Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Mitigation of MAFLD in High Fat-High Sucrose-Fructose Fed Mice by a Combination of Genistein Consumption and Exercise Training
Authors St Aubin CR, Fisher AL, Hernandez JA, Broderick TL, Al-Nakkash L
Received 13 January 2022
Accepted for publication 13 July 2022
Published 23 July 2022 Volume 2022:15 Pages 2157—2172
DOI https://doi.org/10.2147/DMSO.S358256
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Chaheyla R St Aubin,1 Amy L Fisher,1 Jose A Hernandez,2 Tom L Broderick,3,4 Layla Al-Nakkash3
1Department of Biomedical Sciences, College of Graduate Studies, Midwestern University, Glendale, AZ, 85308, USA; 2Department of Biochemistry and Molecular Genetics, College of Graduate Studies, Midwestern University, Glendale, AZ, 85308, USA; 3Department of Physiology, College of Graduate Studies Midwestern University, Glendale, AZ, 85308, USA; 4Laboratory of Diabetes and Exercise Metabolism, College of Graduate Studies, Midwestern University, Glendale, AZ, 85308, USA
Correspondence: Layla Al-Nakkash, Department of Physiology, College of Graduate Studies, Midwestern University, 19555 North 59th Avenue, Glendale, AZ, 85308, USA, Tel +1 623 572 3719, Fax +1 623 572 3673, Email [email protected]
Purpose: Metabolic dysfunction-associated fatty liver disease (MAFLD) is fueled by escalations in both sedentary behavior and caloric intake and is noted in obese type 2 diabetic (T2DM) patients. This study aimed to examine the effects of exercise and the phytoestrogen genistein in mice fed a high fat (60% fat) high sugar (55% fructose with 45% sucrose), HFHS diet.
Methods: Male C57BL/6J mice were assigned to five groups: HFHS, HFHS with genistein (600 mg/kg diet, HFHS+Gen), HFHS with moderate exercise (HFHS+Ex), and HFHS with combined genistein and moderate exercise (HFHS-Gen+Ex). Control lean mice were fed standard chow and water. Exercise consisted of 30-minute sessions of treadmill running five days/week for the 12-week study duration. Body weight was assessed weekly. Liver, kidney, fecal pellets and serum were extracted at the end of the study and maintained at − 80°C.
Results: After 12 weeks of treatment, mice in the HFHS group had the highest hepatic lipid content. Plasma levels of glucose, insulin, leptin, cholesterol, amylin, and total fat content were significantly elevated in HFHS mice compared to control mice. HFHS feeding increased protein expression of carnitine palmitoyltransferase 1b (CPT-1b isoform) in gastrocnemius, CPT1a, glucose transporter protein 2 (GLUT2), glucocorticoid receptor (GR), and fructose 1,6-bisphosphate 1 (FBP1) expression in liver. Exercise alone had minor effects on these metabolic abnormalities. Genistein alone resulted in improvements in body weight, fat content, amylin, insulin sensitivity, and liver histopathology, GR, FBP1, and acetyl-CoA carboxylase 1 (ACC1). Combination treatment resulted in additional metabolic improvements, including reductions in hepatic lipid content and lipid area, alanine transferase activity, CPT1b, and CPT1a.
Conclusion: Our results indicate that a HFHS diet is obesogenic, inducing metabolic perturbations consistent with T2DM and MAFLD. Genistein alone and genistein combined with moderate intensity exercise were effective in reducing MAFLD and the aberrations induced by chronic HFHS feeding.
Keywords: Soy isoflavone, genistein, exercise, western diet, diabetes, hepatic steatosis
Introduction
The unprecedented rise in obesity and type 2 diabetes mellitus (T2DM) in the United States caused by sedentary behavior and the excessive consumption of energy-rich saturated fatty acids and sugar-based foods has yielded a burgeoning prevalence of metabolic dysfunction-associated fatty liver disease1 (MAFLD). MAFLD, formerly known as non-alcoholic fatty liver disease (NAFLD), is characterized by excessive lipid infiltration in the liver (hepatic steatosis) in the absence of alcohol consumption, along with one of the three following criteria: overweight/obesity, presence of T2DM, or evidence of metabolic dysregulation (exhibiting 2 of the following abnormalities high waist circumference, high blood pressure, high cholesterol, pre-diabetes, insulin resistance, high plasma C-reactive protein levels).2 Early studies have shown that lipid accumulation in the liver is an early indicator of the presence and development of metabolic diseases, including insulin resistance, atherogenic dyslipidemia, T2DM as well as an increased risk of cardiovascular events.3–5 Population-based and clinical studies indicate a strong association between MAFLD and cardiometabolic diseases,4,5 autonomic dysfunction, expressed as sympathetic/parasympathetic tone imbalance,6 development of cardiac arrhythmias (atrial fibrillation, prolonged QT interval, and premature ventricular contractions), in addition to ischemic heart disease, stroke, and major adverse cardiovascular events.7–10
Given the observation that weight gain and insulin resistance are strongly associated with the prevalence of MAFLD,11–13 current guidelines for the treatment of MAFLD recommend physical activity and dietary modifications aimed at weight loss. In fact, weight loss is the only recognized mainstay of therapy.14 A reduction in body weight between 7% and 10% has been shown to significantly reduce hepatic steatosis and improve hepatic insulin sensitivity and serum transaminase levels.10,15 In more severe liver conditions, plasma hyaluronic acid, a marker of liver fibrosis, was improved in patients with non-alcoholic steatohepatitis following weight loss.15 Exercise, regardless of type or intensity, is known to be effective in the treatment and minimizing the risk of MAFLD even in the absence of weight loss.16–22 Moderate intensity aerobic exercise favoring duration or vigorous exercise training emphasizing intensity, both align with the recommendations for disease prevention,14 and consistently result in reductions in hepatic lipid content and decreased MAFLD.18,23,24 Benefits of regular exercise are attributed to an increase in whole body muscle capacity for fatty acid oxidation and a reduction in lipid accumulation in the liver by reducing de novo lipogenesis.24,25 In addition to these effects, exercise in combination with dietary modifications offers the added benefits of further reducing hepatic lipid accumulation, decreasing fat mass, and regulating hepatic glucose production, thus improving glucose metabolism and insulin sensitivity.26
Genistein, a known phytoestrogen found in soybean products, fava beans, and in a wide variety of plant-derived foods, has been shown to exert several health benefits.27,28 Because of its structural similarity to estrogen, genistein can bind to the estrogen receptor and exert estrogenic effects,29 without increasing prevalence of certain breast cancers.30 Chronic consumption of genistein has been shown to have antioxidant, anti-inflammatory, and potential antidiabetic effects.31,32 Treating obese diabetic rodents with genistein improved β-cell pancreatic function, increased insulin sensitivity, lowered blood glucose, and exerted a hypolipidemic action.33–35 In a model mouse of diet-induced obesity (DIO) and T2DM from high fat feeding, genistein ameliorated the lipid content and the inflammatory response in liver cells.36 We have shown previously that genistein diet provides benefit at the same concentration proposed in this study (600 mg genistein/kg diet): after 4-weeks consumption induces thermogenic and metabolic changes in the ob/ob mouse model.37,38 Based on those studies, it is evident that a dietary intervention with both genistein and regular exercise training could provide maximal benefit on overall metabolic function. The beneficial impact of both genistein and exercise training on metabolic health is supported in a recent study that demonstrated improved histopathologic profile of ovariectomized rats with nonalcoholic steatohepatitis (NASH) induced by a diet consisting of high-fat and high fructose. The results of that study indicate that four weeks of combined treatment with genistein and exercise reduced the severity of steatohepatitis by decreasing oxidative stress, local inflammation, and intrahepatic lipid content of the ovariectomized rats.39
Considering the relationship between weight gain, diet-induced obesity, and physical inactivity on the development of MAFLD,13,18,40 it remains unclear whether dietary genistein or exercise training can each prevent MAFLD induced by chronic high fat-high sugar (HFHS) feeding in mice, or whether combined treatment has an additive effect in preventing this condition. In this study, we examined the effects of genistein, exercise training, and combined treatment after a 12-week period on hepatic liver accumulation, expression of proteins relating to glucose and fatty acid synthesis and regulation in liver and muscle, and on common markers of cardiovascular disease. To induce MAFLD (as evidenced by hepatic steatosis, T2DM and obesity and therefore fitting the criteria described above), young male mice were given a diet rich in saturated fatty acids and allowed access to drinking water ad libitum containing a blend of high sucrose and fructose (HFHS). The sex and age of mice and addition of simple carbohydrates were selected based on the evidence that the rates of childhood obesity and pediatric MAFLD are increased, and singularly reported in young male boys where the consumption of soft drinks is increased41–46
Materials and Methods
Mouse Model of Obesity and Diet
The Midwestern University Institutional Animal Care and Use Committee approved all experimental procedures involving mice in this study. Animal care was conducted in accord with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. Four-week-old male C57BL/6 mice were purchased from Charles River (Wilmington, MA, USA). After a week of acclimatization, mice were randomly divided into five groups: high fat-high sugar (HFHS) diet, HFHS and exercise training (HFHS+Ex), HFHS high fat and genistein (HFHS+Gen), HFHS and combination exercise training and genistein treatment (HFHS+Gen+Ex). Mice fed a standard diet and normal tap water served as controls (Lean). A description of the diets used in this study has previously been described.47 The high sugar water (42 g/L) consisted of 55% fructose and 45% sucrose. Fructose was added to the drinking water because it accelerates lipid accumulation in the liver and promotes leptin resistance.48 Water was changed every other day. The high fat diet contained 60% of energy from lipids, 20% from carbohydrates, and the remaining 20% from protein (Dyets Inc. Bethlehem, PA). Genistein was incorporated in the high fat diet at a concentration of 600 mg/kg diet (Dyets Inc. Bethlehem, PA). This concentration of genistein yields plasma concentrations of low micromolar ranges49 equivalent to the consumption of a cup of soy milk per day and is therefore reasonable to achieve this dose in humans.49,50
All mice were given food and water ad libitum. Body weight of mice was recorded weekly. Food and water intake were measured over a 24-hour period (at weeks 5, 7, 9 and 11) and at such time points five mice/group were housed separately for the 24-hour duration and the average data for the five mice is shown in Table 1. Mice were housed 2–3 per cage with 12:12-hour light–dark cycle. At the completion of the study and 48 hours after the last exercise session to eliminate the effects of insulin sensitivity,51 mice were euthanized by asphyxiation in an atmosphere of 100% CO2, followed immediately by surgical thoracotomy inducing pneumothorax. Blood was collected by cardiac puncture, immediately centrifuged to obtain the plasma and stored at −80°C for analyses. Liver and skeletal muscle (gastrocnemius) were immediately extracted, frozen in liquid N2, then stored at −80°C until use. A segment of liver was embedded in Optimal Cutting Temperature compound for sectioning (O.C.T., Fisher HealthCare, Houston, TX, USA) Inguinal, peritoneal, and visceral fat pads were carefully excised and weighed to determine total fat pad weight of mice.
 |
Table 1 The Effects of Exercise Training, Genistein, and Combined Exercise and Genistein on Basic Physical Characteristics and Caloric Intake |
Exercise Training Protocol
Exercise training consisted of daily treadmill running (Exer 3/6, Columbus Instruments, Columbus, Ohio, USA), 30 minutes at 12 m/min, five days/week, for a total duration of 150 minutes/week, as previously described.52 Mice were initially acclimated to daily 10-min sessions of treadmill running for a period of one week. After acclimation to running, week 1 consisted of 10 m/min for 15 minutes, and in week 2 duration of running was increased to 30 minutes at 10 m/min. For the remaining weeks of the study (3 to 12), the intensity of exercise was increased to 12 m/min for a 30 minutes duration (5 days/week), corresponding to an estimated oxygen consumption of ~45–48 mL/kg/min based on treadmill belt speed.53 This exercise protocol was designed to mimic the recommendations for physical activity by the American Diabetes Association54 and endorsed by the American Association for the Study of Liver Disease and the American Gastroenterological Association.14
Glucose Tolerance Test
Glucose tolerance tests (GTT) were administered to overnight fasted mice at week 11 of the study. Following a baseline glucose reading (time 0 minutes), mice were administered an intraperitoneal bolus of glucose (2 mg/g body weight). Thereafter, glucose readings were obtained from the tail vein at the following four time points (15, 30, 60, and 120 minutes) after the intraperitoneal bolus. Blood glucose levels were measured using a standard glucose monitoring system (True Metrix, Trividia Health Inc. Fort Lauderdale, FL). The area under the curve (AUC) of the blood glucose measures was determined to assess the degree of insulin resistance in mice.
Western Blot Analysis
Tissues were homogenized per mg in 1 mL Tissue PE LBTM (G-Biosciences, St. Louis, MO, USA) containing 10 µL of EDTA (G-Biosciences, St. Louis, MO, USA) and 10 µL mammalian protease arrest (G-Biosciences, St. Louis, MO, U.S.A.). Homogenizations were performed at 4◦C using a disperser drill (IKA Works, Inc., Wilmington, NC, U.S.A.). Samples were analyzed for protein content using a PierceTM BCA protein assay kit (Thermo Scientific, Rockford, IL, USA) to normalize protein content loaded per sample. Westerns were run as previously described using standard Western blot protocols. Samples were loaded at a known protein concentration and run on NuPAGE® 4–12% Bis-Tris gels (ThermoFisher Scientific, Waltham, MA, USA) at 125–150 volts for 2 hours on ice. Wet transfers were at 2 hours at 30 volts at 4°C or dry transferred using the iBlot 2 Gel Transfer device (Thermo Fisher Scientific, Waltham, MA, USA). The blots were incubated with primary antibody CPT1B (1:1000, Abcam, Cambridge, MA, U.S.A.), GLUT2 (1:500, Santa Cruz, Dallas, TX), GLUT4 (1:500, Santa Cruz, Dallas, TX), GR (1:1000, Cell Signaling Technology Danvers, MA and Abcam Cambridge, MA), FBP1 (1:1000, My BioSource, San Diego, CA), ACC1 (1:1000, Cell Signaling Technology Danvers, MA), CPT1A (1:1000, Abcam Cambridge, MA) and FAS (1:1000, Abcam Cambridge, MA) overnight at 4°C in 5% milk in Tris-buffered saline solution + 0.1% Tween (TBST). Membranes were probed with either actin anti-actin primary antibody (1:3000, EMD Millipore, Billerica, MA, USA), or with GAPDH (1:10,000; Millipore Sigma) for 1 hour at room temperature (either used as controls for normalization). After washing, blots were incubated with secondary antibody, anti-rabbit immunoglobulin (Ig)G (H + L) Dylight™ 800 Conjugate (1:15,000, Thermo Fisher Scientific, Inc., Waltham, MA, USA), for 1 hour at room temperature. Membranes were imaged and band intensity quantified using Odyssey-Clx (Li-COR, Lincoln, NE, USA) and Image Studio Software (Li-COR Biosciences, Inc.).
Hepatic Histology and Morphology
Prior to liver sectioning, the cutting block and blade were acclimated for ~ 30 minutes to −20◦C inside the cryostat chamber. Tissues were sectioned (10 µm) on a Leica CM 1860 cryostat with Thermo Scientific MX35 Premier microtome blades, onto a slide (Fisher Scientific Superfrost Excell microscope slides, Pittsburgh, PA, U.S.A). Immediately after tissue sectioning, tissues were stained with Oil Red O kit (Abcam, Cambridge, MA, USA), briefly: slides were immersed in 10% neutral buffered formalin, NBF (2 minutes), dipped in water five times, submerged in Oil Red O (40 minutes), dipped in tap water until slides were clear (except for the tissue), slides were immersed in Mayer’s solution hematoxylin (5 seconds), dipped in tap water until clear, and dipped in 1% acid alcohol (5 times), followed by tap water (1 minute). Coverslips were applied using an aqueous mounting medium, Vectashield H1000 (Vector Laboratories, Burlingame, CA, U.S.A.). Images were obtained at 20X magnification on an Olympus IX73 inverted microscope. Images were analyzed using Image J Pro software (NIH) to quantify fat droplet size, area and diameter. Three images from different locations of the liver were averaged per mouse.
Serum and Fecal Assays
Plasma samples were assayed for insulin (Alpco Diagnostics, Salem, NH). Leptin, resistin, and amylin were analyzed using a Milliplex Mouse Metabolic Magnetic Bead Panel (EMD Millipore Corporation, Billerica, MA) according to manufacturer instructions. Alanine transaminase activity (Cayman Chemical Company, Ann Arbor, MI), triglyceride, glucose (Wako Diagnostics, Mountain View, CA) and cholesterol (Cayman Chemical Company, Ann Arbor, MI), were assessed according to manufacturer specifications. Fecal samples were removed from the distal end of the large intestine in euthanized mice and assayed for corticosterone (Cayman Chemical Company, Ann Arbor, MI) per manufacturer’s instructions.
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM). Group mean differences were determined using analysis of variance (ANOVA) followed by Tukey’s post hoc test. Analyses were performed using GraphPad software (GraphPad Software, Inc., La Jolla, CA, USA). A value of P < 0.05 was considered statistically significant.
Results
Effect of HFHS Diet, Genistein and/or Exercise on Body and Tissue Weights
Body and tissue weights are shown in Table 1. Mice were weighed at the beginning of the study (day zero, start body weight), and all groups started at the same body weight. Weights were recorded weekly thereafter until the end of the week 12. Chronic HFHS feeding resulted in a significant 1.41-fold increase in final body weight (50.19 ± 1.18 g, n = 10, P<0.05) compared to lean controls (33.66 ± 1.87 g, n = 10). The HFHS-induced weight gain was significantly mitigated by 20% with genistein (Gen) supplementation and by 33% with genistein and exercise combined (Gen+Ex). To assess whether the increases in body weight were associated with improvements in fat accumulation, total visceral adipose tissue content was evaluated (Table 1). HFHS feeding significantly increased adipose content 2.24-fold compared to lean controls. Adipose tissue weight was significantly reduced with Gen (20%) and combined Gen+Ex (45%) compared to mice fed HFHS. Exercise alone had no effect on adiposity. HFHS feeding significantly increased liver weight 1.94-fold compared to lean controls. Liver weight was significantly reduced with Ex (33%). Interestingly, Gen and Gen+Ex significantly prevented the gain in liver weight (52% and 56% respectively) compared to mice fed HFHS, yielding a “lean-like” phenotype.
Food and water intake (and determination of caloric intake) are presented in Table 1 as the average recorded over weeks 5, 7, 9, and 11 of the study. Average daily food intake (expressed as actual grams of food or caloric intake) between the groups was comparable (Table 1). Average daily water intake and the corresponding water caloric intake was comparable between leans and HFHS-fed mice. However, Gen+Ex treatment resulted in a significant increase in water intake and water-associated calories compared to HFHS mice (Table 1).
Effect of HFHS Diet, Genistein and/or Exercise on GTTs and Serum Profile
GTTs were performed during week 11 of the study to assess the effects of treatment on insulin sensitivity. As shown in Figure 1, administration of an intraperitoneal bolus of glucose caused blood glucose levels to rise in all groups. The total excursion in blood glucose as determined by area under the curve (AUC), was highest in mice fed HFHS diet and also with Ex. At 120 minutes, blood glucose levels remained elevated and significantly higher in these two groups compared to lean mice (Figure 1A and B). Blood glucose levels returned to normoglycemic levels at 120 minutes in mice fed either Gen or Gen+Ex. Of note, the total excursion in blood glucose levels was the lowest in the Gen+Ex group. Fasting serum glucose levels (Table 2) were significantly elevated by 1.6-fold with HFHS feeding (235.7±26.1 mg/dL, n = 4, P< 0.05) compared to lean mice (148.5±11.6 mg/dL, n = 4, P< 0.05). Ex had no effect on fasting glucose, whereas Gen and Gen+Ex both significantly reduced fasting glucose levels compared to HFHS and thereby maintained glucose at lean levels. Serum insulin levels (Table 2) were significantly increased by 2.24-fold with HFHS diet compared to leans. Insulin levels were significantly reduced by 40% with Gen+Ex.
 |
Table 2 The Effects of Exercise Training, Genistein, and Combined Exercise and Genistein on Serum Profile |
We assessed the effects of HFHS diet on three key hormones that play a role on insulin and glucose regulation (Table 2). Leptin levels were significantly increased 2-fold with HFHS diet compared to leans, and treatments were without effect. HFHS diet did not modify serum resistin levels and Gen was without effect. However, Ex increased resistin levels by 1.73-fold (Table 2). Amylin levels were significantly increased 4.6-fold with HFHS feeding compared to leans and both Gen and Gen+Ex significantly decreased amylin levels compared to the HFHS group (Table 2).
As shown in Table 2, chronic HFHS feeding yielded a 2-fold increase in serum cholesterol levels compared to leans. Gen treatment was beneficial and significantly diminished serum cholesterol levels by 33%. Triglycerides levels were significantly reduced by 34% in the HFHS diet group versus lean controls and Ex returned levels to those noted in leans.
Effect of HFHS Diet, Genistein and/or Exercise on Hepatic Steatosis
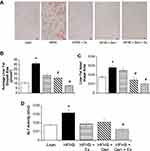
Representative images of the effects HFHS and treatment on fat accumulation in liver evaluated by oil-red-O staining are shown in Figure 2. Chronic consumption of HFHS diet-induced hepatic steatosis (Figure 2A). Hepatic fat area was increased by 40% and fat droplet size was ~4-fold higher after HFHS feeding compared to lean controls. Both Gen and combined Gen+Ex treatment prevented fat accumulation, and mitigated HFHS-induced increases in fat droplet size and area (ie, yielded a more normal liver fat profile, Figure 2B and C). Serum levels of alanine aminotransferase activity, ALT, a marker of hepatic injury, reflected the beneficial effects of combined Gen+Ex treatment on hepatic steatosis and hepatic damage despite consuming HFHS diet (Figure 2D).
Effect of HFHS Diet, Genistein and/or Exercise on Hepatic and Skeletal Muscle Protein Expression of CPT1B, GLUT4, GLUT2, GR, FBP1 and Fecal Corticosterone
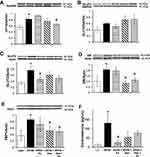
Given the correlation of lipid accumulation in the liver and with insulin resistance in skeletal muscle,55 we measured, by Western blot techniques, the expression levels of proteins involved in lipid metabolism in gastrocnemius muscle. There were no differences between treatment groups in protein expression of lipoprotein lipase, adipose tissue lipase, and peroxisome proliferator-activated receptor-delta (data not shown). Protein expression of CPT1b was also measured under HFHS-induced caloric overload (Figure 3A). CPT1b expression in gastrocnemius muscle was significantly increased 35% by HFHS feeding compared to lean controls and was significantly mitigated by 21% with Gen+Ex treatment. In gastrocnemius muscle GLUT4 expression was shown to be comparable between all groups (Figure 3B). Hepatic GLUT2 expression was significantly elevated 2.2-fold with HFHS feeding compared to leans and Ex treatment prevented this (Figure 3C).
Hepatic GR expression and fecal corticosterone levels were measured to reflect the glucocorticoid status of mice following treatment. GR expression was significantly increased 2-fold HFHS fed mice compared to lean controls and both Gen and Gen+Ex diminished this to maintain lean-type GR levels (Figure 3D). Expression of the rate-limiting enzyme in gluconeogenesis, FBP1 (Figure 3E) was significantly elevated 2.4-fold by HFHS diet compared to lean controls, and both Gen and Gen+Ex prevented this increase. Fecal corticosterone levels were significantly increased 10-fold with HFHS diet, and this increase was mitigated by Ex (Figure 3F). These data suggest that glucocorticoid synthesis was enhanced by HFHS-feeding.
Effect of HFHS Diet, Genistein and/or Exercise on Hepatic Protein Expression of ACC1, CPT1a and FAS
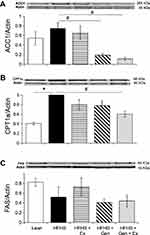
Interestingly, expression of acetyl-CoA carboxylase-1, ACC1, was comparable between HFHS fed and lean control mice. Gen and Gen+Ex both significantly decreased expression of ACC1 by 75% and 85%, respectively (Figure 4A). Expression of carnitine CPT1a was significantly increased 2.5-fold by HFHS diet compared to lean controls and Gen+Ex significantly mitigated this (Figure 4B). Expression of FAS was unchanged between all groups (Figure 4C).
In summary, 12 weeks of HFHS feeding in mice induced, for the most part, the expected changes that reflect insulin resistance, obesity and T2DM. MAFLD was evident in liver of these mice following a diet consisting of HFHS. HFHS feeding increased body weight, adipose tissue content, and plasma glucose levels remained elevated following the GTT challenge. Mice were also hyperinsulinemic, hyperleptinemic, and hypercholesterolemic. In liver of HFHS-fed mice, expression of GR was increased and coupled with the increase fecal corticosterone content, increased glucocorticoid production is suggested with this obesogenic diet. Expression of CPT1 in gastrocnemius muscle and liver were significantly increased with HFHS feeding. In liver, HFHS caused an increased expression of GLUT2, FBP1, and GR, aberrations contributing to hyperglycemia. Exercise had some benefit in preventing the onset of the diabetic phenotype, but the main effects occurred with genistein treatment alone or in combination with exercise. Both forms of treatment, either separately or in combination, were effective in reducing the obese diabetic phenotype, insulin resistance, and MAFLD. A summary of the effects of HFHS feeding and treatment is illustrated in Table 3.
 |
Table 3 Summary of the Effects of HFHS Feeding and Treatment on Physical Characteristics, Liver Histology, Serum and Protein Profiles Relating to the Development of MAFDL in Mice |
Discussion
In recent decades, studies have indicated that physical inactivity and the consumption of calorie-rich foods (fat and sugar-based beverages) increases the prevalence of metabolic disorders, including insulin resistance, obesity, and T2DM. Physical inactivity and low cardiorespiratory fitness have been linked to increased severity of MAFLD for individuals with T2DM.20–23,40 Increasing caloric expenditure in the form of regular exercise with a focus on healthy dietary intervention is an inexpensive, safe, and effective approach known to mitigate these metabolic consequences and prevent the development of both insulin resistance and hepatic steatosis.56,57 In this study, we show that a diet rich in fat, sucrose, and fructose, leads to the development of insulin resistance, increased gluconeogenic profile, severe obesity, and T2DM with hepatosteatosis in mice.
Exercise training had no dramatic effects on the obese diabetic phenotype induced by HFHS feeding in mice, while genistein treatment alone provided significant protection. We note that food intake in mice fed HFHS with genistein was lower (albeit not significantly so) compared to those fed a HFHS diet alone. An anorectic effect of genistein has been reported by others, and while the mechanism for this is unclear it is possible that genistein may have direct effects on brain centers regulating satiety or indirectly by enhancing the secretion of incretins from the gastrointestinal tract and pancreatic amylin (known to induce satiety). Recent reports show that GLP-1 secretion is enhanced with genistein treatment.58,59 We also found that genistein treatment alone decreased adipose tissue weight, which may be the result of lower food intake and modification of molecular pathways involved in the control adipose tissue stores. Decreased food intake may lead to reduced feeding and adjustment in the leptin setpoint regulating satiety. A decrease in leptin secretion from adipose tissue stores is consistent with this effect; indeed, we found that HFHS-fed mice fed genistein had lower blood leptin levels compared to HFHS-fed mice. Exercise induced a trend for a greater food intake in mice, which could be attributed to an increased energy expenditure. Our main findings indicate that intervention consisting of regular exercise training combined with dietary modification is beneficial and prevents several of the detrimental effects of HFHS feeding in mice. Combined exercise and genistein treatment mitigated the HFHS-induced increase in adipose tissue weight, improved insulin sensitivity and the hepatic gluconeogenic profile, and importantly, prevented the development of hepatic steatosis in mice.
In the United States, 1 in 5 adults exhibit the metabolic perturbations of fatty liver disease and even more frequently in patients with obesity and T2DM.12,60,61 Obesogenic diets (high fat and/or sucrose/fructose) have been increasingly utilized to generate NAFLD/MAFLD-like models. However, inconsistent outcomes are likely attributed to variances in percent fat used and/or the type of fat, inclusion of sugar (or not) and/or the type of sugar utilized (sucrose, fructose, or both), along with study duration. Nevertheless, use of obesogenic diets has provided useful preclinical models exhibiting comparable human NAFLD/MAFLD metabolic characteristics (obesity, inflammation, insulin resistance, hyperglycemia).62,63 The progression of nonalcoholic fatty liver disease is thought to start as steatosis, and progress into NASH, fibrosis, cirrhosis, and eventual hepatic carcinoma. Clinically, less than 30% of patients with hepatic steatosis may continue to develop NASH (inflammation, and oxidative stress).64 Interestingly, comparing clinical evidence to animal model relevance, liver inflammation and fibrosis are generally noted in either longer duration high fat diet studies or those including both high fat and high sugar combinations to yield NAFLD/MAFLD and accelerate the progression of liver fibrosis.65–68
The influence of exercise training on fatty liver is largely dependent on exercise intensity;16,17,22,23 high intensity continuous aerobic exercise and high intensity-interval training regimens are known to reduce fatty acid content in liver.20,69 The mechanism explaining this beneficial effect is an improvement in insulin resistance, which is a contributing factor in the development of steatosis.3 Our results support this idea that prevention of the steatosis is, in part, dependent on the exercise stimulus since the intensity of exercise utilized was moderate, and mice remained hyperglycemic and hyperinsulinemic, indicating persistent insulin resistance after exercise training. Histological analysis of liver obtained from treadmill-trained mice in our study revealed some persistent fat accumulation. It is noteworthy that HFHS-fed mice exhibited reduced exercise capacity (attempts to increase exercise intensity of exercise were met with an unwillingness to run by mice). This is consistent with our previous studies using the db/db mouse,52,70 exhibiting insulin resistance after exercise training and a reduction in exercise capacity.52,70,71 Reduced exercise capacity, in patients with T2DM is inversely related to the accumulation of fat in the liver and is a predictor of increased MAFLD severity.18,21,40 In addition, no demonstrable weight loss was observed with exercise training alone.
Lipid accumulation in muscle following HFHS feeding is associated with an increased expression of oxidative enzymes to facilitate disposal of fatty acids.72 HFHS feeding in mice causes insulin resistance and an increase in the expression of FATCD36 binding protein and CPT1, reflecting increased fatty acid uptake and mitochondrial oxidation, respectively, as compensatory mechanisms.72,73 We observed an increased capacity of fatty acid oxidation, expressed as CPT1b, in skeletal muscle from mice fed HFHS, which is consistent with other studies.72 In response to combination treatment, however, CPT1b expression remained at levels seen in lean control mice, suggesting decreased fatty acid supply and oxidation. Interestingly, a reciprocal ~75% increase in GLUT4 expression in skeletal muscle was observed, suggesting an improvement in glucose metabolism with combined genistein and exercise training. Although glucose oxidation was not directly measured in muscle, fasting blood glucose and insulin levels coupled with the rapid return of blood glucose to baseline following the GTTs with the combination treatment are indicative of increased insulin sensitivity, consistent with improved glucose metabolism.
Growing evidence indicates that genistein protects against the development of fatty liver in models of obesity and diabetes induced by high fat feeding alone, or in combination with sucrose or fructose by reducing common cardiovascular risk factors; obesity, hyperinsulinemia, hyperglycemia, and expression of hepatic proinflammatory markers.33–35,74–76 Our results are consistent with those previous studies and support the notion that chronic treatment with this phytoestrogen improves insulin sensitivity in T2DM, and improves the gluconeogenic profile in HFHS-fed mice.77,78 Glucocorticoids activate hepatic glucose production and enhance cortisol secretion in T2DM.79,80 Cortisol (or corticosterone in rodents) binding to the GR receptor increases expression of gluconeogenic genes, including the rate-limiting enzyme FBP1.81 Here, we demonstrate that total protein expression of GR and FBP1 and fecal corticosterone levels were elevated with HFHS feeding (consistent with enhanced glucocorticoid activity). We assessed fecal corticosterone as an index of glucocorticoid status (thereby bypassing issues related to variations in corticosterone levels during the diurnal cycle), and to confirm that glucocorticoid status (and glucose tolerance) was improved with treatment. We have recently shown that genistein improved glucose tolerance in obese diabetic ob/ob mice by decreasing corticosterone level and glucocorticoid amplification in tissues via 11ß-HSD-I and II.37,82
These metabolic perturbations in protein expression were absent in mice treated with genistein alone or in combination with exercise training, indicating reduced gluconeogenesis. This is consistent with a previous study showing that inhibition of FBP1 was effective in reducing hepatic gluconeogenesis and plasma glucose concentrations in Zucker diabetic fatty rats with overt diabetes.83 Inhibiting endogenous glucose production would clearly reduce the long-term metabolic consequences of persisting hyperglycemia. As with all dietary interventional studies, there are limitations with respect to the direct translation of animal model data to purported clinical use.
While insulin resistance is the main driving force in the development of lipogenesis in liver and severity of MAFLD,11 the pathobiology of MAFLD involves several metabolic pathways, including hepatic sterol regulator element binding protein 1 (SREBP-1) as the main transcription factor controlling lipogenesis and therefore MAFLD.84,85 Diets enriched with fructose or saturated fat have been shown to stimulate SREBP-1, FAS and ACC1 synthesis, leading to lipid accumulation in liver.36,86 On the other hand, reports have shown that genistein suppresses hepatic expression of SREBP-1, ACC1, FAS and as well as genes involved in triglyceride and cholesterol synthesis in whole liver and primary human hepatocytes.36,87–89 Our histological evidence shows a decrease in liver fat area with genistein treatment, accompanied by a reduction in ACC1, suggesting decreased conversion of acetyl-CoA to fatty acids. While expression of SREBP-1 was not determined, based on the effects of HFHS feeding and genistein treatment on ACC1 expression, it is likely that the changes in hepatic lipid content could be occurring through the mechanisms regulated by SREBP-1. Similarly, evidence from previously published rodent studies suggests that exercise training also reduces the expression of elongases, FAS and ACC1, thereby preventing hepatosteatosis,16,90–94 although we did not observe this exercise-induced benefit in mice. Discrepancies may relate to variations in one or more of the following: diet composition (high fat diet alone limit liver injury),41 rodent strain, modality/volume/duration of exercise training (using swim training or high-intensity exercise or voluntary wheel running regimens).90–94 Interestingly, in the current study, when exercise was combined with genistein treatment, greater protection against liver fat accumulation in the presence of HFHS feeding was observed. The histological improvements in the relevant markers of injury, including ALT activity, with combined treatment reflected a greater beneficial effect. While experimental studies in rodents clearly show efficacy of combined genistein and exercise training treatment, future clinical studies will be required to explore whether genistein and exercise can provide similar protective effects in humans with hepatic steatosis.
Conclusion
In conclusion, increased consumption of diets containing a high content of fat and refined sugars has led to an unprecedented rise in the prevalence of insulin resistance and diabetes along with an associated development of fatty liver disease. Using HFHS feeding to mimic these conditions, our results indicate that the isoflavone genistein and exercise training afford vital protection against obesity-induced dysfunction, preventing insulin resistance, and the metabolic complication of hepatic steatosis.
Abbreviations
ACC, acetyl CoA carboxylase; ALT, alanine transferase; AUC, area under the curve; CMCVD, common markers of cardiovascular disease; CPT, carnitine palmitoyltransferase; Ex, exercise training; FAS, fatty acid synthase; FBP, fructose-1,6-bisphosphate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Gen, genistein; GLUT, glucose transporter protein; GR, glucocorticoid receptor; GTT, glucose tolerance test; HFHF, high fat high fructose; HFHS, high fat high sugar; MAFLD, metabolic dysfunction-associated liver disease; NAFDL, nonalcoholic associated liver disease; NASH, nonalcoholic steatohepatitis; SREBP-1, sterol regulator element binding protein 1; T2DM, type 2 diabetes mellitus.
Acknowledgments
Amy Fisher and Chaheyla St Aubin were supported through the Biomedical Sciences Program, College of Graduate Studies. This work was supported by Midwestern University Intramural funds (TLB, L.A.), Diabetes Action and Research Education Foundation (L.A.) and the Midwestern Arizona Alzheimer’s Consortium (TLB, L.A.). We thank Mr. Tatum Banayat for helpful technical assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Booth FW, Roberts CK, Thyfault JP, Ruegsegger GN, Toedebusch RG. Role of inactivity in chronic diseases: evolutionary insight and pathophysiological mechanisms. Phys Rev. 2017;97:1351–1402.
2. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consencus statement. J Hepatol. 2020;73(1):202–209. doi:10.1016/j.jhep.2020.03.039
3. Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14(1):32–42. doi:10.1038/nrgastro.2016.147
4. Fabbrini E, Magkos F, Mohammed SS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106(36):15430–15435. doi:10.1073/pnas.0904944106
5. Kim D, Choi S-Y, Park EH, et al. Nonalcoholic fatty liver disease is associated with coronary artey calcification. Hepatology. 2012;56(2):605–613. doi:10.1002/hep.25593
6. Targher G, Mantovani A, Grander C, et al. Association between non-alcoholic fatty liver disease and impaired cardiac sympathetic/parasympathetic balance in subjects with and without type 2 diabetes- The Cooperative Health Research in South Tyrol (CHRIS)-NALFD sub-study. Nutr Metab Cardiovasc Dis. 2021;31(12):3464–3473. doi:10.1016/j.numecd.2021.08.037
7. Gong H, Liu X, Cheng F. Relationship between non-alcoholic fatty liver disease and cardiac arrhythmia: a systematic review and meta-analysis. J Int Med Res. 2021;49(9):3000605211047074. doi:10.1177/03000605211047074
8. Park JH, Kim G, Kim H, et al. The association of hepatic steatosis and fibrosis with heart failure and mortality. Cardiovasc Diabetol. 2021;20(1):197. doi:10.1186/s12933-021-01374-8
9. Simon TG, Roelstraete B, Hagstrom H, Sundstrom J, Ludvigsson JF. Non-alcoholic fatty liver disease and incident major adverse cardiovascular events: results from a nationwide histology cohort. Gut. 2021:325724. doi:10.1136/gutjnl-2021-325724
10. Musso G, Gambino R, Cassader M, Pagano GA. Meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver. Hepatology. 2010;52(1):79–104. doi:10.1002/hep.23623
11. Bril F, Barb D, Portillo-Sanchez P, et al. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology. 2017;65(4):1132–1144. doi:10.1002/hep.28985
12. Diehl AM, Day C, Longo DL. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377(21):2063–2072. doi:10.1056/NEJMra1503519
13. Manne V, Handa P, Kowdley KV. Pathophysiology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Clin Liver Dis. 2018;22(1):23–37. doi:10.1016/j.cld.2017.08.007
14. Association AG. American gastroentrological association medical position statement: nonalcoholic fatty liver disease. Gastroenterol. 2002;123(5):1702–1704. doi:10.1053/gast.2002.36569
15. Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study o fthe effects of lifestyle modification and vitamin E. Hepatology. 2003;38(2):413–419. doi:10.1053/jhep.2003.50316
16. van der Windt DJ, Sud V, Zhang H, Tsung A, Huang H. The effects of physical exercise on fatty liver disease. Gene Exp. 2018;18(2):89–101. doi:10.3727/105221617X15124844266408
17. Cho J, Kim S, Lee S, Kang H. Effect of training intensity on nonalcoholic fatty liver disease. Med Sci Sports Exerc. 2015;47(8):1624–1634. doi:10.1249/MSS.0000000000000595
18. Keating SE, George J, Johnson NA. The benefits of exercise for patients with non-alcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2015;9(10):1247–1250. doi:10.1586/17474124.2015.1075392
19. Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systemic review. J Hepatol. 2012;56(1):255–266. doi:10.1016/j.jhep.2011.06.010
20. Hallsworth K, Thoma C, Hollingsworth KG, et al. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: a randomized controlled trial. Clin Sci. 2015;129(12):1097–1105. doi:10.1042/CS20150308
21. Kantartzis K, Thamer C, Peter A, et al. High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut. 2009;58(9):1281–1288. doi:10.1136/gut.2008.151977
22. Bacchi E, Negri C, Targher G, et al. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetes subjects with nonalcoholic fatty liver disease (the RAED2 Randomized Trial). Hepatology. 2013;58(4):1287–1295. doi:10.1002/hep.26393
23. Keating SE, Hackett DA, Parker HM, et al. Effect of aerobic training dose on liver fat and visceral adiposity. J Hepatol. 2015;63(1):174–182. doi:10.1016/j.jhep.2015.02.022
24. Trefts E, Williams AS, Wasserman DH. Exercise and the regulation of hepatic metabolism. Prog Mol Biol Transl Sci. 2015;135:203–225.
25. Kiens B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev. 2006;86(1):205–243. doi:10.1152/physrev.00023.2004
26. Golabi P, Locklear CT, Austin P, et al. Effectiveness of exercise in hepatic fat mobilization in non-alcoholic fatty liver disease: systematic review. World J Gastroenterol. 2016;22(27):6318–6327. doi:10.3748/wjg.v22.i27.6318
27. Zaheer K, Humayoun Akhtar M. An updated review of dietary isoflavones: nutrition, processing, bioavailability and impacts on human health. Crit Rev Food Sci Nutr. 2017;57(6):1280–1293. doi:10.1080/10408398.2014.989958
28. Sharifi-Rad J, Quispe C, Imran M, et al. Genistein: an integrative overview of its mode of action, pharmacological properties, and health benefits. Oxid Med Cell Longev. 2021;2021:3268136. doi:10.1155/2021/3268136
29. Nazari-Khanamiri F, Ghasemnejad-Berenji M. Cellular and molecular mechanisms of genistein in prevention and treatment of diseases: an overview. J Food Biochem. 2021;45(11). doi:10.1111/jfbc.13972
30. Ziegler RG. Phytoestrogens and breast cancer. Am J Clin Nutr. 2004;79(2):183–184. doi:10.1093/ajcn/79.2.183
31. Park Y-J, Ko JW, Jeon S, Kwon YH. Protective effects of genistein against neuronal degeneration in ApoE −/− mice fed a high-fat diet. Nutrients. 2016;8(11):1–11. doi:10.3390/nu8110692
32. Rajput MS, Sarkar PD. Modulation of neuro-inflammatory condition, acetlcholinesterase and antioxidant levels by genistein attenuates diabetes associated cognitive decline in mice. Chem Biol Interact. 2017;268:93–102. doi:10.1016/j.cbi.2017.02.021
33. Lee YM, Choi JS, Kim MH, Jung MH, Lee YS, Song J. Effects of dietary genistein on hepatic lipid metabolism and mitochondrial function in mice fed high-fat diets. Nutrition. 2006;22(9):956–964. doi:10.1016/j.nut.2005.12.014
34. Choi JS, Song J. Effect of genistein on insulin resistance, renal lipid metabolism, and antioxidative activities in ovariectomized rats. Nutrition. 2009;25(6):676–685. doi:10.1016/j.nut.2008.11.027
35. Kim MH, Kang KS, Lee YS. The inhibitory effect of genistein on hepatic steatosis is linked to visceral adipocyte metabolism in mice with diet-induced non-alcoholic fatty liver disease. Br J Nutr. 2010;104(9):1333–1342. doi:10.1017/S0007114510002266
36. Zamani-Garmsiri F, Hashemnia SMR, Shabani M, Bagherieh M, Emamgholipour S, Meshkani R. Combination of metformin and genistein alleviates non-alcoholic fatty liver disease in high-fat diet-fed mice. J Nutr Biochem. 2021;87:108505. doi:10.1016/j.jnutbio.2020.108505
37. Rockwood SS, Broderick TL, Al-Nakkash L. Feeding obese diabetic mice a genistein diet induces thermogenic and metabolic change. J Med Food. 2018;21(4):332–339. doi:10.1089/jmf.2017.0084
38. Rockwood SS, Mason D, Lord R, Lamar P, Prozialeck WC, Al-Nakkash L. Genistein diet improves body weight, serum glucose and triglyceride levels in both male and female ob/ob mice. Diabetes Metab Syndr Obess. 2019;12:20111–22021.
39. Witayavanitkul N, Werwatganon D, Chayanupatkul M, Klaikeaw N, Sanguanrungsirikul S, Siriviriyakul P. Genistein and exercise modulated lipid peroxidation and improved statohepatitis in ovariectomized rats. BMC Complement Altern Med. 2020;20(1):162. doi:10.1186/s12906-020-02962-z
40. Sabag A, Keating SE, Way KL, et al. The association between cardiorespiratory fitness, liver fat and insulin resistance in adults with or without type 2 diabetes: a cross sectional-analysis. BMC Sports Sci Med Rehabil. 2021;13(1):40. doi:10.1186/s13102-021-00261-9
41. Abdelmalek MF, Suzuki A, Guy C, et al. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1961–1971.
42. Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79(4):537–543. doi:10.1093/ajcn/79.4.537
43. Harrington S. The role of sugar-sweetened beverage consumption in adolescent obesity: a review of the literature. J Sch Nurs. 2008;24(1):3–12. doi:10.1177/10598405080240010201
44. Forshee RA, Storey ML. Total beverage consumption and beverage choices among children and adolescents. Int J Food Sci Nutr. 2003;54(4):297–307. doi:10.1080/09637480120092143
45. Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388–1393. doi:10.1542/peds.2006-1212
46. Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42(3):641–649. doi:10.1002/hep.20842
47. Ortega-Santos CP, Al-Nakkash L, Whisner CM. Exercise and/or genistein treatment impact gut microbiota and inflammation after 12 weeks on a high-fat, high-sugar diet in C57BL/6 mice. Nutrients. 2020;12(11):3410. doi:10.3390/nu12113410
48. Shapiro A, Mu W, Roncal C, Cheng K-Y, Johnson RJ, Scarpace PJ. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am J Physiol Regul Integr Comp Physiol. 2008;295(5):R1370–1375. doi:10.1152/ajpregu.00195.2008
49. Al-Nakkash L, Clarke LL, Rottinghaus GE, Chen YJ, Cooper K, Rubin LJ. Dietary genistein stimulates anion secretion across female murine intestine. J Nutr. 2006;136(11):2785–2790. doi:10.1093/jn/136.11.2785
50. Xu X, Wang HJ, Murphy PA, Cook L, Hendich S. Daidzein is a more bioavailable soymilk isoflavone than is genistein in adult women. J Nutr. 1994;124(6):825–832. doi:10.1093/jn/124.6.825
51. James DE, Burleigh KM, Kragen EW, Chisholm DJ. Effect of acute exercise and prolonged training on insulin response to intravenous glucose in vivo in rat. J Appl Physiol. 1983;55(6):1660–1664. doi:10.1152/jappl.1983.55.6.1660
52. Broderick TL, Sennott JM, Gutkowska J, Jankowski M. Anti-inflammatory and angiogenic effects of exercise training in cardiac muscle of diabetic mice. Diabetes Metab Syndr Obes. 2019;12:565–573. doi:10.2147/DMSO.S197127
53. Hoydal MA, Wislofff U, Kemi OJ, Ellingsen O. Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur J Cardiovasc Prev Rehabil. 2007;14(6):753–760. doi:10.1097/HJR.0b013e3281eacef1
54. Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes: the American college of sports medicine and the American diabetes association: joint position statement. Diabetes Care. 2010;33(12):e147–167. doi:10.2337/dc10-9990
55. Kato K-I, Takeshita Y, Misu H, Zen Y, Kaneko S, Takamura T. Liver steatosis is associated with insulin resistance in skeletal muscle rather than in the liver in Japanese patients with non-alcoholic fatty liver disease. J Diabetes Investig. 2015;6:158–163. doi:10.1111/jdi.12271
56. Eckard C, Cole R, Lockwood J, et al. Prospective histopathologic evaluation of lifestyle modification in nonalcoholic fatty liver disease: a a randomized trial. Therap Adv Gastroenterol. 2013;6(4):249–259. doi:10.1177/1756283X13484078
57. Jin YJ, Kim KM, Hwang SS, et al. Exercise and diet modification in non-obese non-alcoholic fatty liver disease: analysis of biopsies of living liver donors. J Gastroenterol Hepatol. 2012;27(8):1341–1347. doi:10.1111/j.1440-1746.2012.07165.x
58. Rehman K, Ali MB, Akash MSH. Genistein enhances the secretion of glucagon-like peptide-1 (GLP-1) via downregulation of inflammatory responses. Biomed Pharmacother. 2019;112:108670. doi:10.1016/j.biopha.2019.108670
59. Zheng W, Li L, Li H. Phytochemicals modulate pancreatic islet B cell function through glucagon-like peptide-1-related mechanisms. Biochem Pharmacol. 2021;197:114817. doi:10.1016/j.bcp.2021.114817
60. Kwon YM, Oh SW, Hwang SS, Lee CB, Kwon H, Chung GE. Association of nonalcoholic fatty liver disease with components of metabolic syndrome according to body mass index in Korean adults. Am J Gastroenterol. 2012;107(12):1852–1858. doi:10.1038/ajg.2012.314
61. Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the United States and the rest of the world. Clin Liver Dis. 2016;20(2):205–214. doi:10.1016/j.cld.2015.10.001
62. Hebbard L, George J. Animal models of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2011;8(1):35–44. doi:10.1038/nrgastro.2010.191
63. Ouyang X, Cirillo P, Sautin Y, et al. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008;48(6):993–999. doi:10.1016/j.jhep.2008.02.011
64. Bertot LC, Adams LA. The natural course of non-alcoholic fatty liver disease. Int J Mol Sci. 2016;17:774.
65. Jahn D, Kircher S, Hermanns HM, Geier A. Animal models of NAFLD from a hepatologist’s point of view. Biochim Biophys Acta Mol Basis Dis. 2019;1865(5):943–953. doi:10.1016/j.bbadis.2018.06.023
66. Zhong F, Zhou X, Xu J, Gao L. Rodent models of nonalcoholic fatty liver disease. Digestion. 2020;101(5):522–535. doi:10.1159/000501851
67. Berardo C, Di Pasqua LG, Cagna M, Richelmi P, Vairetti M, Ferrigno A. Nonalcolhic fatty liver disease and non-alcoholic steatohepatitis: current issues and future perspectives in preclinical and clinical research. Int J Mol Sci. 2020;21(24):9646. doi:10.3390/ijms21249646
68. Zhang H, Leveille M, Courty E, Gunes A, Nguyen BN, Estall JL. Differences in metabolic and liver pathobiology induced by two dietary mouse models of nonalcoholic fatty liver disease. Am J Physiol Endocrinol Metab. 2020;319(5):E863–E876. doi:10.1152/ajpendo.00321.2020
69. Ainsworth BE, Haskell WL, Herrmann SD, et al. compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581. doi:10.1249/MSS.0b013e31821ece12
70. Broderick TL, Jankowski M, Gutkowska J. The effects of exercise training and caloric restriction on the cardiac oxytocin natriuretic peptide system in the diabetic mouse. Diabetes Metab Syndr Obes. 2017;10:27–36. doi:10.2147/DMSO.S115453
71. Morton GJ, Kaiyala KJ, Fisher JD, Ogimoto K, Schwartz MW, Wisse BE. Identification of a physiological role for leptin in the regulation of ambulatory activity and wheel running in mice. Am J Physiol Endocrinol Metab. 2011;300(2):E392–401. doi:10.1152/ajpendo.00546.2010
72. Song G-Y, Ren L-P, Chen S-C, et al. Similar changes in muscle lipid metabolism are induced by chronic high-fructose feeding and high-fat feeding in C57BL/J6 mice. Clin Exp Pharmacol Physiol. 2012;39(12):1011–1018. doi:10.1111/1440-1681.12017
73. Turner N, Bruce CR, Beale SM, et al. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes. 2007;56(8):2085–2092. doi:10.2337/db07-0093
74. Yang JY, Lee SJ, Park HW, Cha YS. Effect of genistein with carnitine administration on lipid parameters and obesity in C57Bl/6J mice fed a high-fat diet. J Med Food. 2006;9(4):459–467. doi:10.1089/jmf.2006.9.459
75. Yalniz M, Bahcecioglu IH, Kuzu N, et al. Preventative role of genistein in an experimental non-alcoholic steatohepatitis model. J Gastroenterol Hepatol. 2007;22(11):2009–2014. doi:10.1111/j.1440-1746.2006.04681.x
76. Arunkumar E, Karthik D, Anuradha CV. Genistein sensitizes hepatic insulin signaling and modulates lipid regulatory genes through p70 ribosomal S6 kinase-1 inhibition in high-fat-high fructose diet-fed mice. Pharm Biol. 2013;51(7):815–824. doi:10.3109/13880209.2013.766896
77. Consoli A, Nurjhan N, Capani F, Gerich J. Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes. 1989;38(5):550–557. doi:10.2337/diab.38.5.550
78. Firth RG, Bell PM, Marsh HM, Hansen I, Rizza RA. Postprandial hyperglycemia in patients with noninsulin-dependent diabetes mellitus. Role of hepatic and extrahepatice tissues. J Clin Invest. 1986;77(5):1525–1532. doi:10.1172/JCI112467
79. Pereira CD, Azevedo I, Monteiro R, Martins MJ. 11B-hydroxysteroid dehydrogenase type-1: relevance of its modulation in the pathophysiology of obesity, the metabolic syndrome and type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14(10):869–881. doi:10.1111/j.1463-1326.2012.01582.x
80. Krarup T, Krarup T, Hagen C. Do patients with type 2 diabetes mellitus have an increased prevalence of Cushing’s syndrome. DIabets Metab Res Rev. 2012;28(3):219–227. doi:10.1002/dmrr.2262
81. Zhang X, Yang S, Chen J, Su Z. Unravelling the regulation of hepatic gluconeogenesis. Front Endocrinol. 2019;9:802. doi:10.3389/fendo.2018.00802
82. Tagawa N, Kubota S, Kobayashi Y, Kato I. Genistein inhibits glucocorticoid amplification in adipose tissue by suppression of 11Beta-hydroxysteroid dehydrogenase type 1. Steroids. 2015;93:77–86. doi:10.1016/j.steroids.2014.11.003
83. van Poelje PD, Potter SC, Chandramouli VC, Landau BR, Dang Q, Erion MD. Inhibition of fructose 1,6-bisphosphatase reduces excessive endogenous glucose production and attenuates hyperglycemia in zucker diabetic fatty rats. Diabetes. 2006;55(6):1747–1754. doi:10.2337/db05-1443
84. Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109(9):1125–1131. doi:10.1172/JCI0215593
85. Li Y, Xu S, Mihaylova MM, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin resistant mice. Cell Metab. 2011;13(4):376–388. doi:10.1016/j.cmet.2011.03.009
86. Zhang C, Chen X, Zhu RM, et al. Endoplasmic reticulum stress is involved in hepatic SREBP-1c activation and lipid accumulation in fructose-fed mice. Toxicol Lett. 2012;212(3):229–240. doi:10.1016/j.toxlet.2012.06.002
87. Choi YR, Shim JH, Kim MJ. Genistin: a novel potent anti-adipogenic and anti-lipogenic agent. Molecules. 2020;25(9):2042. doi:10.3390/molecules25092042
88. Seidman L, Kruger A, Kegel-Hubner V, Seehofer D, Damm G. Influence of genistein on hepatic lipid metabolism in an in vitro model of hepatic steatosis. Molecules. 2021;26(4):1156. doi:10.3390/molecules26041156
89. Shukla A, Brandsch C, Bettzieche A, Hirche F, Stangl GL, Eder K. Isoflavone-poor soy protein alters lipid metabolism of rats by SREBP-mediated down-regulation of hepatic genes. J Nutr Biochem. 2007;18(5):313–321. doi:10.1016/j.jnutbio.2006.05.007
90. Wu H, Jin M, Han D, et al. Protective effects of aerobic swimming training on high-fat diet induced nonalcoholic fatty liver disease: regulation of lipid metabolism via PANDER-AKT pathway. Biochem Biophys Res Commun. 2015;458(4):862–869. doi:10.1016/j.bbrc.2015.02.046
91. Suk M, Shin Y. Effect of high-intensity exercise and high-fat diet on lipid metabolism in the liver of rats. J Exerc Nutr Biochem. 2015;19(4):289–295. doi:10.5717/jenb.2015.15122303
92. Tsuzuki T, Shinozaki S, Nakamoto H, et al. Voluntary exercise can ameliorate insulin resistance by reducing iNOS-mediated S-nitrosylation of Akt in the liver in obese rats. PLoS One. 2015;10(7):e0132029. doi:10.1371/journal.pone.0132029
93. Rector RS, Thyfault JP, Morris RT, et al. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka long-Evans Tokushima fatty rats. Am J Physiol Gastrointest Liver Physiol. 2008;294(3):G619–626. doi:10.1152/ajpgi.00428.2007
94. Cho J, Lee I, Kim D-H, et al. Effect of aerobic exercise training on non-alcoholic fatty liver disease induced by a high fat diet in C57BL/6 mice. J Exerc Nutr Biochem. 2014;18(4):339–346. doi:10.5717/jenb.2014.18.4.339
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.