Back to Journals » OncoTargets and Therapy » Volume 12
MiR-4766-5p Inhibits The Development And Progression Of Gastric Cancer By Targeting NKAP
Authors Wei Y, Wang Y, Zang A, Wang Z, Fang G, Hong D
Received 20 June 2019
Accepted for publication 16 September 2019
Published 16 October 2019 Volume 2019:12 Pages 8525—8536
DOI https://doi.org/10.2147/OTT.S220234
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Yaning Wei,1,* Yanan Wang,2,* Aimin Zang,1 Zhiyu Wang,1 Guotao Fang,1 Dan Hong1
1Hebei Key Laboratory of Cancer Radiotherapy and Chemotherapy, Department of Medical Oncology, Affiliated Hospital of Hebei University, Baoding City, Hebei Province 071000, People’s Republic of China; 2Department of Medical Pathology, Affiliated Hospital of Hebei University, Baoding City, Hebei Province 071000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dan Hong
Hebei Key Laboratory of Cancer Radiotherapy and Chemotherapy, Department of Medical Oncology, Affiliated Hospital of Hebei University, No. 648 Dongfeng East Road, Lianchi District, Baoding City, Hebei Province 071000, People’s Republic of China
Tel +86 31 2598 3042
Email [email protected]
Purpose: It is widely known that some specific microRNAs can regulate the expressions of genes in gastric cancer cells at the post-transcriptional level. Previous studies have identified that miRNA-4766-5p was involved in tumor cell proliferation and can be an independent prognostic indicator for malignant pleural mesothelioma. However, the mechanism underlying gastric cancer via the miRNA-4766-5p pathway remains to be blank.
Methods: We investigated the expression of miR-4766-5p in gastric cancer tissues and cells through qRT-PCR. We used RNAi to change the expressions of miR-4766-5p in gastric cancer cell lines, AGS and MKN45. Quantitative real-time polymerase chain reaction (qRT-PCR) was employed to detect the mRNA expression of miR-4766-5p. We identified cell proliferation by CCK8 and clone formation assays. We analyzed the cell apoptosis and cycle through flow cytometry. At last, we used a dual-luciferase reporter assay to illustrate the interaction between miR-4766-5p and NKAP and used Western blot to determine the protein expression of signaling pathways.
Results: We found that 1) miR-4766-5p was down-regulated in gastric cancer tissues and cells lines; 2) miR-4766-5p inhibited cell proliferation of gastric cancer cell lines significantly; 3) miR-4766-5p significantly inhibited cell migration and invasion of gastric cancer cells; 4) miR-4766-5p induced gastric cancer cell apoptosis. 5) NKAP was a direct target gene of miR-4766-5p; and 6) miR-4766-5p induced inactivation of AKT/mTOR pathway.
Conclusion: The above results indicate that miR-4766-5p suppressed the proliferation and metastasis of gastric cancer cells through targeting NKAP. Our findings could probably contribute to the diagnostics and prognostics of gastric cancer through new methodologies.
Keywords: miR-4766-5p, progression, gastric cancer, NKAP
Introduction
It is widely known that gastric cancer (GC) is one of the most malignant cancer types, with a huge amount of people dying from it every year.1–3 Gastric cancer is usually originated from the lining of the stomach, and its early symptoms are not clearly noticeable. In its early stage, it tends to be mixed and considered as regular stomach diseases, which poses hurdles to the early diagnostics. Despite the advances and developments in GC diagnostics and therapies, it is still very critical and necessary to keep seeking novel and applicable diagnostics, prognostics, and treatment methodologies.
Previous reports have demonstrated that miRNAs are playing very important roles in human cancers such as lung cancer,4 ovarian cancer,5 hepatocellular carcinomas.6 A large number of studies have found that miRNAs are closely related to the occurrence and development of tumors, and are thought to play the role of oncogenes or tumor suppressor genes.7,8 More and more attention has been paid to the role of miRNAs in gastric cancers, and accumulating evidence suggests that miRNAs may act as potential biomarkers for diagnostics and therapies.9,10 However, only limited clinical studies have revealed several types of miRNAs, with a massive number of miRNAs, remained to be investigated. MiR-4766-5p is a newly discovered member of the microRNA family. As far as we know, only one reports have studied the functions of miR-4766-5p in breast cancer.11 In 2018, Yiran Liang found that the knockdown of miR-4766-5p could significantly promote cell growth, metastasis, and chemoresistance.11 It revealed that miR-4766-5p had a direct target of SIRT1, which would be suppressed by the overexpression of miR-4766-5p. MiR-4766-5p, this novel discovered microRNA, had raised our curiosity about its functions and role in the regulation or promotion of gastric cancer.
NKAP has been reported to be a transcriptional repressor for notch signaling, which is critically demanded in the development of T cell.12,13 In 2011, FC. Hsu found that NKAP participated actively in the maturation of T cell and is involved in the T cell’s functioning competency.13 For gastric cancers, only a few researchers have investigated the NKAP identification. In 2010, CUI Juan revealed that NKAP may be related with gastric cancer as its expression was up-regulated during the development of GC.14 As described in previous studies, NKAP knockdown will lead to an increase in cellular pre-mRNA proportion and chromosome misalignment which further cause cell cycle arrest.15,16 However, the accurate mechanism by which NKAP functions in tumor remains unclear. From these studies, we noticed that NKAP has the potential role in gastric cancer oncogene, which raised our interested in the exploration of NKAP in GC.
In this paper, we aim to reveal the functions of miR-4766-5p in gastric cancer and its underlying molecular association of NKAP. To the best of our knowledge, we are the first to target the associations of miR-4766-5p and NKAP in gastric cancer. We were focused to reveal the following topics: i. the expression of miR-4766-5p in gastric cancer cells; ii. the role of miR-4766-5p in GC’s regulation or promotion; and iii. the inner molecular association between miR-4766-5p and NKAP, and their collaborative functions in GC development. The results identified that miR-4766-5p inhibits the development and progression of gastric cancer by targeting NKAP through the AKT/mTOR pathways. We believe these findings could help unveil new diagnostic and therapeutic strategies in gastric cancer.
Materials And Methods
Gastric Cancer Tissue Samples
We obtained 30 pairs of human primary gastric cancer tissues and their matched normal adjacent tissues from 30 patients who were receiving radical surgery. The patients were recruited from the Affiliated Hospital of Hebei University. The duration from July 2010 to Feb 2015 and their tumor stages were determined based on the Seventh Edition of the Cancer Staging Manual by the American Joint Committee on Cancer. The inclusion criteria were: 1) pathologically confirmed gastric carcinomas; 2) preoperative MRI including DWI. The exclusion criteria were: 1) any adjuvant chemotherapy or radiotherapy between biopsy and surgery; 2) a minimum diameter of the tumor being less than 5 mm (insufficient to support drawing an ROI); 3) incomplete pathological information; 4) inadequate image quality for postprocessing, due to artifacts. Prior to experiments, we got the written informed consent from all patients, and this study was approved by the Ethics Committee of the Affiliated Hospital of Hebei University. After receiving the tissues, we immediately snap-frozen them and stored at 80°C for future analysis.
Cell Culture
The gastric cancer cell lines of MGC-803, AGS, MKN45 and SGC-790 (ScienCell Research Laboratories, US) and human normal gastric epithelial cell line of GES-1 (Jiniou Bio, China) were seeded into RPMI 1640 medium, with 10% FBS (Gibco, US), 100 U/mL penicillin and 100 µg/mL streptomycin (Lonza, US). We cultured the cells under 37 C with 5% CO2.
Cell Transfection
siRNAs against miR-4766-5p, miR-4766-5p-siRNA1, miR-4766-5p-siRNA2, and miR-4766-5p-siRNA2, and negative control siRNA (NC) were synthesized by Ribobio Co., Ltd. (Guang Zhou, Guangdong, China). We constructed the overexpressing plasmid vector, pcDNA3.1-miR-4766-5p, from GeneChem Co., Ltd. (Pudong, Shanghai, China). Cell transfection was performed through Lipofectamine 2000 reagents (Invitrogen, Carlsbad, CA, USA) following by the manual from the vendor.
qRT-PCR Analysis
Total RNAs from tissues or cells were extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) by strictly following the manufacturer’s instructions. We synthesized cDNA through the Reverse Transcription System (Promega, Madison, USA). We conducted RT-PCR using the SYBR Green Master Mixture (Roche, America) reagent in ABI 7500 Real-time PCR instrument. β-actin was acted as an internal control. We calculated relative expression levels and normalized them using the 2−ΔΔCt methods.
CCK8 Assay
CCK8 assay was performed to test cell proliferation. After cell plantation in 96-well plate, we transfected AGS and MKN45 cells with pNC, siNC, pCMV-MIR- miR-4766-5p or si-miR-4766-5p. Every 24 hrs, we incubated the cells in each well with 10 μL CCK-8 solutions for 2 hrs. The results were measured at 450 nm’s OD value using a microplate reader.
Clone Formation Assay
After transfection for 24 h, we seeded gastric cancer cells in a 6 cm diameter’s culture dish at a density of 200 cells per well until visible by naked eyes. Then the supernatants were removed, and the cells were stained in crystal violet for 20 min. We captured the colonies’ images using a digital camera.
Transwell Assays
Next, we aim to assess cell invasive ability in vitro. Gastric cancer cells were seeded on Matrigel-coated transwell with 500 μL serum-free medium. We filled the lower chamber with 500 μL of medium containing 10% FBS. After incubation for 24 hrs, the cells on the upper surface were wiped with cotton wool. Then, we stained the invaded cells by crystal violet for 5 min. We took the photos of the cells and counted the numbers. Lastly, the transwell migration assay was performed with the same steps excluding Matrigel.
Flow Cytometry Detection For Cell Apoptosis
We utilized the Annexin V-FITC Apoptosis Detection Kit (Thermo Fisher Scientific) to measure cell apoptosis using standard protocols. Briefly, transfected cells were harvested, centrifuged, and washed with precooled PBS. Then we sequentially stained the cells with Annexin V-FITC for 15 min and PI for 5 min in a dark environment. After that, we performed flow cytometry and used FlowJo software (TreeStar, San Carlos, CA, USA) to analyze cell apoptosis.
Dual-Luciferase Reporter Assay
MiR-4766-5p and NKAP, miR-4766-5p mimic or NC mimic with pcDNA-NC, pcDNA-NKAP-3ʹUTR-wt or pcDNA-NKAP-3ʹUTR-mut were co-transfected into gastric cancer cells. We determined the relative luciferase activities using a dual-luciferase reporter assay kit (Promega, USA).
Western Blot
After 48 hr’s transfection, the proteins of gastric cancer cells were extracted using RIPA buffer containing 1% protease inhibitors (Roche, Basel, Switzerland). We used the BCA assay to determine protein concentration. The proteins were separated using SDS–PAGE at 20 μg/lane and transferred them to a nitrocellulose membrane (Millipore, Bedford, MA). Then we blocked the membrane with 5% non-fat milk for 2 hrs, incubated them with primary antibodies at 4 °C for one night, and the second antibodies for 1 hr at room temperature. After washing with TBST 3 times, we detected the protein bands by ECL. The primary antibodies to anti-NKAP, anti-AKT, anti-p-AKT, anti-mTOR, anti-p-mTOR, anti-P70S6K, and anti-GAPDH were purchased Abcam. We utilized Image J to quantify the density of each band.
Animal Studies
The female BALB/c nude mice (4–6 weeks) were purchased from Shanghai LAC Laboratory Animal Co. Ltd. (Shanghai, China). pcDNA3.1 or pcDNA3.1-miR-4766-5p transfected AGS cells were injected into the posterior flank of mice subcutaneously at n=6 each group. Tumor volume was monitored at indicated times. Finally, the mice were executed and tumors were resected.
Immunohistochemistry (IHC) Analysis
Paraffin-embedded tumor blocks were cut into sections with 4 μm thickness. Deparaffinization, rehydration and antigen retrieval (microwave, 30 min) were performed before incubation with anti-NKAP or anti-Ki67 (20 min, 1:500). The tumor sections were then developed by using a DAB method. After doubled staining with hematoxylin, dehydration with gradient ethanol, and mounting with neutral resins, tissue sections were observed and imaged in 5 random fields at a microscope. The antibody for anti-Ki67 was purchased from Abcam.
Statistical Analysis
All data were presented as mean ± standard deviation and the statistical analyses were perform using SPSS (19.0 vision, Chicago, IL, USA). We evaluated the differences by the Student t-test and one-way ANOVA. The significant difference was set as P<0.05.
Results
MiR-4766-5p Is Down-Regulated In Gastric Cancer Tissues And Cells
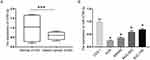
We used the qRT-PCR assay to identify the expression of miR-4766-5p in gastric cancer tissues and normal tissues. As shown in Figure 1A, miR-4766-5p expression was significantly lower in gastric cancer tissues than their para-carcinoma tissue tissues. Figure 1B shows the expression of miR-4766-3p in cell lines of CES-1, AGS, MKN45, MGC-803, SGC-790. The cell line analysis also indicated that AGS and MKN45 cells exhibited the lowest expression of miR-4766-5p. These two results confirmed that miR-4766-5p is down-regulated in gastric cancer tissues and cells.
Inhibition Of Gastric Cancer Cell Proliferation, Migration, And Invasion By miR-4766-5p
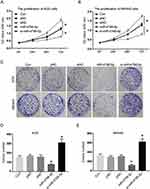
Next, we aim to determine whether miR-4766-5p is in gastric cancer progression. We transfected si-miR-4766-5p into the human gastric cancer cells and performed the CCK-8 assay. Figure 2A and B revealed that cell proliferation was significantly inhibited when silencing miR-4766-5p. To validate the result, we also performed the clone formation assay in gastric cancer cells. Figure 2C shows the clone formation assay for AGS cells and MKN45 cells in control, pNC, siNC, miR-4766-5p, and si-miR-4766-5p. Figure 2D and E show the colony numbers of AGS cells and MKN45 cells. From the results, we found that miR-4766-5p dramatically reduced clone numbers of gastric cancer cells. These data indicated that miR-4766-5p displayed a negative role in gastric cancer cell proliferation.
Knockdown Of miR-4766-5p Significantly Increased Cell Migration And Invasion
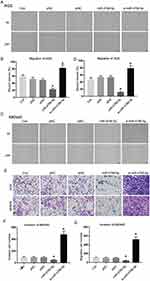
Next, we wanted to reveal the migration and invasion properties of gastric cancer cells by the effect of miR-4766-5p. Here, transwell assays were performed to analyze cell migration and invasion of gastric cancer cells. As shown in Figure 3A, si-miR-4766-5p significantly increases the velocity of cell migration and invasion in AGS cells. The invasion cell numbers also show that the invasion and migration numbers were increased by the silencing of miR-4766-5p (Figure 3B). In Figure 3C and D, a similar phenomenon was observed for MKN45. Figure 3E–G show the migration and invasion results. The above results confirmed that knockdown of miR-4766-5p significantly increased the cancer cell migration and invasion.
Induction Of Gastric Cancer Apoptosis By miR-4766-5p
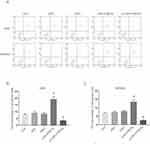
Next, we aim to determine whether cell apoptosis could make contributions to the inhibiting of miR-4766-5p. We analyzed the cell apoptosis of AGS and MKN45 cells using flow cytometry. Figure 4A shows the flow cytometry results for AGS and MKN45. Figure 4B and C show the percentage of apoptotic cells in AGS and MKN45. Our results indicated that miR-4766-5p significantly increased the percentage of total apoptotic cells in gastric cancer cells.
NKAP Was A Direct Target Gene Of miR-4766-5p
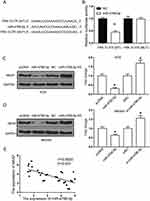
We used the online software of TargetScan for the searching of the endogenic target gene of miR-4766-5p. Figure 5A shows that miR-4766-5p had some sharing sequences with the 3′-UTR of NKAP. It unveiled the fact that NKAP might be a direct target of miR-4766-5p. Next, we constructed wild-type NKAP luciferase vector (NKAP -WT) and mutant type NKAP luciferase vector (NKAP -MUT) and introduced them into gastric cancer cells. This is to identify the interactions between miR-4766-5p and NKAP. Figure 5B shows the relative luciferase activity. We found that the activity was greatly reduced by the introduction of miR-4766-5p mimics, in gastric cancer cells transfected with the NKAP-WT vector. However, the mutation of the matching sites in the 3′-UTR of NKAP did not affect luciferase activity, which was followed by the up-regulation of miR-4766-5p. In addition, we performed Western blot assay in Figure 5C and D. We found that the overexpression of miR-4766-5p could greatly reduce NKAP expression. However, the down-regulation of miR-4766-5p would significantly promote the expression of NKAP in gastric cancer cells. In Figure 5E, we performed a correlation analysis between the expression of miR-4766-5p and the expression of NKAP. We found that the expression of miR-4766-5p was correlated with the expression of NKAP negatively.
MiR-4766-5p Inhibited The Activation Of AKT/mTOR Pathways
Finally, we hoped to explore the effect of miR-4766-5p on the down-regulation of gastric cancer cells. We investigated AKT/mTOR signaling pathways. Previous reported have shown that AKT/mTOR signaling pathway was critical in regulating tumor cell proliferation, cell cycle, apoptosis, and movements. Figure 6A showed the Western blot results of AGS and MKN45 in control, pNC, siNC, miR-4766-5p, and si-miR-4766-5p, respectively. Figure 6B and C then illustrated the phosphorylated levels of AKT and mTOR for AGS and MKN45. We found that the phosphorylated levels of AKT and mTOR in gastric cancer cells were significantly down-regulated by miR-4766-5p. In addition, the expression of downstream effector p70S6K was negatively regulated by miR-4766-5p.
Overexpression Of miR-4766-5p Inhibited Gastric Tumor Growth By Regulating NKAP In Vivo
To validate the function of MiR-4766-5p in vivo, a xenograft model was established in nude mice. pcDNA or pcDNA-miR-4766-5p transfected human AGS cancer cells were injected subcutaneously into the nude mice. Tumor growth and tumor size were monitored and measured regularly. We observed that overexpression of miR-4766-5p significantly inhibited tumor growth compared with a pcDNA group (Figure 7A and B, P<0.05). Moreover, the qRT-PCR analysis suggested that the expression of miR-4766-5p was significantly elevated in the cervical tumor when miR-4766-5p was overexpressed (Figure 7C). IHC staining showed that the expression of NKAP and Ki67 was significantly inhibited by overexpression of miR-4766-5p (Figure 7D). Taken together, these data suggest that knockdown of miR-4766-5p inhibits cervical tumor growth by regulating NKAP, which was consistent with our findings in vitro.
Discussion
There has been growing interest in the investigations of microRNAs’ role and functions in many types of cancers such as breast cancer,17 lung cancer,18 pancreatic cancer.19 Gastric cancer is one of the most harmful human diseases, which causes deaths from a considerable population every year.1–3 Researchers have paid a lot of efforts in finding various types of miRNAs’ working mechanism in the regulation of promotion of gastric cancers. In recent years, scientists have revealed that some specific microRNAs can regulate the expressions of genes in gastric cancer cells at the post-transcriptional level, and can act as diagnostic biomarkers for GC.20–23
MiRNA-4766-5p is a newly discovered miRNA with very limited investigation reported published. In 2018, Yiran Liang et al revealed that miR-4766-5p could greatly decrease the growth, metastasis, and chemoresistance of breast cancer cells.11 The authors found that miR-4766-5p directly targeted SIRT1, and suppressed its regulation in tumor cells. This phenomenon, together with the mystery of this new microRNA, made us curious about whether it could also contribute to gastric cancers.
Our first experiments employed qRT-PCR to monitor the expression of miR-4766-5p in gastric cancer tissues and cells. We found that it is indeed down-regulated in GC cells. In 2010, T. Ueda et al had thoroughly discussed the relationship between the expression of microRNA and the progression and prognosis of GC.9 It found that many microRNAs had an abnormal correlation with tumorigenesis, especially got down-regulated in the tumors’ progression and prognosis. Not to mention, many researchers had reported the down-regulation of many miRNAs like miR-15b, miR-21, and miR-218 in gastric cancers.20–23 Although we are the first to determine miR-4766-5p’s role in GC, our results are still lying in the same theoretical direction with previous studies carried by other scientists.
In 2011, YX. Song had reported that miR-148b was down-regulated in GC, and simultaneously, it suppressed the GC through the inhibition of cell profanation.24 In 2018, M. Xie revealed that gastric cancer could be suppressed by miRNA-1 in the angiogenesis-related growth factors.25 We performed the CCK-8 assay to examine the proliferation of AGS cells and MKN45 cells and found that miR-4766-5p inhibited the proliferation, migration, and invasion of gastric cancer. Our colony formation assays revealed that the knockdown of miR-4766-5p could greatly increase the colony numbers, giving evidence that miR-4766-5p reversed the functions in cell cloning. Our results confirmed the findings that many microRNAs are acting as tumor suppressors in gastric cancers.
Previous reports had indicated that the knockdown of some specific microRNAs could greatly promote tumor cell migrations and invasions in GC. For example, microRNA-623 was reported to inhibit these two properties of GC tumor cells and enhanced the chemo-sensitivity to GC medications.26 Besides, in 2018, X. Deng stated that miR-34a could regulate the apoptosis of GC through the targeting of a regulator.27 Our transwell assays analyzed the cell migration and invasion properties, and the cell numbers from migration and invasion both increased significantly in the group of knockdown of miR-4766-5p. We also conducted flow cytometry to investigate the cell apoptotic percentage, and found that miR-4766-5p may induce this phenomenon. These results further contributed to the GC regulation by introducing a new microRNA member.
NKAP was discovered as a transcriptional repressor for notch signaling.12,13 It is critically required in the development and maturation of T cell. The authors including FC. Hsu found that NKAP is closely related in the maturation of T cell and is involved in the T cell’s functioning competency in a positive way.13 However, for gastric cancer, only CUI Juan gave slight evidence that NKAP might have some relation with gastric cancer. Its expression was up-regulated during the development of GC.14 Our study bridges this gap between NKAP and gastric cancer. We used the online gene database and predicted that miR-4766-5p had a shared sequence with NKAP. The luciferase was greatly reduced in the miR-4766-5p in gastric cancer cells transfected with NKAP-WT vector. The overexpression of miR-4766-5p could reduce NKAP expression, and down-regulation of miR-4766-5p would remarkably promote the expression of NKAP in gastric cancer cells. These results proved that NKAP is a direct target of miR-4766-5p. Their inner association and interaction could regulate the progressing of GC cells.
Previous studies had found many signaling pathways were utilized in gastric cancers. The signaling pathways could be inhibited in GC, including JNK,28 PI3K/AKT/mTOR,29 and AKT/mTOR.30 In 2015, Zhi Zhao et al found that the activation of the Akt/mTOR pathway promoted cell growth and inhibits apoptosis in gastric cancer cells.30 The Western blots results showed protein expression in various cell lines. We found that the phosphorylated levels of AKT and mTOR in gastric cancer cells were remarkably down-regulated by miR-4766-5p. Besides, the expression of downstream effector p70S6K was also negatively regulated by miR-4766-5p. These results are consistent with earlier reports, and confirmed that miR-4766-5p could inhibit the AKT/mTOR signaling pathway.
Conclusions
The above results and analysis have demonstrated that miR-4766-5p was down-regulated in gastric cancer tissues and cells lines. Cell migration and invasion of gastric cancer cells could be greatly inhibited by miR-4766-5p. We found that miR-4766-5p suppressed the proliferation and metastasis of gastric cancer cells through targeting NKAP and the inhibiting of the AKT/mTOR signaling pathway.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12(3):354. doi:10.3748/wjg.v12.i3.354
2. Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. New England Journal of Medicine. 2001;345(11):784–789. doi:10.1056/NEJMoa001999
3. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process—first American Cancer Society award lecture on cancer epidemiology and prevention. Cancer Res. 1992;52(24):6735–6740.
4. Yanaihara N, Caplen N, Bowman E, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9(3):189–198. doi:10.1016/j.ccr.2006.01.025
5. Iorio MV, Visone R, Di Leva G, et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67(18):8699–8707. doi:10.1158/0008-5472.CAN-07-1936
6. Murakami Y, Yasuda T, Saigo K, et al. Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene. 2006;25(17):2537. doi:10.1038/sj.onc.1209283
7. Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350. doi:10.1038/nature02871
8. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. cell. 2004;116(2):281–297. doi:10.1016/s0092-8674(04)00045-5
9. Ueda T, Volinia S, Okumura H, et al. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 2010;11(2):136–146. doi:10.1016/S1470-2045(09)70343-2
10. Tsujiura M, Ichikawa D, Komatsu S, et al. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer. 2010;102(7):1174. doi:10.1038/sj.bjc.6605571
11. Liang Y, Song X, Li Y, et al. A novel long non-coding RNA-PRLB acts as a tumor promoter through regulating miR-4766-5p/SIRT1 axis in breast cancer. Cell Death Dis. 2018;9(5):563. doi:10.1038/s41419-018-0582-1
12. Pajerowski AG, Nguyen C, Aghajanian H, Shapiro MJ, Shapiro VS. NKAP is a transcriptional repressor of notch signaling and is required for T cell development. Immunity. 2009;30(5):696–707. doi:10.1016/j.immuni.2009.02.011
13. Hsu F-C, Pajerowski AG, Nelson-Holte M, Sundsbak R, Shapiro VS. NKAP is required for T cell maturation and acquisition of functional competency. J Exp Med. 2011;208(6):1291–1304. doi:10.1084/jem.20101874
14. Juan C, Li F, Puett D, Hong C, Xu Y. Protein markers identification for gastric cancer diagnosis. Google Patents. 2010.
15. Burgute BD, Peche VS, Steckelberg AL, et al. NKAP is a novel RS-related protein that interacts with RNA and RNA binding proteins. Nucleic Acids Res. 2014;42(5):3177–3193. doi:10.1093/nar/gkt1311
16. Li T, Chen L, Cheng J, et al. SUMOylated NKAP is essential for chromosome alignment by anchoring CENP-E to kinetochores. Nat Commun. 2016;7:12969. doi:10.1038/ncomms12969
17. Lehmann U, Hasemeier B, Christgen M, et al. Epigenetic inactivation of microRNA gene hsa‐mir‐9‐1 in human breast cancer. J Pathol. 2008;214(1):17–24. doi:10.1002/path.2251
18. Yang M, Shen H, Qiu C, et al. High expression of miR-21 and miR-155 predicts recurrence and unfavourable survival in non-small cell lung cancer. Eur J Cancer. 2013;49(3):604–615. doi:10.1016/j.ejca.2012.09.031
19. Ji Q, Hao X, Zhang M, et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4(8):e6816. doi:10.1371/journal.pone.0006816
20. Xia L, Zhang D, Du R, et al. miR‐15b and miR‐16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int J Cancer. 2008;123(2):372–379. doi:10.1002/ijc.23501
21. Feng R, Chen X, Yu Y, et al. miR-126 functions as a tumour suppressor in human gastric cancer. Cancer Lett. 2010;298(1):50–63. doi:10.1016/j.canlet.2010.06.004
22. Kogo R, Mimori K, Tanaka F, Komune S, Mori M. Clinical significance of miR-146a in gastric cancer cases. Clin Cancer Res. 2011;17(13):4277–4284. doi:10.1158/1078-0432.CCR-10-2866
23. Li B-S, Zhao Y-L, Guo G, et al. Plasma microRNAs, miR-223, miR-21 and miR-218, as novel potential biomarkers for gastric cancer detection. PLoS One. 2012;7(7):e41629. doi:10.1371/journal.pone.0041629
24. Song Y-X, Yue Z-Y, Wang Z-N, et al. MicroRNA-148b is frequently down-regulated in gastric cancer and acts as a tumor suppressor by inhibiting cell proliferation. Mol Cancer. 2011;10(1):1. doi:10.1186/1476-4598-10-93
25. Xie M, Dart DA, Guo T, et al. MicroRNA-1 acts as a tumor suppressor microRNA by inhibiting angiogenesis-related growth factors in human gastric cancer. Gastric Cancer. 2018;21(1):41–54. doi:10.1007/s10120-017-0721-x
26. Jiang L, Yang W, Bian W, et al. microRNA-623 targets cyclin D1 to inhibit cell proliferation and enhance the chemosensitivity of cells to 5-fluorouracil in gastric cancer. Oncol Res Featuring Preclinical Clin Cancer Ther. 2018;27(1):19–27. doi:10.3727/096504018X15193469240508
27. Deng X, Zheng H, Li D, et al. MicroRNA-34a regulates proliferation and apoptosis of gastric cancer cells by targeting silent information regulator 1. Exp Ther Med. 2018;15(4):3705–3714. doi:10.3892/etm.2018.5920
28. Almasi S, Kennedy BE, El-Aghil M, et al. TRPM2 channel–mediated regulation of autophagy maintains mitochondrial function and promotes gastric cancer cell survival via the JNK-signaling pathway. J Biol Chem. 2018;293(10):3637–3650. doi:10.1074/jbc.M117.817635
29. Tapia O, Riquelme I, Leal P, et al. The PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance. Virchows Arch. 2014;465(1):25–33. doi:10.1007/s00428-014-1588-4
30. Zhao Z, Han F, Yang S, Wu J, Zhan W. Oxamate-mediated inhibition of lactate dehydrogenase induces protective autophagy in gastric cancer cells: involvement of the Akt–mTOR signaling pathway. Cancer Lett. 2015;358(1):17–26.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.