtained from 6 patients at the Department of Obstetrics and Gynecology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (Union Hospital, Wuhan, China) during 2017–2018. Patients had not received any radiotherapy or chemotherapy before the biopsy was performed. Cases included chemotherapy-sensitive patients (n=3; patient who relapsed within 6 months after the end of 6 courses of chemotherapy treatment) and chemotherapy-resistant patients (n=3; patient who had no recurrence or progression within 6 months after the end of 6 courses of chemotherapy treatment). All ovarian cancer patients were diagnosed with epithelial ovarian carcinoma following histological examination. Collection and archiving of patient data and the use of human tissue samples were carried out with written informed consent, and this study was approved by the Ethics Committee of the hospital.
Cell culture and ethical statement
The human A2780T and A2780 cell lines were kindly donated from the Department of Obstetrics and Gynecology of Wuhan Tongji Hospital (Wuhan, China). The IC50 of A2780T and A2780 are 0.042 μM and 2.213 μM, respectively. A2780 cells were routinely cultured in DMEM (Life Technologies, Carlsbad, CA, USA) supplemented with 10% FBS (Gibco, Carlsbad, CA, USA) in a humidified atmosphere of 5% CO2 at 37°C. For A2780T cells, 0.1 μM paclitaxel was added to the medium to maintain the resistance. The use of two cell lines in this study was approved by the Ethics Committee of the hospital (Union Hospital, Wuhan, China). Mitotracker Deep Red FM was purchased from Molecular Probes (Invitrogen, Carlsbad, CA, USA), and the mitochondrial fluorescent probe Rho123 was purchased from Sigma-Aldrich (St. Louis, MO, USA). P-gp inhibitor cyclosporin A (CsA) was obtained from Absin (Shanghai, China). Mitochondrial membrane potential assay kit JC-1 was purchased from Beyotime Biotechnology (Beijing, China). Mouse monoclonal anti-P-gp/Mdr-1 (D-11: sc-55510) was purchased from Santa Cruz Biochemistry (, Dallas, TX, USA). Anti-HSP60 (12165P), anti-cytochrome c (4280T), and anti-TOM20 (42406S) were purchased from Cell Signal Technology (Danvers, MA, USA). Anti-β-actin mouse monoclonal antibody was obtained from BD Biosciences (San Jose, CA, USA).
Preparation of mitochondria fraction
Mitochondria were extracted according to the method provided by Solazzo et al.6 Briefly, 1×108 cells were harvested and washed twice with ice-cold PBS, and the pellets were resuspended with 600 μL ice-cold buffer A (20 mM HEPES [pH 7.5], 1.5 mM MgCl2, 10 mM KCl, 1 mM EDTA, 1 mM ethylene glycol bis (2-aminoethyl ether) tetraacetic acid, 1 mM dithiothreitol, 0.1 mM phenylmethanesulfonyl fluoride, and 1% cocktail [Sigma]) for 10 mins and then added 200 μL 1 M sucrose to make the final concentration at 250 mM. Thereafter, the cell suspension was passed five times through a 26-gauge needle fitted to a syringe quickly crushing cells to make at least 80% of the cells broken. Large plasma membrane pieces, nuclei, and unbroken cells were removed by centrifuging at 1,000× g at 4°C for 10 mins. The supernatant was subjected to 10,000× g at 4°C for 20 mins. The pellet (mitochondria) was rinsed with the above buffer A containing sucrose. The supernatant was recentrifuged at 1,00,000× g at 4°C for 1 hr to generate cytosol. The pellet of mitochondria and cytosol was kept at −80°C for later use.
Identify the purity and integrity of isolated mitochondria
The integrity and purity of the obtained mitochondrial fraction were identified by transmission electron microscopy (TEM) and western blot. Preparation of ultrathin sections and immunoelectron microscopy analysis were performed as previously described.31 Briefly, the pellet of mitochondria was fixed with 2.5% glutaraldehyde at 4°C for at least 30 mins and then washed with PBS. Afterward, 1% osmic acid was used for fixation over 1 hr, dehydrated by acetone gradient followed by resin embedding. The purity and integrity of mitochondria in ultrathin section was observed by TEM (Olympus, Tokyo, Japan). The protein lysates of cytosol and mitochondria were extracted by acetone and dissolved in 8 M urea.35 The protein concentration was determined by bicinchoninic acid (BCA) method (Beyotime, Haimen, China). For western blot, equal amount of protein of two fractions (mitochondria and cytosol) was separated by 10% SDS-PAGE and transferred onto 0.22 μm polyvinylidene fluoride (PVDF) membrane (Bio-Rad, Hercules, CA, USA). The membrane was blocked (5% skim milk), and then probed with primary antibody anti-HSP60 (1:1,000), anticytochrome c (1:1,000), and anti-β-actin (1:5,000) at 4°C overnight, respectively. Then, Horseradish Peroxidase (HRP)-conjugated antirabbit and mouse IgG antibody were used as secondary antibody. Then, the immunoreactive proteins on the membranes were exposed to Bio-Max MR film (Bio-Rad). HSP-60 and cytochrome c proteins were used as markers for the purity of mitochondrial preparations.6
Measurement of isolated mitochondrial membrane potential by JC-1 kit
Isolated mitochondria were suspended in JC-1 buffer solution and incubated with PBS, JC-1 and JC-1+carbonyl cyanide 3-chlorophenylhydrazone (CCCP), respectively, at 37°C for 15 mins. CCCP is a chemical inhibitor of oxidative phosphorylation, which can decrease the potential of mitochondria membrane. The mitochondria solution was centrifuged at 800× g at 4°C for 5 mins and rinsed twice. The pellets were resuspended and then analyzed by Flow Cytometry (Beckman Coulter, Brea, CA, USA; MoFloTMXDP).
Immunocytochemistry analysis of the expression of P-gp in whole cells
Cells seeded in 24-well plates were fixed in 3.7% paraformaldehyde and penetrated with 0.2% Txion-100, followed by 3% hydrogen peroxide at room temperature (RT) for 10 mins, respectively. Cells were blocked with 5% normal goat serum, then incubated with anti-P-gp antibody (1:100) and secondary antibody (1:4000) at 37℃ for 30 mins, respectively. Then cells were dyed with DAB at RT for 2 mins followed by hematoxylin. Finally, the plate was washed with running water at RT for 15 mins. Finally, cells were photographed by optical microscope (Olympus IX71). Experiments were performed in triplicates.
In situ analysis of P-gp expression by confocal laser scanning and fluorescence microscopy
The A2780 and A2780T cells were seeded on glass coverslips overnight and then stained with 200 nM Mitotracker Deep Red FM (Invitrogen) (a cell-permeable mitochondria selective dye) at 37°C for 20 mins. Paraffin-embedded specimens were routinely dewaxed and antigen repaired. Cells or tissues were fixed and penetrated as mentioned in the section “Immunocytochemistry analysis of the expression of P-gp in whole cells”. Following blocking with 5% normal goat serum for 1 hr, cells were incubated with anti-P-gp antibody (1:100) and tissues were incubated with anti-P-gp (1:200) and anti-TOM20 (1:200) antibody overnight at 4°C and then immunostained with secondary antibody, respectively. The nuclei were stained by DAPI (1:5,000). Finally, the coverslips were mounted and observed under confocal laser scanning microscope (CLSM) (Nikon, Tokyo, Japan; A1-Si). The specimens were observed under fluorescence microscopy (Olympus IX71).
Western blotting analysis the expression of P-gp in isolated mitochondria
Cells were harvested and lysed with RIPA (Beyotime P0013 C), and then centrifuged at 12,000× g at 4°C for 15 mins. The protein concentration was determined by BCA method. Equal amount protein was separated by 10% SDS-PAGE and transferred onto PVDF membrane. The membrane was incubated with the indicated anti-P-gp antibody (1:1,000) overnight, and the other steps were the same as described in the section “Identify the purity and integrity of isolated mitochondria”.
Function assay of mitochondrial P-gp by flow cytometry
The pump function of mitochondrial P-gp was measured by the efflux of fluorescent probe Rhodamine 123 (Rho123) as described previously.31 Briefly, isolated mitochondria extracted from A2780 and A2780T cells were resuspended with ice-cold buffer A (preparation as above), then were divided into two groups to detect the Rho123 fluorescence intensity (control group and intervention group), according to whether adding P-gp inhibitor CsA or not. The fluorescence of unstained mitochondria was regarded as blank value. In the intervention group, 5 μM CsA was pre-added at RT for 30 mins; the control group received an equal volume of vehicle DMSO. In order to detect Rho123 intake rate, the Rho123 (5 μg/mL) was added at RT for 4 mins in dark and then resuspended in 500 μL buffer A. To detect Rho123 efflux rate, after a 4-min incubation in Rho123, the mitochondria were centrifuged at 450× g at 4°C for 5 mins and then resuspended in 500 μL buffer A and incubated at RT for another 6 mins, allowing Rho123 efflux from the mitochondria. The fluorescence intensity was determined by the Flow Cytometer (Beckman Coulter MoFloTMXDP). The experiment was repeated at least three times.
Statistical analysis
The experimental data for Rho123 fluorescence intensity are provided as means ± SD. An independent-samples t-test was performed using the statistical software SPSS13.0. P<0.05 was considered significant.
Results
Identification of the purity, integrity and activity of isolated mitochondria
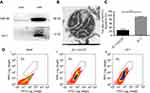
The purity, integrity and activity of mitochondria isolated from A2780 cells were assessed using WB, electron microscopy, and JC-1 accumulation assays, respectively (Figure 1). HSP60 and cytochrome c marked the purity of isolated mitochondria.33 Western blot analysis showed that HSP-60 and cytochrome c were predominantly expressed in the mitochondrial rather than in the cytosol, confirming the high purity and integrity of isolated mitochondria without leaking of cytochrome c into the cytosol during the process of purification (Figure 1A). Electron microscopy analysis showed that the isolated mitochondria had a complete bilayer membrane and ridge structure (Figure 1B). Flow cytometry analysis indicated that the ratio of phycoerythrin (PE)/fluorescein isothiocyanate (FITC) fluorescence in the JC-1 group, referencing the potential of isolated mitochondria, was significantly higher than that in the JC-1 + CCCP group (P<0.001, Figure 1C and D). The CCCP treatment served as a positive control, as CCCP is a potent oxidative phosphorylation uncoupler, resulting in the loss of membrane potential on both sides of the mitochondrial inner membrane. These results demonstrated the high quality of the isolated mitochondria.
P-gp expression in the mitochondria
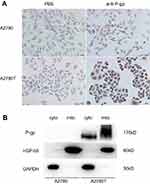
The results of immunocytochemistry demonstrated that P-gp was expressed in the cell membrane, cytoplasm, and nuclei of A2780T cells but not in the A2780 cells (Figure 2A). Western blot showed that the P-gp was highly expressed in the mitochondria and cytosol of A2780T, while it was negatively expressed in A2780 cells (Figure 2B).
Co-localization of p-gp and mitochondria in A2780T cells and ovarian cancer tissues
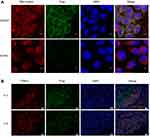
To estimate intuitively whether P-gp locates at mitochondria or not, A2780 and A2780T cells were stained with MitoTracker Deep Red FM (red fluorescence) and anti-P-gp MAb D-11 (green fluorescence) and analyzed by a confocal microscopy (Figure 3A). Dual-channel excitation of both fluorophores was employed to observe P-gp and mitochondrial co-localization, as shown by fluorescent orange. There is apparent co-localization of P-gp and mitochondria in A2780T cells with a Pearson’s correlation coefficient R of 0.6. In contrast, no obvious expression of P-gp was found in the mitochondria of A2780 cells (Figure 3A).
In order to verify the above experimental results, we conducted immunofluorescence experiments on clinical specimens. As shown in Figure 3B, P-gp is highly expressed in ovarian cancer tissues, and the expression of P-gp in tissues of drug-resistant patients is higher than that in sensitive patients. P-gp (green fluorescence) is co-localized with mitochondrial outer membrane marker TOM20 (red fluorescence). Orange is the overlying fluorescence, which represents the co-localization of P-gp and mitochondria.
The active efflux function of mitochondrial P-gp
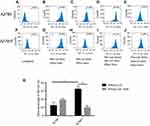
The pump function of mitochondrial P-gp was assessed by flow cytometry, via comparing the intensity of Rho123 in isolated mitochondria with or without adding P-gp inhibitor CsA (Figure 4). Rho123 is a fluorescent dye that selectively stains the mitochondria of living cells and can be specifically transported by P-gp.36 In both cell lines, the fluorescence of unstained isolated mitochondria was detected as blank value (Figure 4A, F). In control group without CsA treatment, the Rho123 intake rate of mitochondria (exhibited as the intensity of fluorescence) isolated from both cell lines increases beyond 90%, which partly suggested the integrity of isolated mitochondria (Figure 4B, G). After 6 mins efflux, the retained fluorescence of mitochondria isolated from A2780T was significantly lower than that from A2780 (P=0.03, Figure 4C, H, K). However, in the intervention group with CsA pretreatment, the retained fluorescence intensity of mitochondria isolated from A2780T was significantly higher than that of the control group (P=0.015, Figure 4I, J, K). This phenomenon was not found in mitochondria isolated from A2780 (P>0.05, Figure 4D, E, K).
Discussion
Acquired drug resistance of the tumor is a key factor in the relapse of patients who respond well to the initial chemotherapy treatment. One of the main mechanisms is overexpression of P-gp in cancer cells, which contributes to the MDR phenotype37 and seems as a promising molecular target to overcome MDR. However, many attempts to find a successful therapeutic approach to inhibit the drug efflux pump for overcoming MDR in cancer have largely failed to date.38 Intracellular location of P-gp may partially explain this phenomenon. A previous study demonstrated that P-gp is expressed in intracellular membranes, such as nucleus,39 ER,40 Golgi apparatus,41 endosome,42 and lysosomes,43 which may influence the intracellular trafficking of drugs. Different from those organelles, mitochondria are the center of cellular metabolism and energy production. And mitochondrial apoptosis pathway is the specific target of many chemotherapeutic agents. Therefore, we supposed that P-gp in mitochondrial membrane plays roles in redistributing drugs and protecting mitochondria against apoptosis. However, due to the restrictions of mitochondria purification, the expression and function of P-gp in mitochondria still remain controversial. Our study found that P-gp is expressed in mitochondria and pumps out drugs from mitochondria.
Ensuring the purity and completeness of mitochondria is a difficult task in the mitochondria extraction process due to the variety of subcellular organelles. However, because of the expression of P-gp on the cell membrane and other organelle membranes, the purity and integrity of mitochondrial extraction is particularly important for the study of subsequent mechanism underlying mitochondrial MDR. In this study, we used modified sucrose density gradient centrifugation to obtain purified mitochondria. Western blot analysis confirmed that cytochrome c was only expressed in mitochondria which suggested that there is no leaking of cytochrome c out of mitochondria during the purification process.6 In addition, HSP-60 was more expressed in the mitochondria than in the cytosol. Electron microscopy images indicated that purified mitochondria had complete bilayer membrane and clear ridge structure. To confirm the activity of the purified mitochondria, we used JC-1 kit to measure the membrane potential of isolated mitochondria. Flow cytometry analysis showed the isolated mitochondria possessed high membrane potential, which was reversed when adding CCCP (a potent oxidative phosphorylation uncoupler). All the above confirmed the successful extraction of mitochondria.
This study shows that P-gp expressed in mitochondria isolated from the human acquired paclitaxel-resistant ovarian cancer cells A2780T but not in their parental drug-sensitive cells A2780. CLSM revealed that P-gp labeled by green fluorescence was located in the cell membrane of A2780T as well as perinuclear region, co-localizing with red fluorescent-labeled mitochondria (orange fluorescence). However, no green fluorescence was found in A2780 cells. This was confirmed by our western blot analysis of mitochondria and cytosol protein, which showed a specific band in 170 kD in both parts of A2780T but not in A2780. Our results were consistent with the previous study conducted by Chen et al,44 who used stable isotope labeling by amino acids in cell culture (SILAC)-based quantitative proteomic strategy to compare mitochondrial protein expression in doxorubicin-resistant OVCAR8 cells and found increased expression of P-gp in the mitochondria, twenty-four fold higher than that in sensitive cells. Considering the limitations of cell experiment, we validated the co-localization of P-gp and mitochondria using human ovarian cancer tissues. Different from the cell line, P-gp was expressed in both sensitive and resistant ovarian cancer tissues, with higher expression in the latter.
There were conflicting views concerning the role of intracellular P-gp in drug resistance. Fu et al found that intracellular compartments (ER and Golgi) contributed to drug sequestration and re-localization of P-gp from plasma membrane to intracellular organelles could be a potential way to overcome MDR.40 Munteanu et al demonstrated that mitochondrial P-gp was involved in drug accumulation but not in its efflux function.45 However, in the results of our study, extracellular experiments using flow cytometry confirmed that the isolated mitochondria of A2780T could efflux Rho123 (a mitochondrial fluorescent probe), and the phenomenon could be partly reversed by P-gp inhibitor CsA. While in drug-sensitive cell A2780, Rho123 accumulated and maintained in mitochondria, the intensity of which has not been changed after CsA treatment. Confusingly, there were some differences between Figure 4C and E. We suspect that CsA may affect mitochondrial membrane potential, causing the outflow of Rho123 in mitochondria. This needs further research to confirm. The results indicated that the role of mitochondrial P-gp in A2780T was to efflux drugs from mitochondria, thus protecting mitochondria from proapoptotic factors induced by chemotherapy agents.
Our study demonstrated that P-gp is expressed in mitochondria of paclitaxel-resistant ovarian cell and pumps out drugs from mitochondria. This suggests that P-gp may work as antiapoptotic factor, as well as drug-efflux pump, to protect mitochondria against apoptosis. This provides a good explanation for the following findings: research by Ni Chonghaile et al proposed that differential mitochondrial priming, which means the pretreatment proximity of tumor cell mitochondria to the apoptotic threshold, may be a determinant of differential chemosensitivity.46 They also found that chemoresistant cancers and normal tissues were poorly primed compared with the chemosensitive cancers. In the study of cross-talking between stromal and cancer cells, Pasquier et al demonstrated that cancer cells and endothelial cells could exchange mitochondria between each other; thus cancer cells acquire mitochondria and become chemoresistant.47 These results suggested that cancer cells could transmit resistant phenotypes through mitochondria transportation. Therefore, exploring the functional status and proteomics of mitochondria in drug-resistant cells, such as P-gp, may be the key to understand the underlying mechanisms of MDR.
Conclusion
In summary, our study strongly demonstrated that P-gp is highly expressed in mitochondria in taxol-resistant ovarian cancer cells and mediates drug efflux from mitochondria. The presence and efflux effect of P-gp in mitochondria of human ovarian cells probably protect cancer cells from chemotherapy. The potential antiapoptotic effect of P-gp on mitochondria is worthy of further study.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (grant number: 81201720) and Hubei Provincial Natural Science Foundation of China (grant number: 2018CFB726)
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vaughan S, Coward JI, Bast RC
2. Holmes D. Ovarian cancer: beyond resistance. Nature. 2015;527(7579):S217. doi:10.1038/nature15724
3. Coleman MP, Forman D, Bryant H, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995-2007 (the international cancer benchmarking partnership): an analysis of population-based cancer registry data. Lancet (London, England). 2011;377(9760):127–138. doi:10.1016/S0140-6736(10)62231-3
4. Kunjachan S, Rychlik B, Storm G, Kiessling F, Lammers T. Multidrug resistance: physiological principles and nanomedical solutions. Adv Drug Deliv Rev. 2013;65(13–14):1852–1865. doi:10.1016/j.addr.2013.09.018
5. Fantappie O, Masini E, Sardi I, et al. The MDR phenotype is associated with the expression of COX-2 and iNOS in a human hepatocellular carcinoma cell line. Hepatology (Baltimore, Md). 2002;35(4):843–852. doi:10.1053/jhep.2002.32469
6. Solazzo M, Fantappie O, D’Amico M, et al. Mitochondrial expression and functional activity of breast cancer resistance protein in different multiple drug-resistant cell lines. Cancer Res. 2009;69(18):7235–7242. doi:10.1158/0008-5472.CAN-08-4315
7. Johnatty SE, Beesley J, Gao B, et al. ABCB1 (MDR1) polymorphisms and ovarian cancer progression and survival: a comprehensive analysis from the ovarian cancer association consortium and the cancer genome atlas. Gynecol Oncol. 2013;131(1):8–14. doi:10.1016/j.ygyno.2013.07.107
8. Rocco A, Compare D, Liguori E, et al. MDR1-P-glycoprotein behaves as an oncofetal protein that promotes cell survival in gastric cancer cells. Lab Invest. 2012;92(10):1407–1418. doi:10.1038/labinvest.2012.100
9. Silva R, Vilas-Boas V, Carmo H, et al. Modulation of P-glycoprotein efflux pump: induction and activation as a therapeutic strategy. Pharmacol Ther. 2015;149:1–123. doi:10.1016/j.pharmthera.2014.11.013
10. Januchowski R, Wojtowicz K, Sujka-Kordowska P, Andrzejewska M, Zabel M. MDR gene expression analysis of six drug-resistant ovarian cancer cell lines. Biomed Res Int. 2013;2013:1–10. doi:10.1155/2013/241763
11. Meschini S, Calcabrini A, Monti E, et al. Intracellular P-glycoprotein expression is associated with the intrinsic multidrug resistance phenotype in human colon adenocarcinoma cells. Int J Cancer. 2000;87(5):615–628.
12. Kim HR, Lee KY, Ahn SG, et al. Transcriptional regulation, stabilization, and subcellular redistribution of multidrug resistance-associated protein 1 (MRP1) by glycogen synthase kinase 3alphabeta: novel insights on modes of cadmium-induced cell death stimulated by MRP1. Arch Toxicol. 2015;89(8):1271–1284. doi:10.1007/s00204-014-1381-9
13. Tan AS, Baty JW, Dong LF, et al. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell Metab. 2015;21(1):81–94. doi:10.1016/j.cmet.2014.12.003
14. Guaragnella N, Giannattasio S, Moro L. Mitochondrial dysfunction in cancer chemoresistance. Biochem Pharmacol. 2014;92(1):62–72. doi:10.1016/j.bcp.2014.07.027
15. Colak S, Zimberlin CD, Fessler E, et al. Decreased mitochondrial priming determines chemoresistance of colon cancer stem cells. Cell Death Differ. 2014;21(7):1170–1177. doi:10.1038/cdd.2014.37
16. Paterson JK, Gottesman MM. P-Glycoprotein is not present in mitochondrial membranes. Exp Cell Res. 2007;313(14):3100–3105. doi:10.1016/j.yexcr.2007.04.019
17. Fu D, Arias IM. Intracellular trafficking of P-glycoprotein. Int J Biochem Cell Biol. 2012;44(3):461–464. doi:10.1016/j.biocel.2011.12.009
18. Ho GT, Aird RE, Liu B, et al. MDR1 deficiency impairs mitochondrial homeostasis and promotes intestinal inflammation. Mucosal Immunol. 2018;11:120–130.
19. Sarosiek KA, Ni Chonghaile T, Letai A. Mitochondria: gatekeepers of response to chemotherapy. Trends Cell Biol. 2013;23(12):612–619. doi:10.1016/j.tcb.2013.08.003
20. Kutuk O, Letai A. Displacement of Bim by Bmf and Puma rather than increase in Bim level mediates paclitaxel-induced apoptosis in breast cancer cells. Cell Death Differ. 2010;17(10):1624–1635. doi:10.1038/cdd.2010.41
21. Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11(9):621–632. doi:10.1038/nrm2952
22. Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 2000;256(1):42–49. doi:10.1006/excr.2000.4838
23. Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25(34):4798–4811. doi:10.1038/sj.onc.1209608
24. Wang TH, Wang HS, Soong YK. Paclitaxel-induced cell death: where the cell cycle and apoptosis come together. Cancer. 2000;88(11):2619–2628.
25. Janssen K, Pohlmann S, Janicke RU, Schulze-Osthoff K, Fischer U. Apaf-1 and caspase-9 deficiency prevents apoptosis in a bax-controlled pathway and promotes clonogenic survival during paclitaxel treatment. Blood. 2007;110(10):3662–3672. doi:10.1182/blood-2007-02-073213
26. Galluzzi L, Senovilla L, Vitale I, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31(15):1869–1883. doi:10.1038/onc.2011.384
27. Takara K, Tsujimoto M, Kokufu M, Ohnishi N, Yokoyama T. Up-regulation of MDR1 function and expression by cisplatin in LLC-PK1 cells. Biol Pharm Bull. 2003;26(2):205–209. doi:10.1248/bpb.26.205
28. Demeule M, Brossard M, Beliveau R. Cisplatin induces renal expression of P-glycoprotein and canalicular multispecific organic anion transporter. Am J Physiol. 1999;277(6 Pt 2):F832–840. doi:10.1152/ajprenal.1999.277.6.F832
29. Hassan HE, Myers AL, Lee IJ, Coop A, Eddington ND. Oxycodone induces overexpression of P-glycoprotein (ABCB1) and affects paclitaxel’s tissue distribution in sprague dawley rats. J Pharm Sci. 2007;96(9):2494–2506. doi:10.1002/jps.20893
30. Kamazawa S, Kigawa J, Kanamori Y, et al. Multidrug resistance gene-1 is a useful predictor of paclitaxel-based chemotherapy for patients with ovarian cancer. Gynecol Oncol. 2002;86(2):171–176.
31. Shen Y, Chu Y, Yang Y, Wang Z. Mitochondrial localization of P-glycoprotein in the human breast cancer cell line MCF-7/ADM and its functional characterization. Oncol Rep. 2012;27(5):1535–1540. doi:10.3892/or.2012.1671
32. Ling X, He Y, Zhang G, Zhou Y, Yan B. Increased P-glycoprotein expression in mitochondria is related to acquired multidrug resistance in human hepatoma cells depleted of mitochondrial DNA. Int J Oncol. 2012;40(1):109–118. doi:10.3892/ijo.2011.1181
33. Solazzo M, Fantappie O, Lasagna N, Sassoli C, Nosi D, Mazzanti R. P-gp localization in mitochondria and its functional characterization in multiple drug-resistant cell lines. Exp Cell Res. 2006;312(20):4070–4078. doi:10.1016/j.yexcr.2006.09.005
34. Mo Q, Zhang Y, Jin X, et al. Geldanamycin, an inhibitor of Hsp90, increases paclitaxel-mediated toxicity in ovarian cancer cells through sustained activation of the p38/H2AX axis. Tumour Biol. 2016;37(11):14745–14755. doi:10.1007/s13277-016-5297-2
35. Chen X, Cui Z, Wei S, et al. Chronic high glucose induced INS-1beta cell mitochondrial dysfunction: a comparative mitochondrial proteome with SILAC. Proteomics. 2013;13(20):3030–3039. doi:10.1002/pmic.201200448
36. Pavek P, Staud F, Fendrich Z, et al. Examination of the functional activity of P-glycoprotein in the rat placental barrier using rhodamine 123. J Pharmacol Exp Ther. 2003;305(3):1239–1250. doi:10.1124/jpet.102.048470
37. Sharom FJ. ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics. 2008;9(1):105–127. doi:10.2217/14622416.9.1.105
38. Binkhathlan Z, Lavasanifar A. P-glycoprotein inhibition as a therapeutic approach for overcoming multidrug resistance in cancer: current status and future perspectives. Curr Cancer Drug Targets. 2013;13:326–346. doi:10.2174/15680096113139990076
39. Calcabrini A, Meschini S, Stringaro A, Cianfriglia M, Arancia G, Molinari A. Detection of P-glycoprotein in the nuclear envelope of multidrug resistant cells. Histochem J. 2000;32(10):599–606.
40. Fu D, Bebawy M, Kable EP, Roufogalis BD. Dynamic and intracellular trafficking of P-glycoprotein-EGFP fusion protein: implications in multidrug resistance in cancer. Int J Cancer. 2004;109(2):174–181. doi:10.1002/ijc.11659
41. Molinari A, Calcabrini A, Meschini S, et al. Detection of P-glycoprotein in the Golgi apparatus of drug-untreated human melanoma cells. Int J Cancer. 1998;75(6):885–893.
42. Fu D, Roufogalis BD. Actin disruption inhibits endosomal traffic of P-glycoprotein-EGFP and resistance to daunorubicin accumulation. Am J Physiol Cell Physiol. 2007;292(4):C1543–1552. doi:10.1152/ajpcell.00068.2006
43. Rajagopal A, Simon SM. Subcellular localization and activity of multidrug resistance proteins. Mol Biol Cell. 2003;14(8):3389–3399. doi:10.1091/mbc.e02-11-0704
44. Chen X, Wei S, Ma Y, et al. Quantitative proteomics analysis identifies mitochondria as therapeutic targets of multidrug-resistance in ovarian cancer. Theranostics. 2014;4(12):1164–1175. doi:10.7150/thno.8502
45. Munteanu E, Verdier M, Grandjean-Forestier F, et al. Mitochondrial localization and activity of P-glycoprotein in doxorubicin-resistant K562 cells. Biochem Pharmacol. 2006;71(8):1162–1174. doi:10.1016/j.bcp.2006.01.006
46. Ni Chonghaile T, Sarosiek KA, Vo TT, et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334(6059):1129–1133. doi:10.1126/science.1206727
47. Pasquier J, Guerrouahen BS, Al Thawadi H, et al. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J Transl Med. 2013;11:94. doi:10.1186/1479-5876-11-94
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.