Back to Journals » Cancer Management and Research » Volume 11
Mismatch repair status and high expression of PD-L1 in nasopharyngeal carcinoma
Authors Zhao L, Liao X, Hong G, Zhuang Y, Fu K, Chen P, Wang Y, Chen H, Lin Q
Received 8 November 2018
Accepted for publication 28 January 2019
Published 19 February 2019 Volume 2019:11 Pages 1631—1640
DOI https://doi.org/10.2147/CMAR.S193878
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Ahmet Emre Eşkazan
Liang Zhao,1,* Xiyi Liao,1,* Ganji Hong,1 Yanzhen Zhuang,2 Kaili Fu,1 Peiqiong Chen,2 Yuhuan Wang,2 Haojun Chen,3 Qin Lin1
1Department of Radiation Oncology, Xiamen Cancer Hospital, The First Affiliated Hospital of Xiamen University, Teaching Hospital of Fujian Medical University, Xiamen, China; 2Department of Pathology, The First Affiliated Hospital of Xiamen University, Teaching Hospital of Fujian Medical University, Xiamen, China; 3Department of Nuclear Medicine & Minnan PET Center, Xiamen Cancer Hospital, The First Affiliated Hospital of Xiamen University, Teaching Hospital of Fujian Medical University, Xiamen, China
*These authors contributed equally to this work
Purpose: To analyze the mismatch repair (MMR) status and PD-L1 expression in nasopharyngeal carcinoma (NPC), and investigate whether PD-L1 and MMR status could be used as a biomarker for predicting response of immune checkpoint blockades (ICBs) treatment.
Patients and methods: A total of 108 patients were initially histopathologically diagnosed with NPC between December 2017 and September 2018. All tissue specimens were collected before any treatment. Tumor tissue MMR status was determined by both immunohistochemistry and PCR. The expression of PD-L1 in NPC tissue was analyzed immunohistochemically. High PD-L1 expression in tumor cells (TC) or tumor-infiltrating immune cells (TIIC) was defined as ≥50% of corresponding cells with membranous staining.
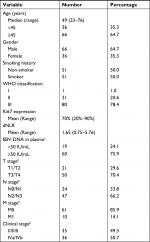
Results: Tissue samples were obtained from 102 patients after written informed consent was obtained. Seventy-one (69.6%) patients were treated in our hospital after diagnosis. Disease in stages I–III accounted for 35 (49.3%) cases, while stage IVa–IVb was identified in 36 (50.7%) cases. Only two of 102 patients were identified as MMR-deficient (dMMR) by IHC and PCR. High PD-L1 expression in TC was confirmed in 77 of the 102 (75.5%) NPC cases, while only 13 of the 102 (12.7%) NPC cases were considered to exhibit high PD-L1 expression in TIIC. PD-L1 expression in TC was positively correlated with T stage (P=0.033), while PD-L1 expression in TIIC was negatively associated with plasma Epstein–Barr virus DNA load (P=0.021), N stage (P=0.009), M stage (P=0.014), and clinical stage (P=0.001).
Conclusion: dMMR is a rare event in NPC and may not be a prospective biomarker to predict the effectiveness of treatment with ICBs in clinical practice. It was also determined that high PD-L1 expression in NPC is quite common and the importance of distinguishing PD-L1 expression in TC and TIIC was highlighted.
Keywords: immunotherapy, biomarker, microsatellite instability, PD-L1, tumor-infiltrating immune cells
Introduction
As we approach the era of cancer immunotherapy, immune checkpoint blockades (ICBs) have changed the landscape of advanced tumor therapy by harnessing the power of the immune system to cure cancer.1 One of the ICBs, which targets to blockade the programmed cell death protein 1 (PD-1) or programmed cell death ligand 1 (PD-L1), has dramatically improved the disease outcome of several malignancies, such as melanoma, non-small-cell lung cancer (NSCLC), colorectal cancer, lymphomas, renal cell carcinoma, and head and neck squamous cell carcinoma (HNSCC).2–6 Although the PD-1/PD-L1 blockade enables persistent control of diseases and even cures, considerable time is required before a response is elicited, the treatment is costly, and an approximate objective response rate (ORR) of only 20% is achieved in solid tumors.2,4,7,8
Oncologists have investigated many strategies to promote the effectiveness of ICBs. One of the main methods is the development of prospective biomarkers of response to ICBs; another approach is the development of a reasonable combination therapy. PD-L1 expression on tumor cells has been explored as a prospective biomarker to identify subgroup patients since the early phase of the development of these ICBs. In the Keynote-001 Phase I study of PD-1 blockade in advanced NSCLC patients, Garon et al9 found that patients with PD-L1-positive tumors (defined as ≥50% of tumor cells with PD-L1 membranous staining) had a higher ORR and longer progression-free survival (PFS) and overall survival (OS) compared with patients with PD-L1-negative tumors. Researchers observed similar results in a Phase III study of Keynote-024 and defined the cutoff value of PD-L1 expression as 50%.10 The US Food and Drug Administration (FDA) approved pembrolizumab as a first-line treatment for patients with metastatic NSCLC whose tumoral PD-L1 expression exceeded 50% on the basis of these clinical results. Other biomarkers, such as deficient mismatch repair (dMMR), have also been developed for use in precision cancer immunotherapy. A Phase II study of the clinical effectiveness of PD-1 inhibitor showed that the immune-related ORR was 40% in patients with dMMR colorectal cancer (CRC), whereas the ORR was 0% in patients with proficient mismatch repair (pMMR) CRC.11 The ORR in patients with dMMR non-CRC was similar to that of patients with dMMR CRC.11 This observation was confirmed in an expanded study of patients with advanced dMMR cancers across 12 different tumor types.12 Based on these studies, pembrolizumab was approved by the FDA for treatment of patients with microsatellite instability-high (MSI-H) or dMMR solid tumors in May 2017. However, nasopharyngeal carcinoma (NPC) was not included in the 12 cancers assessed in that trial.12
NPC is distinct from other head and neck cancers and is characterized by a unique group of geographical, etiological, and biological features.13 Although the 5-year OS in early-stage disease is over 80%, the 5-year OS of recurrent and distant metastatic NPC is poor,14 suggesting that a substantial unmet need exists for better treatment strategies. Few clinical trials of ICB treatment for advanced NPC have been completed, with ORRs of only 20.5% (9/44) and 25.9% (7/27);15,16 effective prospective biomarkers were not identified in either study.
In this study, we comprehensively analyzed the MMR status in patients diagnosed with NPC using immunohistochemistry (IHC) and polymerase chain reaction (PCR,) and analyzed their PD-L1 status to clarify the role of MMR proteins as predictive biomarkers for ICBs.
Materials and methods
Patients and tissue samples
A total of 108 patients were initially histopathologically diagnosed with NPC between December 2017 and September 2018 at the First Affiliated Hospital of Xiamen University. Six patients disagreed to join with our research. Tissue samples were obtained from 102 patients after written informed consent was obtained. All tissue specimens were collected before any treatments. The immunostained tissue sections were scored by two independent investigators who were blinded to the clinical data. Complete blood cell counts were detected for every patient within 30 days before biopsy. The Epstein–Barr virus (EBV) DNA load in plasma was analyzed by PCR if approved by the individual patient. Clinical stage was determined by the physician according to the eighth edition of the American Joint Committee on Cancer (AJCC) staging of NPC. This study was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital of Xiamen University and conducted in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
MMR status
Tumor tissue MMR status was determined by both IHC and PCR. The formalin-fixed, paraffin-embedded (FFPE) tumor tissue sections were subjected to IHC examination using primary antibodies against MLH1 (G168-15 clone, MXB), MSH6 (44 clone, MXB), MSH2 (FE11 clone, Dako, Glostrup, Denmark), and PMS2 (EP51 clone, Dako). DNA was extracted from tumor tissues and peripheral blood. We used the MSI Multiplex System (Promega, Madison, WI, USA) with five mononucleotide markers for the detection of MSI: NR-21, BAT-26, BAT-25, NR-24, and MONO-27.17 Separation and detection of amplified fragments were performed on an ABI PRISM 3500 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) following the manufacturer’s protocol. Allelic patterns or genotypes of paired normal and tumor tissues were compared, and samples with more than two microsatellites were considered MSI-H. In addition, two pentanucleotide markers (Penta C and Penta D) were attached to identify sample mix-ups and/or contamination.
PD-L1 IHC, EBV status, and Ki67 expression
FFPE NPC tissues were sectioned to 4-µm thickness. For immunohistochemical detection of PD-L1, we used the BenchMark GX automated slide stainer to stain the sections with PD-L1 antibody (SP263, Ventana, Oro Valley, AZ, USA) according to the manufacturer’s recommended protocol. Positive control (placenta) and negative control samples were run simultaneously with each specimen. EBV status was determined by EBV-encoded small RNA (EBER) in situ hybridization using standard protocols. For Ki67 expression detection, the FFPE tumor tissue sections were incubated with a monoclonal anti-Ki67 antibody (MIB-1, MXB) at a dilution of 1:100 at 4°C overnight according to the manufacturer’s instructions.
Statistical analysis
All statistical analyses were conducted by using SPSS 22.0 statistical analysis software. The distribution of PD-L1 expression in tumor cells (TC) and tumor-infiltrating immune cells (TIIC) was significantly different from the normal distribution (Shapiro–Wilk test); therefore, we used Spearman rank correlation for analyses. The test was used to analyze the association of PD-L1 expression in TC or TIIC with clinical characteristics of NPC patients, where appropriate. All tests were two-sided, and a P-value less than 0.05 was considered statistically significant.
Results
Characteristics of the patients
The clinical characteristics of the 102 NPC patients in this study are summarized in Table 1. The median age of the patients was 49 years (range=23–76 years). The majority of NPC patients were men (66, 64.7%). Fifty-one (50%) patients were current or former smokers. Histologically, one NPC patient was WHO I type, 20 patients were WHO II type, and 81 were WHO III type. The median derived neutrophil–lymphocyte ratio (dNLR) was 1.65 (range=0.75–5.76), and the median Ki67 expression was 70% (range=20%–90%). The total number of EBV-infected patients in the overall NPC cohort was 101. Sites for NPC collection included the nasopharynx, lymph nodes, lungs, and epidural tissue. A total of 79 (77.5%) patients were assessed for EBV DNA load. Seventy-one (69.6%) patients were treated in our hospital after diagnosis. Disease in stages I–III accounted for 35 (49.3%) cases, while stage IVa–IVb was identified in 36 (50.7%) cases.
MMR status
One of 102 patients was identified as dMMR by IHC; the MSH2 and MSH6 IHC were negative (Figure 1). This patient was a 41-year-old woman with WHO III type and stage IVa (T3N3M0) NPC. We marked this female patient as patient I. She was a non-smoker and had a plasma EBV DNA level of 1.48×104 IU/mL. PD-L1 expression was observed in 100% of TC and 10% of TIIC. Interestingly, the MMR protein IHC of a male patient was inconsistent, and we marked this male patient as patient II. Both of our pathologists considered some cancer nests negative for MMR proteins, while other cancer nests were positive in the same section (Figure 2). We suspected that this phenomenon was due to tumor heterogeneity or chimerism of patient II. The peripheral blood chromosome karyotype of patient II was analyzed with a negative result. MMR protein IHC of serial sections was performed again with cytokeratin (CK)–5/6 IHC. The cancer nest areas negative for MMR proteins were confirmed as the tumor cell region, and the MMR protein-positive area was determined to not be a tumor cell region by CK5/6 IHC (Figure 3). Thus, IHC of all four MMR proteins (MLH1, PMS2, MSH2, and MSH6) was negative, indicating that patient II had a dMMR tumor. This patient was a 63-year-old male heavy smoker with WHO II type and stage IVb (T4N0M1) NPC. His plasma EBV DNA level was 1.23×103 IU/mL. PD-L1 expression was observed in 60% of TC and 30% of TIIC. PCR also indicated that these two patients had MSI tumors (Figure 4), whereas the other patients had microsatellite stable (MSS) tumors.
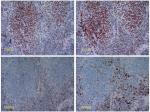  | Figure 1 Immunohistochemistry of the MMR proteins. The tumor cells were assessed for expression of PMS2 (+), MLH1 (+), MSH2 (−), and MSH6 (−). Abbreviation: MMR, mismatch repair. |
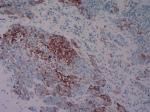  | Figure 3 Immunohistochemistry of the cytokeratin (CK)–5/6 protein. The cells in the bottom left are tumor cells, whereas those in the top right are lymphocytes. |
PD-L1 expression
The proportion of PD-L1 expression in TC and TIIC is presented in Table 2. The typical IHC staining of PD-L1 with low and high expression is revealed in Figure 5. Negative PD-L1 expression in TC was observed in one of the 102 (1%) NPC cases. High PD-L1 expression, defined as ≥50% of TC with membranous staining, was confirmed in 77 of the 102 (75.5%) NPC cases. PD-L1 expression in TC was not associated with age, gender, smoking history, Ki67 expression, dNLR, N stage, M stage, and clinical stage. Although the cutoff of PD-L1 expression in TIIC was identical to that in TC, only 13 of the 102 (12.7%) NPC cases were considered to exhibit high PD-L1 expression. PD-L1 expression in TC was only correlated with T stage (P=0.033; coefficient =0.254). Furthermore, the PD-L1 expression in TIIC was significantly negatively associated with plasma EBV DNA load (P=0.021; coefficient =–0.259), N stage (P=0.009; coefficient =–0.309), M stage (P=0.014; coefficient =–0.291), and clinical stage (P=0.001), but not significantly related to age, gender, smoking history, Ki67 expression, dNLR, or T stage (Table 3).
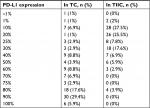  | Table 2 PD-L1 expression in TC and TIIC Abbreviations: PD-L1, programmed death-1 ligand; TC, tumor cells; TIIC, tumor-infiltrating immune cells. |
Discussion
Despite the encouraging success of immunotherapy in recent years, the effectiveness of this therapy varies widely among individuals and different tumor types. In May 2017, pembrolizumab was approved for patients with MSI-H or dMMR solid tumors based on two Phase II clinical trials.11,12 This was the first time the FDA approved a cancer treatment based on a common genomic biomarker rather than a tissue origin approach. However, the suitability of MMR status as a biomarker for ICBs in NPC patients was unknown. We determined the MMR status in a cohort of unselected patients using IHC and the same PCR method that was used in the two referenced trials.11,12 Our study cohort comprised patients at the First Affiliated Hospital of Xiamen University in Southern China, a region in which NPC is considered endemic. As far as we know, this is the first study to detect the MMR status using both IHC and the MSI Multiplex System in a reasonable sample size of patients with NPC.
Five mononucleotide markers (BAT-25, BAT-26, NR-21, NR-24, and MONO-27) were previously recommended as the best markers for detection of MSI-H colorectal tumors from a set of 266 mono-, di-, tetra-, and penta-nucleotide repeat microsatellite markers.17 The MSI Multiplex System is both highly specific and sensitive and amenable to high-throughput analysis of colorectal tumors. Examination of MSI using this system may not be suitable for tumors other than colorectal tumors. However, the Phase II trial mentioned above used this MSI panel to investigate 12 types of tumors.12 A study including 56 NPC patients demonstrated that dMMR is a rare event (2%, 1/56),18 consistent with our results. Their PCR method targeted three mononucleotide markers (BAT26, BAT25, and CAT25) and three dinucleotide markers (D5S.346, D17S.250, and D2S.123).18 Notably, BAT26, BAT25, and CAT25 are included in the five mononucleotide markers we used in the MSI Multiplex System.
Although the gold standard for detecting MSI is genetic testing by PCR, the College of American Pathologists (CAP) recommends an initial IHC workup to detect the presence or absence of MMR proteins (MLH1, MSH2, MSH6, and PMS2).19 Loss of expression of the MMR proteins in IHC is generally associated with mutation (or methylation) of the corresponding genes, which cannot be determined with MSI testing. In addition, IHC testing is much more widely available both in laboratories and hospitals. In our cohort, the expression of MSH2 and MSH6 was absent in IHC of one patient, and expression of all four proteins (MLH1, MSH2, MSH6, and PMS2) was lost in IHC of another patient. Interestingly, the phenomenon of the half-positive, half-negative MMR protein IHC in this patient was initially suspected as tumor heterogeneity or chimerism. The peripheral blood chromosome karyotype of this patient was negative. Once MMR IHC was performed again in conjunction with CK5/6 IHC in serial sections, we confirmed that the MMR positivity appeared in lymphocytes instead of tumor cells. The nasopharynx is characteristically full of lymphocytes; because nasopharyngeal tumor cells may be as small as lymphocytes, lymphocytes in close proximity may be wrongly considered a tumor cell nest. One disadvantage of MMR IHC is that the results may be affected by conditions of tissue fixation, which is not required for PCR.20 Thus, we recommend MMR IHC for initial screening to identify dMMR or MSI tumors in NPC patients. Then, PCR can be used to identify the highly suspected or uncertain populations. The two patients with loss of expression of MSH2 and MSH6 and the four MMR proteins in IHC, respectively, were both identified as MSI-H patients with NPC by the MSI Multiplex System. dMMR tumors have more potential to encode “non-self” immunogenic antigens and contain prominent lymphocyte infiltrates, which may be more susceptible to ICBs.11,21,22 Yarchoan et al23 pooled the response data of 27 tumor types or subtypes from the largest published studies that evaluated the ORR of ICBs and found a strong relationship between the tumor mutational burden (TMB) and the effectiveness of ICB therapies across multiple cancers. In fact, loss of function in MMR genes is correlated with high TMB in tumors.24,25 However, the reverse is not true: MSI-H generally occurs as a subset of high TMB, and other factors such as mutations in the POLD1 and POLE genes, ultraviolet light exposure, and smoking can result in high TMB.26–29 One study that analyzed 62,150 samples indicated that only 16% of samples with high TMB were classified as MSI-H.26 Our data, which represented the largest population compared with that of published studies on the MMR status of NPC patients, showed that dMMR in NPC is a rare event. Our previous study reported that pMMR in NPC is susceptible to ICBs, and one patient with extensively metastatic NPC showed a complete response and is alive as of this study.30 It is also worth noting the NSCLC is not included in the 12 different types of cancer which the Phase II trial mentioned above,11 although the PD-1/PD-L1-based therapy is generally useful in patients with positive PD-L1 (≥50%) for NSCLC regardless of MMR status in a Phase III study of Keynote-024.10 Taken together, dMMR is very rare and may not be suitable as a biomarker to predict the effect of ICBs in NPC patients.
Several studies have demonstrated that PD-L1 is frequently expressed in NPC,31–34 which was supported by our results. However, PD-L1 expression in tumor sections was not distinguished between TIIC and TC in the majority of related studies. Based on our results, PD-L1 expression in TC and TIIC might exhibit different correlations with clinical characteristics and be regulated by distinct mechanisms. Our study demonstrated that PD-L1 expression in TC was significantly associated with the primary tumor stage, which may predict the poor prognosis. However, PD-L1 expression in TIIC was negatively associated with lymph node stage, distant metastasis, clinical stage, and plasma EBV DNA load. All four factors are related to adverse prognosis. These findings were in agreement with the previous studies.35–37 Analyzing the reason, PD-L1 expression in TC could be upregulated by tumor-intrinsic mechanisms such as constitutive activation of oncogenic signaling pathways and related signaling pathways, independently of inflammatory signals in the tumor microenvironment.38,39 However, transcriptome analysis results demonstrated that PD-L1 expression in TIIC can be driven via adaptive mechanisms including exogenous inflammation-mediated immune responses within the tumor microenvironment and reflects pre-existing immunity.36,39 In other words, TIIC expressing PD-L1 are more strongly correlated with cancer immune response compared with TC, which means PD-L1 expression in TIIC may be a favorable prognostic factor. A meta-analysis involving 18 studies of 3,674 patients suggested that PD-L1 expression in TIIC was related to better survival in cancer patients.36 These different mechanisms may partly explain the varied prognostic indication of PD-L1 expression in TC and TIIC.
Plasma EBV DNA fragments are short DNA fragments that are released into the circulation by the apoptosis of cancer cells, and low levels of DNA fragments are released from small-sized tumors into the blood.40 Researchers have found that the concentration of EBV DNA is highly correlated with lymph node status and clinical stage, suggesting that the EBV DNA load is an accurate biomarker for diagnosis and prognosis of NPC in endemic areas.41,42 Notably, in a Phase II trial of PD-1 blockade in 61 unselected patients with metastatic gastric cancer, a dramatic response was observed in EBV-positive tumors, suggesting that EBV-positive cancer may also be actively considered for up-front ICBs.43 However, the dynamic changes of EBV in NPC has no significant correction with ICBs treatment both in the Phase Ib trial of pembrolizumab for NPC patients in the Keynote-028 study and the Mayo Clinic Phase 2 Consortium of Nivolumab for NPC patients.15,16 Both studies explained that the small sample size may be the limitation to demonstrate a statistical significance. The association between PD-L1 expression in TIIC and EBV DNA load observed in our cohort should be explored further. Recently, Lin et al44 has shown that PD-L1-expressing TIIC may determine the clinical efficacy of ICB-mediated tumor regression. It is indispensable to discriminate PD-L1 expression in TC and TIIC; however, most research has focused only on PD-L1 expression in TC.
We collected data from published studies of NPC patients31–34 and used the same cutoff value (50%) as for NSCLC.9,10 Of note, the proportion of high PD-L1 expression (≥50%) in the previous NPC studies was much lower than that in ours (75.5%). These studies used clone E1L3N (Cell Signaling Technology, USA) (Ventana, PA, USA) or clone SP142 (Ventana) as the PD-L1 antibody, and published studies have demonstrated that both antibodies result in fewer stained tumor cells than the antibodies we used (SP263, Ventana).45,46 These studies were also retrospective;31–34 the tumor tissues were from paraffin blocks that were preserved for more than 3 years in specimen banks. Over time, antigen alterations or loss of the specimen in paraffin blocks will affect the IHC results, as has been demonstrated in nucleic acid-based assays.47,48 We highlight that the tumor specimens in our study were subjected to IHC immediately, likely producing more accurate results. Three of four common assays had similar analytical performance in terms of PD-L1 expression in the Blueprint PD-L1 IHC Assay Comparison Project; the antibodies we used were also used in those assays.45 Because ICB clinical trials for NPC are less common than those for other cancers, our PD-L1 expression data from unselected patients using the antibody recommended in the Blueprint PD-L1 IHC Assay Comparison Project can provide valuable information for future clinical trial design. Moreover, high PD-L1 expression implied that NPC might be a suitable candidate for PD-1/PD-L1 ICBs.
This study has some limitations that need to be addressed. First, this was a single-center study with a limited number of patients, and the clinical stage of several patients was not available because they were not hospitalized after diagnosis in our center. Second, the TMB was not detected in our cohort, although it has been considered a hotspot biomarker in many studies. Future validation of our findings with a larger population and TMB detection is warranted.
Conclusion
dMMR is a rare event in NPC and may not be a prospective biomarker to predict the effectiveness of treatment with ICBs in clinical practice. We also determined that high PD-L1 expression in NPC is common, and highlighted the importance of distinguishing PD-L1 expression in TC and TIIC.
Acknowledgment
This study was supported by the Natural Science Foundation of Fujian Province [Grant number 2016J01633] and the National Natural Science Foundation of China [Grant number 81772893].
Disclosure
The authors report no conflicts of interest in this work.
References
Callahan MK, Postow MA, Wolchok JD. Targeting T cell co-receptors for cancer therapy. Immunity. 2016;44(5):1069–1078. | ||
Tan S, Chen D, Liu K, et al. Crystal clear: visualizing the intervention mechanism of the PD-1/PD-L1 interaction by two cancer therapeutic monoclonal antibodies. Protein Cell. 2016;7(12):866–877. | ||
Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315(15):1600–1609. | ||
Medina PJ, Adams VR. PD-1 pathway inhibitors: immuno-Oncology agents for restoring antitumor immune responses. Pharmacotherapy. 2016;36(3):317–334. | ||
Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015 Oct 22;373(17):1627–1639. | ||
Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319. | ||
Ascierto PA, Brugarolas J, Buonaguro L, et al. Perspectives in immunotherapy: meeting report from the immunotherapy bridge (29–30 November, 2017, Naples, Italy). J Immunother Cancer. 2018; 6(1):69. | ||
Grigg C, Rizvi NA. PD-L1 biomarker testing for non-small cell lung cancer: truth or fiction? J Immunother Cancer. 2016;4(1):48. | ||
Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. | ||
Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. | ||
Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. | ||
Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. | ||
Bruce JP, Yip K, Bratman SV, Ito E, Liu FF. Nasopharyngeal cancer: molecular landscape. JCO. 2015;33(29):3346–3355. | ||
Lee AW, Ma BB, Ng WT, Chan AT. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. 2015;33(29):3356–3364. | ||
Ma BBY, Lim WT, Goh BC, et al. Antitumor activity of nivolumab in recurrent and metastatic nasopharyngeal carcinoma: an international, multicenter study of the Mayo Clinic phase 2 Consortium (NCI-9742). J Clin Oncol. 2018;36(14):1412–1418. | ||
Hsu C, Lee SH, Ejadi S, et al. Safety and antitumor activity of pembrolizumab in patients with programmed Death-Ligand 1-positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study. J Clin Oncol. 2017;35(36):4050–4056. | ||
Bacher JW, Flanagan LA, Smalley RL, et al. Development of a fluorescent multiplex assay for detection of MSI-High tumors. Dis Markers. 2004;20(4–5):237–250. | ||
Chang AMV, Chiosea SI, Altman A, Pagdanganan HA, Ma C. Programmed Death-Ligand 1 expression, microsatellite instability, Epstein-Barr virus, and human papillomavirus in nasopharyngeal carcinomas of patients from the Philippines. Head Neck Pathol. 2017;11(2):203–211. | ||
Hashmi AA, Ali R, Hussain ZF, et al. Mismatch repair deficiency screening in colorectal carcinoma by a four-antibody immunohistochemical panel in Pakistani population and its correlation with histopathological parameters. World J Surg Oncol. 2017;15(1):116. | ||
Chen W, Swanson BJ, Frankel WL. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn Pathol. 2017;12(1):24. | ||
Smyrk TC, Watson P, Kaul K, Lynch HT. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Canc. 2001;91(12):2417–2422. | ||
Alexander J, Watanabe T, Wu TT, Rashid A, Li S, Hamilton SR. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. 2001;158(2):527–535. | ||
Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377(25):2500–2501. | ||
Peltomäki P. Role of DNA mismatch repair defects in the pathogenesis of human cancer. J Clin Oncol. 2003;21(6):1174–1179. | ||
Zysman M, Saka A, Millar A, Knight J, Chapman W, Bapat B. Methylation of adenomatous polyposis coli in endometrial cancer occurs more frequently in tumors with microsatellite instability phenotype. Canc Res. 2002;62(13):3663–3666. | ||
Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34. | ||
Alexandrov LB, Ju YS, Haase K, et al. Mutational signatures associated with tobacco smoking in human cancer. Science. 2016;354(6312):618–622. | ||
Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. | ||
Pursell ZF, Isoz I, Lundström EB, Johansson E, Kunkel TA. Yeast DNA polymerase epsilon participates in leading-strand DNA replication. Science. 2007;317(5834):127–130. | ||
Liao X, Zhao L, Wu S, et al. Microsatellite stability and mismatch repair proficiency in nasopharyngeal carcinoma may not predict programmed death-1 blockade resistance. Oncotarget. 2017;8(68):113287–113293. | ||
Chan OS, Kowanetz M, Ng WT, et al. Characterization of PD-L1 expression and immune cell infiltration in nasopharyngeal cancer. Oral Oncol. 2017;67:52–60. | ||
Lee VH, Lo AW, Leung CY, et al. Correlation of PD-L1 expression of tumor cells with survival outcomes after radical intensity-modulated radiation therapy for non-metastatic nasopharyngeal carcinoma. PLoS One. 2016;11(6):e0157969. | ||
Zhang J, Fang W, Qin T, et al. Co-expression of PD-1 and PD-L1 predicts poor outcome in nasopharyngeal carcinoma. Med Oncol. 2015;32(3):86. | ||
Fang W, Zhang J, Hong S, et al. EBV-driven LMP1 and IFN-γ up-regulate PD-L1 in nasopharyngeal carcinoma: implications for oncotargeted therapy. Oncotarget. 2014;5(23):12189–12202. | ||
Qu Y, Wang D, Yang L, Liu HY, Cui W, Che YQ. Expression and clinical significance of programmed death ligand 1 in nasopharyngeal carcinoma. Mol Clin Oncol. 2018;9(1):75–81. | ||
Zhao T, Li C, Wu Y, Li B, Zhang B. Prognostic value of PD-L1 expression in tumor infiltrating immune cells in cancers: a meta-analysis. PLoS One. 2017;12(4):e0176822. | ||
Kim HR, Ha SJ, Hong MH, et al. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Sci Rep. 2016;6(1):36956. | ||
Atefi M, Avramis E, Lassen A, et al. Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin Cancer Res. 2014;20(13):3446–3457. | ||
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Canc. 2012;12(4):252–264. | ||
Lee VH, Kwong DL, Leung TW, et al. The addition of pretreatment plasma Epstein-Barr virus DNA into the eighth edition of nasopharyngeal cancer TNM stage classification. Int J Cancer. 144(7):1713–1722. | ||
Ji MF, Huang QH, Yu X, et al. Evaluation of plasma Epstein-Barr virus DNA load to distinguish nasopharyngeal carcinoma patients from healthy high-risk populations in southern China. Cancer. 2014;120(9):1353–1360. | ||
Chan KC, Zhang J, Chan AT, et al. Molecular characterization of circulating EBV DNA in the plasma of nasopharyngeal carcinoma and lymphoma patients. Canc Res. 2003;63(9):2028–2032. | ||
Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449–1458. | ||
Lin H, Wei S, Hurt EM, et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J Clin Invest. 2018;128(2):805–815. | ||
Hirsch FR, Mcelhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol. 2017;12(2):208–222. | ||
Smith J, Robida MD, Acosta K, et al. Quantitative and qualitative characterization of two PD-L1 clones: SP263 and E1L3N. Diagn Pathol. 2016;11(1):44. | ||
Xie R, Chung JY, Ylaya K, et al. Factors influencing the degradation of archival formalin-fixed paraffin-embedded tissue sections. J Histochem Cytochem. 2011;59(4):356–365. | ||
Wester K, Wahlund E, Sundström C, et al. Paraffin section storage and immunohistochemistry. Effects of time, temperature, fixation, and retrieval protocol with emphasis on p53 protein and MIB1 antigen. Appl Immunohistochem Mol Morphol. 2000;8(1):61–70. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.