Back to Journals » OncoTargets and Therapy » Volume 12
MIR22HG As A Tumor Suppressive lncRNA In HCC: A Comprehensive Analysis Integrating RT-qPCR, mRNA-Seq, And Microarrays
Authors Gao L, Xiong D, He R, Yang X, Lai Z, Liu L, Huang Z, Wu H, Yang L, Ma J, Li S, Lin P , Yang H, Luo D, Dang Y, Chen G
Received 16 August 2019
Accepted for publication 14 October 2019
Published 18 November 2019 Volume 2019:12 Pages 9827—9848
DOI https://doi.org/10.2147/OTT.S227541
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Li Gao,1,* Dan-dan Xiong,1,* Rong-quan He,2 Xia Yang,1 Ze-feng Lai,3 Li-min Liu,3 Zhi-guang Huang,1 Hua-yu Wu,4 Li-hua Yang,2 Jie Ma,2 Sheng-hua Li,5 Peng Lin,6 Hong Yang,6 Dian-zhong Luo,1 Yi-wu Dang,1,* Gang Chen1,*
1Department of Pathology, First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, Zhuang Autonomous Region 530021, People’s Republic of China; 2Department of Medical Oncology, First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, Zhuang Autonomous Region 530021, People’s Republic of China; 3School of Pharmacy, Guangxi Medical University, Nanning, Guangxi, Zhuang Autonomous Region 530021, People’s Republic of China; 4Department of Cell Biology and Genetics, School of Pre-Clinical Medicine, Guangxi Medical University, Nanning, Guangxi, Zhuang Autonomous Region 530021, People’s Republic of China; 5Department of Urology Surgery, First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, Zhuang Autonomous Region 530021, People’s Republic of China; 6Department of Ultrasound, First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, Zhuang Autonomous Region 530021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Gang Chen
Department of Pathology, First Affiliated Hospital of Guangxi Medical University, 6 Shuangyong Road, Nanning, Guangxi 530021, People’s Republic of China
Tel +0086-771-5356534
Fax +86 0771-5356534
Email [email protected]
Yi-wu Dang
Department of Pathology, First Affiliated Hospital of Guangxi Medical University, 6 Shuangyong Road, Nanning, Guangxi 530021, People’s Republic of China
Tel +0086-771-5356534
Fax +86 0771-5356534
Email [email protected]
Introduction: MIR22HG has a reported involvement in the tumorigenesis of a variety of cancers, including hepatocellular carcinoma (HCC). However, the exact molecular mechanism of MIR22HG in HCC has not been clarified.
Methods: In the present study, we integrated data from in-house RT-qPCR, RNA-sequencing, microarray, and literature studies to conduct a comprehensive evaluation of the clinico-pathological and prognostic significance of MIR22HG in an extremely large group of HCC samples. We also explored the potential mechanism of MIR22HG in HCC by analyzing the alteration profiles of MIR22HG in HCC to predict transcription factors (TFs) that may interact with MIR22HG and to annotate the biological functions of genes co-expressed with MIR22HG. MIR22HG expression was also compared in HCC nude mice xenografts before and after a treatment with nitidine chloride.
Results: We found that MIR22HG was downregulated in HCC and that this downregulation correlated with the malignant phenotype of HCC. Comprehensive analysis of the prognostic impact of MIR22HG in HCC revealed a beneficial effect of MIR22HG on the survival outcome of HCC patients. Seven cases of MIR22HG deep deletion occurred in 360 of the cancer genome atlas (TCGA) provisional HCC samples. A total of 22 MIR22HG-TF-mRNA triplets in HCC were predicted by the lncRNAmap. Co-expressed genes of MIR22HG, identified by weighted correlation network analysis (WGCNA), mainly participated in the pathways involving osteoclast differentiation, chemokine signaling pathways, and hematopoietic cell lineage. In vivo experiments demonstrated that nitidine chloride could stimulate MIR22HG expression in HCC xenografts.
Conclusion: In summary, MIR22HG may play a tumor-suppressive role in HCC by coordinating with predicted TFs and co-expressed genes, such as NLRP3, CSF1R, SIGLEC10, and ZEB2, or by being controlled by nitidine chloride.
Keywords: MIR22HG, hepatocellular carcinoma, RT-qPCR, transcription factor, co-expressed genes, nitidine chloride
Introduction
Liver cancer ranks sixth in cancer incidence worldwide and second in tumor-related mortality worldwide, with more than half of the new cases and deaths now being reported in China.1 Hepatocellular carcinoma (HCC) is the predominant type of liver cancer and accounts for approximately 80% of liver cancer.2 In the past decade, the incidence and mortality of HCC has shown an upward trend in both males and females.3 Current treatment options for HCC patients include surgical resection, local ablation, and chemotherapy, and these have achieved certain therapeutic effects. However, these treatment options also cause side effects, such as high recurrence rates after surgical resection and local ablation4 and resistance to drugs, including sorafenib and regorafenib.5–7 Therefore, identification of effective biomarkers and therapeutic strategies is imperative for improving the survival condition of HCC patients.
The rapid development of second-generation sequencing technology has raised awareness of epigenetic causes of human cancers. In particular, non-coding RNAs, such as long-chain non-coding RNAs (lncRNAs) have received wide attention. The lncRNAs are transcripts that are more than 200 nucleotides in length but cannot be translated into proteins.8 These non-coding RNAs play important roles in diverse human cancers and affect tumor biology activities including ranging from cell proliferation and cell to apoptosis.9–11 One lncRNA, MIR22HG, has a reported involvement in the tumorigenesis of a variety of cancers. For example, silencing of MIR22HG triggered apoptosis in lung cancer cells, while upregulation of MIR22HG inhibited the proliferation of endometrial cancer cells.12,13 Studies have shown that overexpression of MIR22HG could significantly suppress the malignant progression of HCC, while indicating good survival outcome of HCC patients,14–16 suggesting promising prospects for the application of MIR22HG in HCC treatment. Nevertheless, these studies had shortcomings, including the use of only limited numbers of HCC specimens for examining the expression level of MIR22HG in HCC and non-cancer tissues and the lack of sufficient diversity in the methods used to evaluate the expression of MIR22HG in HCC and non-cancer tissues. For these reasons, the exact molecular mechanism of MIR22HG in HCC has not yet been clarified.
In the present study, we performed a multidimensional assessment of the clinical significance of MIR22HG in an extremely large group of HCC samples by integrating data from in-house quantitative reverse transcription-polymerase chain reactions (qRT-PCR), RNA-sequencing, microarrays, and literature studies. Comprehensive indexes calculated from the present study, which included the standardized mean difference (SMD) and summarized receiver operating characteristic (SROC) curves, offered a relatively impartial evaluation of the differential expression of MIR22HG in HCC and non-cancer tissues. We also endeavored to uncover the molecular mechanism of MIR22HG in HCC by investigating the alterations in the profiles of MIR22HG in HCC, the transcription factors (TF) interacting with MIR22HG, the biological functions of the co-expressed genes of MIR22HG, and how nitidine chloride influences MIR22HG expression in HCC.
Materials And Methods
Clinico-Pathological Significance Of MIR22HG In HCC
In-House qRT-PCR
HCC and paired non-cancer tissues were collected from March 2018 to March 2019 from 101 HCC patients, aged between 35 and 68 years (12 male and 8 female), who were treated at the First Affiliated Hospital of Guangxi Medical University (Nanning, China). The samples were fixed in 10% buffered formalin for 16 h and then paraffin embedded. All enrolled patients signed informed consent forms, and the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University approved the study.
The process of qRT-PCR has been described in detail in previous studies.17–19 GAPDH served as the reference gene for MIR22HG. Information about the primers for MIR22HG and GAPDH is listed in Table 1.18,19 MIR22HG expression was calculated by the formula:
 |
Table 1 Information On The Primers For MIR22HG And GAPDH |
2−ΔCq =−(CqMIR22HG-CqGAPDH)20
Evaluation Of The Clinicopathological Associations Of MIR22HG In HCC Using mRNA Data
In the present study, we obtained log2(x+0.001)-transformed level 3 transcripts per million reads (TPM) RNA-seq data from 374 HCC and 50 adjacent normal tissues, as well as the clinicopathological information, from The Cancer Genome Atlas (TCGA) data portal (https://portal.gdc.cancer.gov/) (IBM Corp., Armonk, NY, USA). An additional 175 normal liver tissues from Genotype-Tissue Expression (GTEx) (https://www.gtexportal.org/home/) were also included as the non-cancer control, giving mRNA-seq data from a total of 374 HCC and 225 non-cancer tissues.
Integrated SMD Of MIR22HG Expression In HCC And Non-Cancer Tissues
Microarrays published before July 18, 2019 and pertaining to expression data for MIR22HG in HCC and non-cancer tissues were searched in the GEO (https://www.ncbi.nlm.nih.gov/gds/) and ArrayExpress (https://www.ebi.ac.uk/arrayexpress/) databases using the search terms “(C17orf91 OR MIR22HG OR MIR22 host gene) AND (hepato OR liver OR hepatic OR HCC) AND (adenocarcinoma OR carcinoma OR cancer OR neoplasm OR tumor OR tumor OR neoplas OR malignan)”. Studies were included if they offered sufficient MIR22HG expression data (more than five HCC or non-cancer cases) in human HCC and non-cancer samples for the calculation of a SMD. Basic information, as well as expression data and data used to plot SROC curves, were extracted from the included studies according to methods described previously.21 Forest plots of SMDs with the 95% confidence interval (CI) were produced for in-house RT-qPCR data, mRNA-seq, and microarrays, as described previously.21 A series of plots, including SROC, forest plots of sensitivity (SEN), specificity (SPE), positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR), were created using MetaDisc v.1.4.
Literature Study Selection For Comprehensive Analysis Of The Prognostic Value Of MIR22HG In HCC
In addition to the GEO and Arrayexpress databases, studies published as of July 18, 2019 were retrieved from literature databases, including PubMed, ScienceDirect, Ovid, Wiley Online Library, Web of Science, Springerlink, Embass, Chinese VIP, CNKI, Sinomed, and Wang Fang, to evaluate the prognostic significance of MIR22HG in HCC. The search terms for the literature searches were the same as those used for searching microarrays. Studies were included if they met the following requirements: (1) published in Chinese or English and (2) reporting sufficient data, including the hazard ratios (HRs) with 95% CIs for overall survival (OS) for HCC patients with different MIR22HG expression levels. Studies were excluded for the following reasons: (1) publication as letters, case reports, reviews, or conference reports and (2) incomplete prognostic data for MIR22HG in HCC. When duplicate study cohorts were encountered, only the most recent study was included. The first author, method for survival analysis, and HRs with 95% CIs were extracted from the qualified studies. HR values with 95% CIs for mRNA-seq, two microarrays (GSE76427 and E-MTAB-36), and all the included literature studies were merged using the meta package of R software v.3.5.2.
Potential Molecular Mechanism Of MIR22HG In HCC
Gene Alteration Of MIR22HG In HCC Tissue From cBioPortal
The cBioPortal database (http://www.cbioportal.org) was mined for mutation profiles of MIR22HG in HCC.22 The alteration status of MIR22HG in 440 TCGA provisional HCC samples was queried from the OncoPrint module of cBioPortal.
Predicting MIR22HG–TF-mRNA Triplets In HCC
The interaction between TFs and lncRNAs contributed partly to the driving mechanism of HCC; therefore, we hypothesized that TFs might be involved in MIR22HG-related tumorigenesis of HCC. TFs that potentially could bind to MIR22HG were predicted through lncRNAMap. Correlation between the log2 (x+0.001) transformed transcripts per million (TPM) expression of MIR22HG and the predicted TFs in TCGA-HCC cohorts was assessed by Pearson correlation analysis in Graphpad Prism v.8.0.
Co-Expression Analysis Of MIR22HG
We first calculated the differentially expressed genes (DEGs) based on the count data of 374 HCC and 50 adjacent normal samples from TCGA, which was performed using the limma voom package of R software v.3.5.2.23 Genes with a log2 fold change value >1 or <1 and an adjusted P value <0.01 were defined as DEGs. We integrated the log2 (x+0.001) TPM expression value of DEGs and MIR22HG in 374 TCGA-HCC samples into an input matrix for subsequent co-expression analysis. Weighted correlation network analysis (WGCNA) was carried out utilizing the WGCNA package in R software v.3.5.2 for identification of genes that were co-expressed with MIR22HG. A co-expression network of MIR22HG and co-expressed genes was constructed in Cytoskape v.3.7 according to the calculated weight value of more than 0.05. We then analyzed the biological functions, pathways, and disease enrichment of the co-expressed genes using the ClusterProfiler package in R software v.3.5.2. Terms with P value < 0.05 were considered statistically significant.
The Impact Of Nitidine Chloride On Expression Of MIR22HG In HCC
Our team is researching the anti-cancer effect and mechanism of traditional Chinese medicines. Specifically, considerable research data have been accumulated for nitidine chloride, and we have found an inhibitory effect of nitidine chloride on the growth of liver cancer cells.24,25 Taking into account the clinico-pathological action of MIR22HG in HCC, we hypothesized that it might be affected by traditional Chinese medicines such as nitidine chloride. Therefore, we performed in vivo experiments on nude mice to investigate the influence of nitidine chloride on MIR22HG expression in HCC. Male and female nude mice, purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China), were handled according to the Guide for the Care and Use of Laboratory Animals (the Shanghai SLAC Laboratory Animal of China, 2015). Each mouse was inoculated with SMMC7721 cells (1 × 107 cells/mL, 0.2mL in total) by subcutaneous injection into the right armpit. When the injected cells had produced a tumor of approximately 70 mm3 in size, all mice were randomly assigned to either the negative control group for intraperitoneal injection with saline or the treatment group for injection with 7 mg/kg nitidine chloride. After 15 days, the mice were anesthetized, and the tumor tissues were excised and stored at −80°C.
Total RNA was extracted with TRIzol Regent (Invitrogen, USA). RNA purity was determined using the NanoPhotometer® spectrophotometer (IMPLEN, CA, USA). RNA integrity was checked using the RNA Nano 6000 Assay Kit of the Agilent Bioanalyzer 2100 system (Agilent Technologies, CA, USA).
The mRNA sequencing libraries were established using the rRNA-depleted RNA and the NEB Next® Ultra™ Directional RNA Library Prep Kit for Illumina® (NEB, USA), following the manufacturer’s recommendations. The library quality was checked using the Agilent Bioanalyzer 2100 system. After removing reads with adaptors, >5% unknown nucleotides, and low-quality bases, the qualified reads were mapped against human genome references (GRCh37/hg19). Differentially expressed lncRNAs were identified based on the count data of lncRNAs using DESeq2 package in R software v.3.3.2 (|log2FC|>1, P<0.01).
Statistical Analysis
SPSS 22.0 was used for the statistical analyses of mRNA-seq and RT-qPCR data. The expression data for MIR22HG are presented as M ± SD. The expression levels of MIR22HG in HCC and non-cancer tissues determined by RT-qPCR were evaluated by paired sample t-tests and MIR22HG expression levels in HCC and non-cancer tissues from mRNA-seq were evaluated by independent samples t-tests. The significance of differential MIR22HG expression between two groups with different clinicopathological parameters was examined by independent samples t-tests. ROC curves were plotted to assess the discriminatory value of MIR22HG for HCC. AUC values of 0.5–0.7, 0.7–0.9, and 0.9–1.0 indicated a poor, moderate, and high discriminatory capacity, respectively. The prognostic significance of MIR22HG for HCC was examined by dividing all patient samples from mRNA-seq or microarrays (GSE76427 and E-MTAB-36) into two groups according to the median expression level of MIR22HG. Kaplan–Meier survival curves were drawn to compare the survival condition of patients with high or low MIR22HG expression. A P-value of less than 0.05 was considered statistically significant.
Results
Clinico-Pathological Significance Of MIR22HG In HCC
Differential Expression Of MIR22HG In HCC And Non-Cancer Tissues
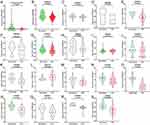
Statistical analysis of RT-qPCR data for 101 HCC and paired non-cancer tissues demonstrated a significant downregulation of MIR22HG in HCC tissues compared with non-cancer tissues (P<0.001) (Table 2, Figure 1). The expression of MIR22HG was also inversely correlated with metastasis and vascular invasion (P<0.001 and P = 0.015) (Table 2, Figure 2). MRNA-seq data for 374 HCC and 225 non-cancer samples also revealed the significant downregulation of MIR22HG in HCC (P<0.001) (Table 3) (Figure 1). A difference was also detected in the MIR22HG expression in groups of TCGA-HCC patients with various grades. Patients with advanced grade HCC (III–IV) had significantly lower expression of MIR22HG when compared with patients with early grade HCC (I–II) (Table 3, Figure 2).
 |
Table 2 Clinico-Pathological Value Of MIR22HG Expression In HCC From RT-qPCR Data |
 |
Table 3 Clinico-Pathological Value Of MIR22HG Expression In HCC From mRNA-Seq Data |
The GEO and Arrayexpress searches retrieved a total of 34 GEO microarrays and three Arrayexpress microarrays with sufficient expression data for MIR22HG in more than five HCC and non-cancer samples; these were considered eligible for the integrated calculation of SMD. The basic information and extracted data for all the included microarrays is summarized in Tables 4 and 5. MIR22HG showed a clearly downregulated expression in most of the microarrays (P<0.05) (Figures 1 and 3). A panel of ROC curves for the in-house RT-qPCR, mRNA-seq, and all included microarrays suggested a good ability of MIR22HG to distinguish HCC from non-cancer tissues (Figures 4 and 5). The integrated SMD for the in-house RT-qPCR, mRNA-seq, and all included microarrays, which together covered an extremely large sample of 2636 HCC and 2072 non-cancer samples, corroborated the downregulated expression of MIR22HG in HCC (SMD = −0.97, 95% CI = −1.17- −0.77, I2 = 88%, P<0.01) (Figure 6). Subgroup analysis revealed that experiment type might be a potential source of heterogeneity because the microarrays in the subgroup of transcription profiling by array showed no heterogeneity (I2 = 0%, P = 0.42) (Figure 6B). The sROC curves and forest plots of SEN, SPE, PLR, NLR, and DOR for in-house RT-qPCR, mRNA-seq and all included microarrays confirmed the moderate capability of MIR22HG to differentiate HCC from non-cancer tissues (Figure 7).
 |
Table 4 Basic Information For All Included Microarrays |
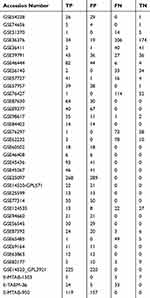 |
Table 5 Data Used To Plot The sROC Curves From All Included Microarrays |
The Prognostic Impact Of MIR22HG On HCC
Kaplan-Meier survival curves were plotted for mRNA-seq data and two microarray studies (GSE76427 and E-MTAB-36) containing overall survival data for MIR22HG in HCC (Figure 8). Three literature studies that provided overall survival data of MIR22HG were enrolled for comprehensive analysis of the prognostic significance of MIR22HG for HCC.15,16,26 Survival data extracted from the three included literature studies are listed in Table 6. Pooled HRs incorporating overall survival data from mRNA-seq, two microarrays, and three literature studies implicated MIR22HG as a protective prognostic factor for HCC (HR = 0.75, 95% CI = 0.64–0.89, I2 = 64%, P<0.01) (Figure 9A). Subgroup analysis and sensitivity analysis reported an unspecified cause of heterogeneity (Figure 9B and C). No significant publication bias was detected in the symmetrical funnel plot (P = 0.517) (Figure 9D).
 |
Table 6 Summary Of Survival Data For The Comprehensive Prognostic Value Of MIR22HG In Hepatocellular Carcinoma (HCC) |
Potential Molecular Mechanism Of MIR22HG In HCC
Gene Alteration Of MIR22HG In HCC Tissue From cBioPortal
Alteration profiles in cBioPortal indicated the occurrence of 18 cases of gene alteration, including two cases of amplification, seven cases of deep deletion, and nine cases of high mRNA, in TCGA provisional HCC samples, accounting for 5% of all the profiled cases (Figure 11A).
Predicting MIR22HG-Transcription Factor (TF)-mRNA Triplets In HCC
A total of 22 MIR22HG-TF-mRNA triplets were predicted by lncRNAmap (Table 7). Of the 22 TFs with potential relationships with MIR22HG, we found significant reverse correlation between MIR22HG and HNF4A expression (r = −0.097, P = 0.045) (Figure 11B).
 |
Table 7 Predicted MIR22HG-TF-mRNA Triplets In Hepatocellular Carcinoma (HCC) From LncMAP |
Co-Expression Analysis Of MIR22HG
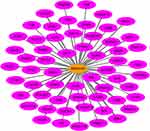
In total, 5942 DEGs were identified by limma voom analysis in TCGA-LIHC cohorts. The WGCNA for the expression matrix of these DEGs and MIR22HG showed co-expression of 59 genes with MIR22HG (weight value >0.05) (Figure 12). Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis for co-expressed genes of MIR22HG disclosed their main enrichment in molecular functions that included protein tyrosine kinase activity, non-membrane spanning protein tyrosine kinase activity, and phosphotyrosine residue binding, as well as clustering in pathways that included osteoclast differentiation, chemokine signaling pathways, and hematopoietic cell lineage (P<0.05) (Figures 13A-C and 14). The co-expressed genes were associated with several diseases, including human immunodeficiency virus infectious disease, gout, and primary immunodeficiency disease (P<0.05) (Figure 13D).
Differentially Expressed lncRNAs In Nitidine Chloride Treated HCC Xenografts
After the quality control of principal component analysis, two NC-treated and three control HCC xenograft tumor tissues were collected for detection of differentially expressed lncRNAs. The tumor volumes in the NC-treated group were significantly reduced compared with those of control group (p-value < 0.05).27 Heatmaps for differentially expressed lncRNAs before and after the nitidine chloride treatment in HCC xenografts showed a significant upregulation of 23 lncRNAs and downregulation of 12 lncRNAs. In particular, MIR22HG was significantly upregulated in nitidine chloride-treated HCC xenograft tissues (log2FC = 1.373, P<0.001) (Figure 10).
Discussion
The novelties of this article are reflected in the following aspects. We integrated data from in-house RT-qPCR, RNA-sequencing, microarray, and literature studies to provide a comprehensive evaluation of the clinico-pathological and prognostic significance of MIR22HG in an extremely large group of HCC samples. We explored the potential mechanism of MIR22HG in HCC by analyzing the alteration profiles of MIR22HG in HCC, by predicting TFs interacting with MIR22HG, and by annotating the biological functions of genes co-expressed with MIR22HG. We also compared the expression of MIR22HG in HCC nude mice xenografts before and after a treatment with nitidine chloride.
When our study is compared with several previous studies that explored the role of MIR22HG in HCC using a single method, one of the highlights of our study is that we conducted a comprehensive appraisal of the clinical significance of MIR22HG in HCC using an extremely large number of samples (2636 HCC and 2072 non-cancer tissues) collected from in-house RT-qPCR, RNA-seq, microarrays, and literature studies. The huge size of our sample group strengthened the reliability of our results. We confirmed downregulation of MIR22HG in HCC, the correlation between MIR22HG expression and the malignant phenotype of HCC, and the beneficial prognostic influence of MIR22HG on HCC, in agreement with the reports by prior research groups.14–16 These results implied that downregulation of MIR22HG results in a loss of its protective effect in HCC and subsequent malignant progression of the tumor, which is no longer restrained by MIR22HG. Consequently, HCC patients with low MIR22HG expression are predicted to show worse survival.
The finding that MIR22HG is downregulated in HCC then raises the question of the nature of the mechanism directing the MIR22HG effects on HCC. Previous studies revealed that MIR22HG could regulate the miR-10a-5p/NCOR2 axis, HMGB1, or HuR to participate in the oncogenesis of HCC.14,15 Our investigation of the molecular basis of MIR22HG in HCC also provided novel insights into the mechanism of MIR22HG in HCC. The finding of seven cases of deep deletion occurring in the TCGA provisional HCC samples from cBioPortal may explain the downregulation of MIR22HG at the transcriptional level. The predicted MIR22HG-TF-mRNA triplets in HCC hinted that a binding reaction between MIR22HG and TFs, such as CREBBP, POU2F2, ZZZ3, SIRT6, and EP300, might participate in the initiation and development of HCC. Co-expression analysis for MIR22HG implicated the MIR22HG-related gene interaction network in HCC.
Several of the genes co-expressed with MIR22HG, including NLRP3, CSF1R, SIGLEC10, and ZEB2, were reported to play crucial roles in the initiation and progression of HCC.28–31 Functional analysis of the co-expressed genes suggested a significant enrichment in molecular functions that included protein tyrosine kinase activity, non-membrane spanning protein tyrosine kinase activity, and phosphotyrosine residue binding, as well as activation of pathways involving hematopoietic cell lineage, viral protein interaction with cytokines and cytokine receptors, and activity of the Rap1 signaling pathway. We noted that the Rap1 signaling pathway was closely associated with the growth, invasion, and apoptosis of HCC cells. Mo et al reported that EYA4 could attenuate the growth and invasion of HCC cells by repression of the NF-κB activity and RAP1 expression.32 In vitro experiments by Zha et al showed that knockdown of Rap1 led to 5-fluorouracil-induced apoptosis in HepG2 cells.33 We postulated that the co-expressed genes may coordinate with MIR22HG to influence molecular functions and pathways essential for the oncogenesis of HCC, thereby affecting the development of HCC.
Unlike studies that investigated the mechanism of MIR22HG in HCC by focusing on the interplay between MIR22HG and specific miRNAs or mRNAs, our study has uncovered a new mechanism for MIR22HG in HCC using traditional Chinese medicine as the breakthrough point. Nitidine chloride is a natural alkaloid compound with proven anti-tumor effects in multiple human cancers, including osteosarcoma, ovarian cancer, acute myeloid leukemia, and HCC.25,34–36 The impact of nitidine chloride on lncRNAs in cancer has never been studied previously, so we conducted in vivo experiments to test whether nitidine chloride treatment might influence the expression of MIR22HG in HCC. The results demonstrated that nitidine chloride could stimulate MIR22HG expression in HCC xenografts, thereby implying that nitidine chloride and MIR22HG might have synergistic effects in the inhibition of HCC tumor growth.
The present study had several limitations that should be pointed out. The effect of MIR22HG on the function of HCC cells should be validated by in vitro or in vivo experiments. Limited by experiment support, expression of lncRNAs was sequenced in only three control and two NC-treated groups, which should be conducted with larger sample size. The interactions between MIR22HG and the predicted TFs, the co-expressed genes, and nitidine chloride also require further experiments. Further work is warranted to address these limitations.
Conclusion
In conclusion, we identified downregulation of MIR22HG and a protective effect of MIR22HG on the clinical progression and prognosis of HCC patients.
Ethics Approval And Informed Consent
All 101 patients with HCC provided signed informed consent and approval of this study was granted by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University. Approval for the use of nude mice in this study was granted by the Ethical Committee of First Affiliated Hospital of Guangxi Medical University (Nanning, Guangxi, China) (2018-KY-NSFC-102).
Data Availability
The datasets generated and/or analyzed during the current study are available in TCGA (https://portal.gdc.cancer.gov/), Arrayexpress (https://www.ebi.ac.uk/arrayexpress/), GEO (https://www.ncbi.nlm.nih.gov/gds/), cBioPortal (https://www.cbioportal.org/), and LncMAP (http://bio-bigdata.hrbmu.edu.cn/LncMAP/survival.jsp).
Acknowledgments
The authors would like to thank TCGA, Arrayexpress, GEO, cBioPortal and LncMAP databases for the availability of the data.
Disclosure
All authors declared no conflicts of interests in this work.
References
1. Fu J, Wang H. Precision diagnosis and treatment of liver cancer in China. Cancer Lett. 2018;412:283–288.
2. Zhao B, Lu Y, Cao X, et al. MiRNA-124 inhibits the proliferation, migration and invasion of cancer cell in hepatocellular carcinoma by downregulating lncRNA-UCA1. Onco Targets Ther. 2019;12:4509–4516.
3. Bertuccio P, Turati F, Carioli G, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol. 2017;67:302–309.
4. Rich NE, Yopp AC, Singal AG. Medical management of hepatocellular carcinoma. J Oncol Pract. 2017;13:356–364.
5. Sun T, Liu H, Ming L. Multiple roles of autophagy in the sorafenib resistance of hepatocellular carcinoma. Cell Physiol Biochem. 2017;44:716–727.
6. Zhu YJ, Zheng B, Wang HY, et al. New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacol Sin. 2017;38:614–622.
7. Tutusaus A, Stefanovic M, Boix L, et al. Antiapoptotic BCL-2 proteins determine sorafenib/regorafenib resistance and BH3-mimetic efficacy in hepatocellular carcinoma. Oncotarget. 2018;9:16701–16717.
8. Zhang Y, Liu J, Lv Y, et al. LncRNA meg3 suppresses hepatocellular carcinoma in vitro and vivo studies. Am J Transl Res. 2019;11:4089–4099.
9. Zeng Z, Xu FY, Zheng H, et al. LncRNA-MTA2TR functions as a promoter in pancreatic cancer via driving deacetylation-dependent accumulation of HIF-1alpha. Theranostics. 2019;9:5298–5314.
10. Zheng J, Zhang H, Ma R, et al. Long non-coding RNA KRT19P3 suppresses proliferation and metastasis through COPS7A-mediated NF-kappaB pathway in gastric cancer. Oncogene. Epub 2019 Aug 13.
11. Bhan A, Soleimani M, Mandal SS. Long Noncoding RNA and Cancer: A New Paradigm. Cancer Res. 2017;77:3965–3981.
12. Su W, Feng S, Chen X, et al. Silencing of long noncoding RNA MIR22HG triggers cell survival/death signaling via oncogenes YBX1, MET, and p21 in lung cancer. Cancer Res. 2018;78:3207–3219.
13. Cui Z, An X, Li J, et al. LncRNA MIR22HG negatively regulates miR-141-3p to enhance DAPK1 expression and inhibits endometrial carcinoma cells proliferation. Biomed Pharmacother. 2018;104:223–228.
14. Wu Y, Zhou Y, Huan L, et al. LncRNA MIR22HG inhibits growth, migration and invasion through regulating the miR-10a-5p/NCOR2 axis in hepatocellular carcinoma cells. Cancer Sci. 2019;110:973–984.
15. Zhang DY, Zou XJ, Cao CH, et al. Identification and functional characterization of long non-coding rna mir22hg as a tumor suppressor for hepatocellular carcinoma. Theranostics. 2018;8:3751–3765.
16. Dong Y, Yan W, Zhang SL, et al. Prognostic values of long non-coding RNA MIR22HG for patients with hepatocellular carcinoma after hepatectomy. Oncotarget. 2017;8:114041–114049.
17. Xiong DD, Li ZY, Liang L, et al. The LncRNA NEAT1 accelerates lung adenocarcinoma deterioration and binds to Mir-193a-3p as a competitive endogenous RNA. Cell Physiol Biochem. 2018;48:905–918.
18. Zhang Y, Huang JC, Cai KT, et al. Long noncoding RNA HOTTIP promotes hepatocellular carcinoma tumorigenesis and development: a comprehensive investigation based on bioinformatics, qRTPCR and metaanalysis of 393 cases. Int J Oncol. 2017;51:1705–1721.
19. Wen DY, Lin P, Pang YY, et al. Expression of the long intergenic non-protein coding RNA 665 (LINC00665) gene and the cell cycle in hepatocellular carcinoma using the cancer genome atlas, the gene expression omnibus, and quantitative real-time polymerase chain reaction. Med Sci Monit. 2018;24:2786–2808.
20. Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1.
21. Dang YW, Zeng J, He RQ, et al. Effects of miR-152 on cell growth inhibition, motility suppression and apoptosis induction in hepatocellular carcinoma cells. Asian Pac J Cancer Prev. 2014;15:4969–4976.
22. He RQ, Gao L, Ma J, et al. Oncogenic role of miR1835p in lung adenocarcinoma: a comprehensive study of qPCR, in vitro experiments and bioinformatic analysis. Oncol Rep. 2018;40:83–100.
23. Law CW, Chen Y, Shi W, et al. voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014;15:R29.
24. Liu LM, Lin P, Yang H, et al. Gene profiling of HepG2 cells following nitidine chloride treatment: an investigation with microarray and connectivity mapping. Oncol Rep. 2019;41:3244–3256.
25. Liu LM, Xiong DD, Lin P, et al. DNA topoisomerase 1 and 2A function as oncogenes in liver cancer and may be direct targets of nitidine chloride. Int J Oncol. 2018;53:1897–1912.
26. Wu Y, Wang PS, Wang BG, et al. Genomewide identification of a novel six-LncRNA signature to improve prognosis prediction in resectable hepatocellular carcinoma. Cancer Med. 2018;7:6219–6233.
27. Fu HX. Exploration the anti-angiogenesis effect of nitidine chloride based on mTOR signal pathway. Guangxi Med Univ. 2017;86.
28. Wei Q, Zhu R, Zhu J, et al. E2-induced activation of the NLRP3 inflammasome triggers pyroptosis and inhibits autophagy in HCC cells. Oncol Res. 2019;27:827–834.
29. Cai H, Zhu XD, Ao JY, et al. Colony-stimulating factor-1-induced AIF1 expression in tumor-associated macrophages enhances the progression of hepatocellular carcinoma. Oncoimmunology. 2017;6:e1333213.
30. Zhang P, Lu X, Tao K, et al. Siglec-10 is associated with survival and natural killer cell dysfunction in hepatocellular carcinoma. J Surg Res. 2015;194:107–113.
31. Gao HB, Gao FZ, Chen XF. MiRNA-1179 suppresses the metastasis of hepatocellular carcinoma by interacting with ZEB2. Eur Rev Med Pharmacol Sci. 2019;23:5149–5157.
32. Mo SJ, Hou X, Hao XY, et al. EYA4 inhibits hepatocellular carcinoma growth and invasion by suppressing NF-kappaB-dependent RAP1 transactivation. Cancer Commun (Lond). 2018;38:9.
33. Zha Y, Gan P, Yao Q, et al. Downregulation of Rap1 promotes 5-fluorouracil-induced apoptosis in hepatocellular carcinoma cell line HepG2. Oncol Rep. 2014;31:1691–1698.
34. Xu H, Cao T, Zhang X, et al. Nitidine chloride inhibits SIN1 expression in osteosarcoma cells. Mol Ther Oncolytics. 2019;12:224–234.
35. Chen S, Yang L, Feng J. Nitidine chloride inhibits proliferation and induces apoptosis in ovarian cancer cells by activating the Fas signalling pathway. J Pharm Pharmacol. 2018;70:778–786.
36. Li P, Yan S, Dong X, et al. Cell cycle arrest and apoptosis induction activity of nitidine chloride on acute myeloid leukemia cells. Med Chem. 2018;14:60–66.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.