Back to Journals » OncoTargets and Therapy » Volume 11
MiR-411 suppresses the development of bladder cancer by regulating ZnT1
Authors Liu Y, Liu T, Jin H, Yin L, Yu H, Bi J
Received 10 May 2018
Accepted for publication 3 September 2018
Published 4 December 2018 Volume 2018:11 Pages 8695—8704
DOI https://doi.org/10.2147/OTT.S173750
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yao Dai
Yang Liu, Tao Liu, Hongwei Jin, Lei Yin, Hongyuan Yu, Jianbin Bi
Urology Surgery, First Affiliated Hospital of China Medical University, Shenyang, China
Background: At present, the molecular genetics of the development and progression of bladder cancer are still unclear. In recent years, the pathological relevance and significance of microRNAs (miRNAs) in bladder cancer have attracted increasing attention.
Methods: The expressions of miR-411 and zinc transporter 1 (ZnT1) in bladder cancer were determined by western blot and real-time PCR. Biological software, luciferase reporter gene, Western blot and real-time PCR were used to determine the regulatory effect of miR-411 on ZnT1. MTT and transwell were used to confirm the regulatory effect of miR-411 on bladder cancer cells. MTT and transwell were used to find how miR-411 modulated the biological activity of bladder cancer cells by regulating ZnT1.
Results: The expression of miR-411 was low in bladder cancer and was negatively correlated with ZnT1. MiR-411 can inhibit the activity and the expression of ZnT1. MiR-411 can inhibit the growth and metastasis of bladder cancer cells. MiR-411 inhibited the growth and metastasis of bladder cancer cells by targeting ZnT1.
Conclusion: The miR-411 target ZnT1 may provide a potential therapeutic target for the treatment of bladder cancer.
Keywords: miR-411, ZnT1, bladder cancer, proliferation, metastasis
Introduction
As one of the most common malignant tumors in humans, the pathogenesis of bladder cancer is complex, involving the expression of a large number of genes, protein dysfunction, and a variety of signal pathways. At present, the molecular genetic mechanisms underlying bladder cancer development are not clear. In recent years, increasing attention has been paid to the role and significance of miRNAs in the carcinogenesis and development of bladder cancer.1–3
At present, many kinds of miRNA have been shown to be closely related to the occurrence and development of tumors. Inhibition of miR-203 in estrogen receptor (ER)-positive breast cancer cells inhibited tumor growth in a preclinical breast cancer model.4 In bladder cancer, miR-145 was shown to have a lower expression level in cancer tissues, whereas the expression of miR-21 was upregulated in cancer tissues.5–8 miR-129, miR-133b, and miR-518c have been shown to be related to the progression of bladder cancer and can be used as tumor markers to monitor the occurrence and development of bladder cancer.9,10 miR-129 can regulate the apoptosis-related pathway and affect the proliferation of bladder cancer cells via GALNT1 and SOX4. The expression of miR-143 was low in bladder cancer, and it can regulate the expression of RAS, which means that it may play a role as a tumor suppressor in bladder cancer. miR-30–3 p, miR-133a, and miR-199a may regulate the growth of bladder cancer by regulating the activity of KRT7.11–17 miR-411 has been shown to be expressed at a low level in various tumors, and microarray data have shown low expression of miR-411 in bladder cancer,18 but there is no specific study on the specific mechanism of miR-411 in bladder cancer.
Abnormal expression of the zinc transporter may mediate several steps in the pathophysiological processes of cancer, such as excessive proliferation and excessive migration of cells.19 Deletion of the zinc transporter gene also remarkably inhibited activation of matrix metalloproteinases (MMPs; such as MMP2) and led to overexpression of cell cycle-related proteins (such as Cyclin D1).20
In our study, we found that the expression of miR-411 in bladder cancer was lower than that in the adjacent tissue, and that ZnT1 expression and miR-411 in bladder cancer patients were negatively correlated. Thereafter, through a series of in vitro experiments, we confirmed that miR-411 can regulate the growth and metastasis of bladder cancer cells by affecting ZnT1.
Materials and methods
Tissue specimens
This study was approved by the Protection of Human Subjects Committee of First Affiliated Hospital of China Medical University. Written informed consent was obtained from all patients involved in the study (19 males, 11 females; age range, 35–78 years; mean age, 51.28; 25 cases of stages I–II, 5 cases of stage III). In total, 30 pairs of human bladder cancer tissue along with corresponding adjacent nonneoplastic tissue were obtained with the patients’ approval. The tissue samples were snap frozen in liquid nitrogen at the time of surgery and stored at −80°C in a freezer.
Cell culture
Human bladder cancer cell lines (BIU87, 5637, and T24) and a human ureteral immortalized cell line (SV-HUC-1) were obtained from the Chinese Academy of Medical Sciences. Cells were maintained in DMEM (Gibco) supplemented with 10% FBS Gibco) at 37°C in a humidified air atmosphere containing 5% carbon dioxide.
Real-time PCR
Total RNA was collected using Trizol (Shenggong, Shanghai, China) from frozen tissues and cell lines according to the manufacturer’s recommendations.21 Mature miRNA analysis was performed using TaqMan miRNA assays (Huibai, Shenyang, China) by Huibai company. miRDB, an online database of predicted miRNA targets (http://www.mirdb.org/miRDB/), was used to predict the target site of the miRNAs. The expression of miR-411 was detected using a Stem-Loop real-time PCR assay as described previously.22,23 Primer sequences were synthesized as shown in Table 1.
  | Table 1 Primer sequences synthesized for use in the experiments |
Western blotting
The cells and tissues were collected and lysed in the radioimmunoprecipitation assay (RIPA) buffer. For each Western blot assay, an equal amount (30 μg) of proteins was electrophoresed on a 10% SDS-PAGE onto a polyvinylidine difluoride membrane. The membranes were blocked with 5% nonfat milk for 1 hour. The membranes were incubated overnight with specific antibodies targeting ZnT1, Cyclin D1, MMP2, and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Proteins were then visualized with peroxidase-conjugated secondary antibodies for 1 hour.
Immunohistochemistry (IHC)
The tissue samples were harvested and fixed in 10% normal buffered formaldehyde for 3 days and then embedded in paraffin. Tissue sections at the fracture site were cut longitudinally at a thickness of 3 μm and then prepared for IHC staining. IHC was performed using anti-ZnT1 antibodies (Santa Cruz Biotechnology).
Dual-luciferase reporter assay
A dual-luciferase reporter assay (involving firefly and Renilla luciferases) was performed to determine whether ZnT1 is a direct target of miR-411. BIU87 cells were seeded into 12-well plates. After 24 hours, the cells were transfected with a luciferase reporter plasmid, miR-411 mimic, negative control (NC), or miR-411 inhibitor (antisense), with three independent experiments for each. At 48 hours posttransfection, the activities of firefly and Renilla luciferases were examined using a Dual-Luciferase Reporter Assay System (Promega Corporation, Beijing, China). The ZnT1 3′-untranslated region (UTR) was cloned into a pGL3 Luciferase Reporter Vector (Shenggong, Shanghai, China), and the construct was designated ZnT1-wt. In addition, a pGL3-ZnT1 mutant 3′-UTR construct (designated ZnT1-mut) was generated by mutating the sequence that is complementary to the seed sequence in the binding region of miR-411. The primers were: ZnT1-wt, F: 5′-CTCCAACGGGCTGAAATTGG-3′, R: 5′-GGGGTCAGGGAAACATGGAT-3′, ZnT1-mut, F: 5′-GGTGGCCAATACCAGCAACT-3′, R: 5′-CAAATGCTTTGCAGGGGTC-3′.
MTT assays
The assessment of cell proliferation was performed using an MTT (3-(4,5-dimethylthiazol-2-yl)–2,5-diphenyltetrazolium bromide) assay with MTT (Shenggong, Shanghai, China), according to the manufacturer’s protocol. Following transfection for 24 hours, cells were seeded into 96-well plates. A 10-μL volume of MTT solution was added into each well. The formazan precipitates were then dissolved in 200 μL of dimethyl sulfoxide (DMSO). The absorbance at 490 nm (A490) of each well was measured using a microplate absorbance reader (Bio-Rad, Beijing, China).
Transwell assay
The migration and invasion potentials of the cells were evaluated using Transwell chambers with an 8-μm pore polycarbonate membrane (Corning Costar, Beijing, China) coated with/without Matrigel. Following transfection for 24 hours, cells were added into the upper chamber. Medium containing 20% FBS was then added to the lower chamber as a chemoattractant. The cells that migrated or invaded were counted under a light microscope. Experiments were carried out at least three times.
Transfection
To stably silence ZnT1, BIU87 cells were transfected with si-ZnT1 (Shanghai GeneChem Company, Shanghai, China), and si-NC was used as a negative control. Protein and RNA were extracted at 48 hours after transfection.
Statistical analysis
All data were analyzed with PRISM 5.0 (GraphPad, Inc., La Jolla, CA, USA). Two-sample t-tests were used to analyze the results. P<0.05 was considered statistically significant.
Ethics approval and informed consent
For the use of clinical materials for research purposes, ethics approval was obtained from the Protection of Human Subjects Committee of First Affiliated Hospital of China Medical University (ref: 2015024). In addition, patient informed consent was obtained in writing according to First Affiliated Hospital of China Medical University regulations. This patient consent involved a statement of consent to participate in the study and to use their tissues for research purposes, as specified in the Declaration of Helsinki.
Results
Expression of miR-411 and ZnT1 in bladder cancer
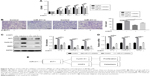
The expression of miR-411 in the 30 patients with bladder cancer was detected by real-time PCR. The result showed that miR-411 was downregulated in bladder cancer (Figure 1A). In addition, miR-411 was associated with the staging of bladder cancer (Table 2). There was also a lower expression of miR-411 in bladder cancer cells (BIU87, 5637, and T24) compared with SV-HUC-1 cells (Figure 1B). Next, we analyzed the expression of ZnT1 in bladder cancer. The results showed that ZnT1 expression in bladder cancer tissue was higher than in adjacent tissues (Figure 1C and D). The expression of ZnT1 increased with increasing TNM classification (Figure 1E). There was also a higher expression of ZnT1 in bladder cancer cells (BIU87, 5637, and T24) compared with SV-HUC-1 cells (Figure 1F and G).
  | Table 2 Relationship between miR-411 and bladder cancer |
Relationship between miR-411 and ZnT1 in bladder cancer
In bladder cancer tissues, we found a negative correlation between miR-411 and ZnT1 (Figure 2A). miRDB predicted that miR-411 targets a region in the 3′-UTR of ZnT1 (Figure 2B). The luciferase reporter gene assay showed that miR-411 can directly affect ZnT1 (Figure 2C). When miR-411 was overexpressed in BIU87 cells, the expression of ZnT1 was assessed by Western blotting and real-time PCR (Figure 2D and E). The results showed that miR-411 inhibited the expression of ZnT1 at the protein and mRNA levels. Moreover, ZnT1 expression was upregulated when miR-411 was inhibited in BIU87 cells (Figure 2F and G).
miR-411 can inhibit the proliferation of bladder cancer cells
An MTT assay was used to examine the effect of miR-411 on BIU87 cells. From the experiments involving the transfection of the miR-411 mimic or inhibitor, we found that miR-411 could inhibit the proliferation of BIU87 cells (Figure 3A and B). We also found that the inhibitory effect of miR-411 on the proliferation of BIU87 cells may be achieved by its inhibitory effect on Cyclin D1 (Figure 3C–F).
miR-411 can inhibit the metastasis of bladder cancer cells
Transwell assays (with or without Matrigel) were used to study whether miR-411 was involved in the metastasis of BIU87 cells (Figure 4A–D). The miR-411 mimic or miR-411 inhibitor was transfected into BIU87 cells (along with the NC) to detect the effect of miR-411 on the metastasis of bladder cancer cells. The results showed that the metastasis of BIU87 cells was significantly inhibited when miR-411 was overexpressed. However, the metastasis of BIU87 cells was promoted when the miR-411 expression was decreased. We also found that the inhibitory effect of miR-411 on the metastasis of BIU87 cells may be achieved by its inhibitory effect on MMP2 (Figure 4E–H).
Effects of miR-411 inhibitor and ZnT1 siRNA on proliferation and metastasis of BIU87 cells
MTT and Transwell assays showed that when ZnT1 was silenced using ZnT1 siRNA, cell proliferation and migration was significantly suppressed, and miR-411 also significantly inhibited the proliferation and migration of BIU87 cells (Figure 5A and B). When both miR-411 and ZnT1 were inhibited, the proliferation and migration ability of the cells was stronger than when ZnT1 alone was silenced. Similar results were obtained in the Western blotting and real-time PCR assays (Figure 5C and D). In conclusion, we conclude that miR-411 can be used to target bladder ZnT1 for the proliferation and migration of bladder cancer (Figure 5E).
Discussion
The expression and functions of miR-411 have been investigated in several types of cancer. However, until now, miR-411 in human bladder cancer has not been examined. Research revealed that miR-411 might be a potential target for lung cancer therapy.24 Data showed that serum miR-411 expression was significantly positively associated with TNM stage in lung cancer. Studies also suggested that high expression of miR-411 was an independent indicator of poor prognosis for non-small cell lung carcinoma patients.24–26 In breast cancer, researchers showed that the miR-411–5 p/GRB2/Ras axis has the potential to provide molecular targets for breast cancer therapy.25 In contrast, in renal cell carcinoma, miR-411 was found to be significantly downregulated and it played a role as a tumor suppressor.26 Thus, studies have demonstrated that miR-411 is important in tumorigenesis and development.
However, there are no previous reports on the functions of miR-411 in bladder cancer. In the present study, it was found that miR-411 suppressed bladder cancer cell proliferation and metastasis. These results indicate that miR-411 has important functions in bladder cancer tumorigenesis and development, and it may have clinical implications for the treatment of bladder cancer.
Zinc is an essential trace element in the tissues and cells of the body. Zinc is involved in the synthesis and catabolism of various proteins and nucleic acids in the body, and zinc regulates the activity of enzymes. Zinc deficiency can lead to a series of diseases, such as body growth retardation, impaired DNA synthesis and repair, and immune dysfunction. Studies have shown that zinc in the blood is lower in bladder cancer patients than in healthy people.24 Zinc content in urine is higher in bladder cancer patients than in healthy people, and the difference is particularly significant in superficial (nonmuscle invasive) bladder cancer.27,28 Therefore, we speculated that zinc content is involved in the occurrence and development of bladder cancer, so the protein regulating zinc metabolism (ZnT1) has become our object of study. Zinc transporters transport zinc ions to or into cells. Recent research has shown that Znt1 is associated with a variety of malignant tumors, such as breast and pancreatic cancer.29 The present study demonstrated the high expression of ZnT1 in bladder cancer, and further showed that miR-411 can play a regulatory role regarding ZnT1.
In this study, first, we used Western blotting and real-time PCR to assess the expression of ZnT1 in bladder cancer tissues and adjacent normal mucosa, and we found that ZnT1 upregulation and miR-411 downregulation are likely to be important mechanisms underlying bladder cancer development. We revealed that miR-411 expression was negatively correlated with ZnT1 in bladder cancer.
Second, we predicted the target genes of miR-411 using bioinformatics, and we then confirmed that ZnT1 was a target gene of miR-411 in bladder cancer using biological experimental methods. Using quantitative real-time PCR, Western blotting, and the luciferase reporter gene method, we found that miR-411 was partially complementary to the ZnT1 mRNA 3′-UTR sequence and inhibited the translation of ZnT1 mRNA, which indicated that ZnT1 was regulated at the posttranscriptional level. The gain of function study showed that enhanced expression of miR-411 could not only inhibit cell proliferation but also cell migration and invasion in vitro.
Studies have shown that ZnT1 can regulate cell growth and migration by regulating a variety of signaling pathways.8 In particular, ZnT1 can regulate the expression of Cyclin D1 and MMP2. Cyclin D1 is an important protein that regulates the cell cycle. Our study found that miR-411 can inhibit cell proliferation by inhibiting the level of Cyclin D1. MMP2 is a common protein that regulates cell invasion and metastasis. Our research also demonstrated that miR-411 can inhibit cell invasion and metastasis by inhibiting the expression of MMP2.
In conclusion, through a series of experiments, we found that miR-411 can inhibit the occurrence and development of bladder cancer by influencing the expression of ZnT1 to a certain extent.
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
This study was supported by the National Natural Science Fund (ref: 81372722).
Disclosure
The authors report no conflicts of interest in this work.
References
Cui X, Kong C, Zhu Y, et al. miR-130b, an onco-miRNA in bladder cancer, is directly regulated by NF-κB and sustains NF-κB activation by decreasing Cylindromatosis expression. Oncotarget. 2016;7(30):48547–48561. | ||
Drayton RM, Dudziec E, Peter S, et al. Reduced expression of miRNA-27a modulates cisplatin resistance in bladder cancer by targeting the cystine/glutamate exchanger SLC7A11. Clin Cancer Res. 2014;20(7):1990–2000. | ||
Duan R, Zhang Z, Zheng F, et al. Combining protein and miRNA quantification for bladder cancer analysis. ACS Appl Mater Interfaces. 2017;9(28):23420–23427. | ||
Muhammad N, Bhattacharya S, Steele R, Ray RB. Anti-miR-203 suppresses ER-positive breast cancer growth and stemness by targeting SOCS3. Oncotarget. 2016;7(36):58595–58605. | ||
Eissa S, Habib H, Ali E, Kotb Y. Evaluation of urinary miRNA-96 as a potential biomarker for bladder cancer diagnosis. Med Oncol. 2015;32(1):413. | ||
Enkelmann A, Heinzelmann J, von Eggeling F, et al. Specific protein and miRNA patterns characterise tumour-associated fibroblasts in bladder cancer. J Cancer Res Clin Oncol. 2011;137(5):751–759. | ||
Feng C, Sun P, Hu J, et al. miRNA-556-3p promotes human bladder cancer proliferation, migration and invasion by negatively regulating DAB2IP expression. Int J Oncol. 2017;50(6):2101–2112. | ||
Hecker N, Stephan C, Mollenkopf HJ, Jung K, Preissner R, Meyer HA. A new algorithm for integrated analysis of miRNA-mRNA interactions based on individual classification reveals insights into bladder cancer. PLoS One. 2013;8(5):e64543. | ||
Zo RB, Long Z. MiR-124-3p suppresses bladder cancer by targeting DNA methyltransferase 3B. J Cell Physiol. Epub 2018 Jun 12. | ||
Zhu Y, Liang S, Pan H, Cheng Z, Rui X. Inhibition of miR-1247 on cell proliferation and invasion in bladder cancer through its downstream target of RAB36. J Biosci. 2018;43(2):365–373. | ||
Hirata H, Ueno K, Shahryari V, et al. Oncogenic miRNA-182-5p targets Smad4 and RECK in human bladder cancer. PLoS One. 2012;7(11):e51056. | ||
Jin N, Jin X, Gu X, et al. Screening biomarkers of bladder cancer using combined miRNA and mRNA microarray analysis. Mol Med Rep. 2015;12(2):3170–3176. | ||
Li W, Liu J, Zou D, et al. Exploration of bladder cancer molecular mechanisms based on miRNA-mRNA regulatory network. Oncol Rep. 2017;37(3):1461–1468. | ||
Pignot G, Cizeron-Clairac G, Vacher S, et al. microRNA expression profile in a large series of bladder tumors: identification of a 3-miRNA signature associated with aggressiveness of muscle-invasive bladder cancer. Int J Cancer. 2013;132(11):2479–2491. | ||
Yang X, Cheng Y, Li P, et al. A lentiviral sponge for miRNA-21 diminishes aerobic glycolysis in bladder cancer T24 cells via the PTEN/PI3K/AKT/mTOR axis. Tumour Biol. 2015;36(1):383–391. | ||
Zhao X, He W, Li J, et al. MiRNA-125b inhibits proliferation and migration by targeting SphK1 in bladder cancer. Am J Transl Res. 2015;7(11):2346–2354. | ||
Zhao X, Li J, Huang S, Wan X, Luo H, Wu D. MiRNA-29c regulates cell growth and invasion by targeting CDK6 in bladder cancer. Am J Transl Res. 2015;7(8):1382–1389. | ||
Zhang X, Zhang M, Cheng J, Lv Z, Wang F, Cai Z. MiR-411 functions as a tumor suppressor in renal cell cancer. Int J Biol Markers. 2017;32(4):454–460. | ||
Mei Z, Yan P, Wang Y, Liu S, He F. Knockdown of zinc transporter ZIP8 expression inhibits neuroblastoma progression and metastasis in vitro. Mol Med Rep. 2018;18(1):477–485. | ||
Choi BY, Jung JW, Suh SW. The emerging role of zinc in the pathogenesis of multiple sclerosis. Int J Mol Sci. 2017;18(10):pii:E2070. | ||
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. | ||
Zhang X, Zhang T, Yang K, Zhang M, Wang K. miR-486-5p suppresses prostate cancer metastasis by targeting Snail and regulating epithelial-mesenchymal transition. Onco Targets Ther. 2016;9:6909–6914. | ||
Ye H, Yu X, Xia J, et al. MiR-486-3p targeting ECM1 represses cell proliferation and metastasis in cervical cancer. Biomed Pharmacother. 2016;80:109–114. | ||
Wang SY, Li Y, Jiang YS, et al. Investigation of serum miR-411 as a diagnosis and prognosis biomarker for non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2017;21(18):4092–4097. | ||
Zhang Y, Xu G, Liu G, et al. miR-411-5p inhibits proliferation and metastasis of breast cancer cell via targeting GRB2. Biochem Biophys Res Commun. 2016;476(4):607–613. | ||
Zhao Z, Qin L, Li S. miR-411 contributes the cell proliferation of lung cancer by targeting FOXO1. Tumour Biol. 2016;37(4):5551–5560. | ||
Tan J, Sun C, Xu K, Wang C, Guo J. Immobilization of ALA-Zn(II) Coordination polymer pro-photosensitizers on magnetite colloidal supraparticles for target photodynamic therapy of bladder cancer. Small. 2015;11(47):6338–6346. | ||
Lin CN, Wang LH, Shen KH. Determining urinary trace elements (Cu, Zn, Pb, As, and Se) in patients with bladder cancer. J Clin Lab Anal. 2009;23(3):192–195. | ||
Chandler P, Kochupurakkal BS, Alam S, Richardson AL, Soybel DI, Kelleher SL. Subtype-specific accumulation of intracellular zinc pools is associated with the malignant phenotype in breast cancer. Mol Cancer. 2016;15(1):2. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.