Back to Journals » International Journal of General Medicine » Volume 14
miR-328-3p, a Predictor of Stroke, Aggravates the Cerebral Ischemia-Reperfusion Injury
Authors Wang S, Jun J, Cong L, Du L, Wang C
Received 20 February 2021
Accepted for publication 14 May 2021
Published 8 June 2021 Volume 2021:14 Pages 2367—2376
DOI https://doi.org/10.2147/IJGM.S307392
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Shun Wang,1 Jiang Jun,2 Liyuan Cong,3 Lutao Du,1 Chuanxin Wang1
1Department of Clinical Laboratory, the Second Hospital, Cheeloo College of Medicine, Shandong University, Jinan, 250012, Shandong, People’s Republic of China; 2Department of Neurosurgery, the Second Hospital, Cheeloo College of Medicine, Shandong University, Jinan, 250012, Shandong, People’s Republic of China; 3Department of Clinical Laboratory, Community Health Service Center, Qingdao, 266000, Shandong, People’s Republic of China
Correspondence: Chuanxin Wang
Department of Clinical Laboratory, the Second Hospital, Cheeloo College of Medicine, Shandong University, Jinan, 250012, Shandong, People’s Republic of China
Tel +86 0531 85875543
Email [email protected]
Background: In the present study, we aimed to identify microRNAs (miRNAs) that affected the prognosis of stroke and assess their biological effects.
Materials and Methods: A high-throughput sequencing (HTS) analysis was performed to screen distinctive miRNAs in serum exosomes of stroke patients, and these miRNAs were subsequently validated using individual quantitative real-time polymerase chain reaction (qRT-PCR) in a cohort consisting of 39 stroke patients and 20 normal controls. Briefly, miR-328-3p agomir or agomir NC was injected into rats before ischemia and reperfusion (I/R) injury. Zea-Longa score, neurological severity score (mNSS), triphenyltetrazolium chloride (TTC) staining, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, transmission electron microscopy, and hematoxylin and eosin (H&E) staining were used to examine the brain injury. Immunohistochemistry was utilized to determine the expressions of TNF-α and IL-6.
Results: The expression of serum exosomal miR-328-3p was significantly reduced in patients with an infarct volume ≥ 10 cm3 (P=0.01). Serum exosomal miR-328-3p was associated with the short-term prognosis (P=0.02), and the level of miR-328-3p was an independent relative factor for short-term prognosis (OR 5.276, P=0.02). The sensitivity of miR-328-3p level higher than 1.24 to predict the severity of the patient’s 1-week prognosis was 70%, and the specificity was 83% (AUC=0.74, P=0.02). The mNSS was higher in the miR-328-3p agomir group compared with the agomir NC group (P=0.03). Neutrophil infiltration was more serious in the miR-328-3p agomir group.
Conclusion: Our study indicated that miR-328-3p played a critical predictive role in the short-term prognosis of stroke, and up-regulation of miR-328-3p aggravated cerebral I/R injury.
Keywords: ischemic stroke, miR-328-3p, prognosis, cerebral ischemia-reperfusion
Introduction
Stroke is a major cause of global disease burden with limited therapies. The in-hospital stroke mortality is estimated to be around 5%, 26–43% of strokes are progressive, and their recurrence rate is about 30%.1 Ischemic stroke leads to the necrosis of cerebral ischemic hypoxic lesions, which in turn produces clinically corresponding neurological deficits.2 A primary goal in the treatment of acute ischemic stroke is revascularization, and 80% of the patients may still progress to reperfusion injury.3 Ischemia and reperfusion are similarly taken into account as a pathological conditions contributing to ischemic stroke.4 Therefore, it is urgently necessary to develop novel diagnostic and therapeutic targets to reduce the high mortality and improve the prognosis of stroke patients.
MicroRNAs (miRNAs), usually 18–25 nucleotides in length, are a super-family of small non-coding RNAs derived from the genome. The abnormal expression of miRNAs is closely related to the occurrence and development of various diseases, such as cerebrovascular disease.5 Circulatory miRNAs are also peripheral biomarkers of diseases such as cancer, stroke, and type 2 diabetes mellitus (T2DM).6–8 Serum circulating miRNA-221-3p and miRNA-382-5p may be used as potential noninvasive biomarkers for the diagnosis of ischemic stroke.9 miR-34b can protect against focal cerebral ischemia-reperfusion (I/R) injury.10 There are abundant and stable miRNAs in blood exosomes.11 Due to the protection of exosomes, miRNAs can be protected from enzymatic degradation, transported in body fluids at long distances, and stably detected, and their expression profiles have obvious specificity.12,13 High-throughput sequencing (HTS) analysis can screen the related miRNAs in serum exosomes of stroke patients.
We have previously investigated serum markers of cerebrovascular disease and their influencing factors.14,15 In the present study, we aimed to identify miRNAs that affected the prognosis of stroke and found miR-328 as a potential candidate. It has been reported that miR-328 is up-regulated in traumatic brain injury.16 Therefore, we assessed the biological effects of miR-328 on cerebral I/R injury to provide potential biomarkers for stroke therapy.
Materials and Methods
Clinical Specimens
A total of 42 stroke patients and 23 normal controls were enrolled from the Second Hospital, Cheeloo College of Medicine, Shandong University between March 2017 and July 2018. The medical history, imaging examinations, and other information of patients were collected. The inclusion criteria were set as follows: 1) stroke patients who met the diagnostic criteria of cerebrovascular disease formulated by the Fourth National Conference of Cerebrovascular Diseases in 1995, and the diagnosis was confirmed by cranial CT or MRI; 2) admitted for the first time, and the onset time was less than 8 h; 3) routine laboratory examination was improved after admission; and 4) patients who did not take antiplatelet drugs and lipid-lowering drugs 1 month before admission. The exclusion criteria were set as follows: patients with severe heart, lung, liver, kidney dysfunction, malignant tumors, and a platelet count <100×109/L or other diseases of the blood system. National Institute of Health stroke scale (NIHSS) score was assessed in 24 h and 7 days after the onset of stroke to evaluate the short-term prognosis of stroke. Stroke progression was defined as any new neurological symptoms/signs or any neurological worsening within 7 days after the stroke onset. The prognosis of patients was also examined at 1-, 3-, 6-, and 12-month follow-up after the onset of stroke. Recurrence and stroke-related death were regarded as poor long-term prognoses. Blood samples of normal controls and patients were obtained from the Department of Clinical Laboratory within 4 h after collection. The serum was centrifuged at 12,000 rpm for 10 min at 4°C to remove the remaining blood cell debris in the supernatant and stored at −80°C until use. RiboTM Exosome Isolation Reagent was used to separate the exosomes in serum, and Particle Metrix’ ZetaView was used to detect extracellular vesicles. The exosomal miRNAs were extracted using QIAGEN miRNeasy Micro Kit. Three normal controls and three stroke patients were selected to analyze and screen differential miRNAs using HTS. Stroke patients’ samples were collected on the 2nd day (acute phase, peak inflammatory response period) and the 10th day (recovery phase). HiSeq/MiSeq HTS was completed by Beijing Nuohe Zhiyuan Biotechnology Co., Ltd. This study was approved by the Human Research Ethics Committees from the Second Hospital of Shandong University (No. KYLL-2017(GJ)P-0015).
Quantitative Real-Time Polymerase Chain Reaction
Exosome extraction from serum samples using ExoQuickTM Solution (EXOQ5A-1; SBI System Biosciences, USA) and RNA isolation using miRNeasy Mini Kit (QIAGEN, #217004) were performed as previously described.17 Purified RNA was reversely transcribed into cDNA using miScript Reverse Transcription Kit (Life Technologies). qRT-PCR was conducted using miScript SYBR Green PCR Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions with three replicates. U6 was utilized as an endogenous control for miRNAs. The relative expression of the target miRNA was calculated utilizing the 2−ΔΔCT method. miR-328-3p forward primer: 5'-TTCGCTTATCTGGCCCTCTCT-3'. reverse primer: 5'-TATGGTTGTTCACGACTGCTTCAC-3'. U6 forward primer: 5ʹ-GCTTCGGCAGCACATATACTAAAAT-3ʹ. U6 reverse primer: 5ʹ- CGCTTCACGAATTTGCGTGTCAT −3ʹ.
Rat Models of Middle Cerebral Artery Occlusion (MCAO) and Reperfusion
Adult male Sprague-Dawley (SD) rats (2 months old) were housed under a 12-h light/dark cycle with free access to water and food. After a 72-h acclimatization period, they were anesthetized with 10% chloral hydrate (0.4 mL/100 g) through intraperitoneal injection. For intracerebroventricular (ICV) surgery, the head of the rat was fixed on the brain stereotaxic device. An intracranial injection was performed after positioning using stereotactic co-ordinates (0.8 mm posterior to the bregma, 1.6 mm lateral to the midline, and 3.5 mm below the outer surface of the skull). Each rat was injected with 10 µL of miR-328-3p agomir or agomir NC (50 OD mimics were all dissolved in DEPC water and provided for six rats). The rat was single-housed after the operation. After 30 min, the MCAO and reperfusion experiments were performed on the same side. The rats that died within 24 h after the operation were excluded. At last, there were 6 rats in each group. This study was approved by the Animal Research Ethics Committees from the Second Hospital of Shandong University (No. KYLL-2017(GJ)A-0042) and followed Guidelines for the ethical review of laboratory animal welfare People’s Republic of China National Standard GB/T 35892–2018.
Examination of Neurological Functions
A Zea-Longa score was used to determine the brain damage in rats after the establishment of the MCAO model. The modified neurological severity score (mNSS) was also employed to examine neurological functions. mNSS was applied to evaluate a combination of motor, sensory, and balance functions. Neurological function was graded on a scale of 0–18 (1–6 is mild, 7–12 is moderate; and 13–18 is severe). All evaluations were performed by three professionals who were blinded to the experimental design.
Transmission Electron Microscopy (TEM)
The rats were anesthetized with chloral hydrate (0.4 mL/100 g), followed by fixation by perfusion with 4% paraformaldehyde. The brains were dissected and fixed in the same solution for 24 h. The ultrastructural changes in the brain were determined using TEM by Wuhan Servicebio Technology CO., Ltd. with an HT7700 (Hitachi, Japan) transmission electron microscope.
Triphenyltetrazolium Chloride (TTC) Staining
TTC staining was utilized to determine the infarct volume of the post-ischemic brain damage. Three brains were isolated in each group and quickly cryopreserved at −20°C for 20 min. The whole-brain tissue was divided into five coronal sections and incubated in 1% TTC solution at 37°C for 30 min in the dark. Subsequently, the sections were immobilized with 4% paraformaldehyde for 24 h and photographed by microscope. Image J was used to quantify the volume ratio of the ischemic area.
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
TUNEL assay was used to test cell apoptotic activity. Each slice was randomly selected to take photos with a field of vision of 200 times. Image-Pro Plus 6.0 software was used to select the same labeled green fluorescent nuclei as the unified standard for judging all positive cells, and DAPI blue nuclei were selected as total cells. Randomly, two photos of each sample were selected to count the proportion of TUNEL-positive cells.
Immunohistochemical Assay and Hematoxylin and Eosin (H&E) Staining
Brain tissue was fixed with 4% paraformaldehyde, embedded in paraffin, and sectioned (5-μm thickness). H&E staining and immunohistochemical assay of FOXO4, tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) were performed and imaged under a light microscope. Image J was used to quantify the expressions of FOXO4, TNF-α, and IL-6.
Statistical Analysis
All statistical analyses were performed using Prism 8 and SPSS 26 statistical software. Non-normally distributed variables were expressed as the median and interquartile range [M (p25, p75)] with the Mann–Whitney test. Normally distributed variables were expressed as mean ± standard deviation using an unpaired two-tailed Student’s t-test. The factors affecting the prognosis of stroke were analyzed by logistic regression analysis. P ˂ 0.05 was considered statistically significant.
Results
The Level of Serum Exosomal miR-328-3p Predicts the Short-Term Prognosis of Stroke
We used an HTS approach to analyze the expressions of miRNAs in serum exosomes from the normal controls and stroke patients on the 2nd day (acute phase, peak inflammation period) and the 10th day (recovery phase). The results showed that there were 58 differential miRNAs between the acute phase and recovery phase in stroke patients, including 30 up-regulated and 28 down-regulated miRNAs (Figure 1A). The differential serum exosomal miRNAs included miR-328-3p, miR-1301-3p, miR-320a, and so on. Among them, 27 miRNAs were also different from the normal controls, including 15 up-regulated and 12 down-regulated miRNAs (Figure 1B).
Next, we verified our results using 20 normal controls and 39 stroke patients (The basic information of patients is shown in Table 1), the expression of serum exosomal miR-328-3p was slightly lower in stroke patients, and the difference was not statistically different (P>0.05) (Figure 2A). Moreover, the expression of serum exosomal miR-328-3p was significantly decreased in stroke patients with an infarct volume ≥10 cm3 (P=0.01). Assessment of NIHSS score was performed at 24 h and 7 days after the onset of stroke. The prognosis of patients was followed up at the 3rd, 6th, and 12th months after the onset of stroke. Recurrence and stroke-related death were regarded as poor long-term prognoses. Serum exosomal miR-328-3p was associated with the stroke progression (P=0.02), the expression of miR-328-3p was lower in patients with good short-term prognosis compared with the patients with poor short-term prognosis (0.73 vs 1.51, P=0.02) (Figure 2B). The sensitivity of miR-328-3p level higher than 1.24 to predict the severity of the patient’s short-term prognosis was 70%, and the specificity was 83% (Figure 2C, AUC=0.74, P=0.02). At the 3rd, 6th, and 12th months of follow-up, serum exosomal miR-328-3p was not associated with long-term prognosis (P=0.73, P=0.63, P=0.35). Further logistic regression analysis demonstrated that serum exosomal miR-328-3p was an independent relative factor for short-term prognosis (OR 5.276, P=0.02, Table 2).
 |
Table 1 The Clinical Characteristics of Stroke Patients and Health Controls |
 |
Table 2 Logistic Regression Analysis of Clinical Predictors in the Early-Onset PSD Patients |
miR-328-3p Aggravates Cerebral I/R Injury
At 30 min before surgery, the rats were intracranially injected with miR-328-3p agomir or agomir NC. The rats were embolized by thread embolization and then reperfused after 2 h of ischemia. A postoperative Zea-Longa score was used to assess the functional results. The difference was not statistically significant (P>0.05) (Figure 2D). At 24 h after the operation, the mNSS was used to evaluate the function. Figure 2E shows that the mNSS in the miR-328-3p agomir group was higher compared with the control group (P=0.03).
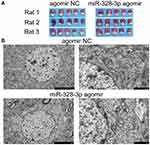
Figure 3A illustrates the TTC staining of brain sections, and there was no significant difference between the two groups (0.23 vs 0.27, P=0.61). Then we used TEM to further observe the brain tissues (Figure 3B) and found that the cell injury was more serious in the miR-328-3p agomir group. The pathological results showed that the structural integrity of the choroid plexus was destroyed, and the neutrophil infiltration in the miR-328-3p agomir group was more serious compared with the control group (Figure 4).
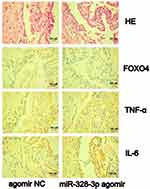
Finally, we used immunohistochemistry to assess the expressions of FOXO4, TNF-α, and IL-6 in tissue sections. Image J was used to quantify the expressions of FOXO4, TNF-α, and IL-6. Figure 4 shows that the expression of FOXO4 in the miR-328-3p agomir group was lower compared with the agomir NC group (P=0.03). On the contrary, the expressions of TNF-α and IL-6 in the miR-328-3p agomir group were higher compared with the agomir NC group, while the difference was not statistically significant (P=0.1, P=0.1).
There was no significant difference in the apoptotic rate of I/R tissue cells between the agomir NC group and miR-328-3p agomir group (10.68 vs 9.07, P>0.05).
Discussion
Cytotoxic edema occurs immediately in a large portion of the malignant stroke ischemic territory. The subsequent damage of the tight junctions leads to the breakdown of the blood-brain barrier (BBB) and vasogenic brain edema, resulting in space-occupying brain swelling. The progressive vasogenic edema leads to midline shift and transtentorial herniation, finally leading to brain stem compression and death. Early prediction of stroke and reduction of reperfusion injury play key roles in reducing the high mortality and improving the prognosis of patients with cerebral infarction (CI).18 Therefore, it is important to identify novel markers for stroke treatment. miRNAs exert momentous effects on posttranscriptional gene modulation by suppressing the translation of their target genes. Previous studies have shown that miRNAs are critical modulators of CI-concerned cellular biological processes, including proliferation, apoptosis and so on.19 Serum miR-124 may serve as a molecular marker for acute ischemic stroke.20 Through the JNK signaling pathway, miR-145 accelerates the proliferation of endothelial progenitor cells in CI rats.21 It has been demonstrated that miR-24 suppresses brain tissue cell apoptosis of rats with CI.22 Electroacupuncture treatment can balance the level of miR-328 to facilitate angiogenesis of ischemic cerebral cortex tissue by modulating the expressions of vascular endothelial growth factor family genes and proteins.23 In our present study, we found that the expression of serum exosomal miR-328-3p exhibited a good correlation with stroke infarct volume. Moreover, we also found that serum exosomal miR-328-3p was associated with the short-term prognosis, and the expression of serum exosomal miR-328-3p was lower in patients with good short-term prognosis compared with the patients with poor short-term prognosis. Therefore, the level of serum exosomal miR-328-3p could be used as a prognostic factor for stroke. However, the specific mechanism underlying the miR-328-3p action on stroke remains largely unexplored.
Ischemic brain injury is caused by a series of pathophysiological events, including inflammation, oxidative stress, and apoptosis. Increasing evidence demonstrates that inflammation is a critical determinant of outcome. The first blood-borne cells to be recruited in the brain are neutrophils, followed by monocytes and lymphocytes. BBB disruption and migration of leukocytes can increase edema and hemorrhagic transformation. It has been reported that early BBB dysfunction predicts neurological outcome following aneurysmal subarachnoid hemorrhage.24 In our present study, the neutrophil infiltration of destroyed choroid plexus in the miR-328-3p agomir group was more compared with the control group. Therefore, miR-328-3p might aggravate cerebral I/R injury and could predict the outcome of stroke. It is well known that exosomes bear proteins and RNAs, mediate intercellular communication between different cell types, and affect normal and pathological conditions. Therefore, the expression of exosomal miR-328-3p in stroke patients might affect the neutrophil infiltration and aggravate inflammatory reactions.
As key cytokines, TNF-α and IL-6 can trigger inflammation and joint damage.25,26 TNF-α plays a key role in the inflammatory cascade, which regulates the immune response, with powerful impacts on various aspects of humoral and cellular immunity. Targeting TNF-α has considerably improved the success in CI treatment.27 Serum miR-124, TNF-α, and IL-1β can be utilized as markers for the early diagnosis of vulnerable carotid plaques in patients with acute CI.28 IL-6, induced by TNF-α and IL-1b, can predict the outcome (functional outcome, mortality, and infection) of stroke.29 In our present study, miR-328-3p increased the expressions of TNF-α and IL-6 in cerebral I/R, and regulated neutrophil infiltration. This might be the reason that miR-328-3p agomir aggravated cerebral I/R injury.
As one member of the forkhead (FOX) transcription factor O family, FOXO4 is involved in many biological processes, including cell proliferation, metabolism, immunity, and apoptosis.30 Studies have shown that FOXO4 is associated with cell apoptosis in many ischemic and metabolic diseases, such as diabetic retinopathy,31 diabetic nephropathy,32 and ischemic limbs.33 Through endothelial Arg1, FOXO4 accelerates early inflammatory response to myocardial infarction.34 However, the expression of FOXO4 in stroke has been rarely reported so far. Besides, FOXO4 is a target gene of miR-328-3p, and it is negatively modulated by miR-328-3p in CI cells and HBV-infected liver cells.35,36 In our present study, overexpressed miR-328-3p obviously stimulated inflammation, and the expression of FOXO4 in the miR-328-3p agomir group was lower compared with the agomir NC group. This finding was consistent with previous studies, while the mechanism of miR-328-3p/FOXO4 axis regulating inflammation remains unclear and further research is needed.
Conclusions
In conclusion, our study showed that miR-328-3p played a critical predictive role in the short-term prognosis of stroke. Moreover, miR-328-3p might aggravate the inflammatory response of cerebral I/R injury. Collectively, our current findings provided valuable insights into the treatment of stroke.
Data Sharing Statement
All data generated or analyzed during this study are available from the corresponding author Chuanxin Wang upon reasonable request.
Ethics Approval and Consent to Participate
The Human Research Ethics Committees from the Second Hospital of Shandong University approved this study (No. KYLL-2017(GJ)P-0015). All participants provided written informed consent, and the procedures were conducted following the principles of the Declaration of Helsinki.
Acknowledgments
The study was supported by the Youth Program of National Natural Science Foundation of China (No. 82000559), Key R & D projects in Shandong Province (No. 2019GSF108147), and Natural Science Foundation of Shandong Province (No. ZR2019PH105).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Dr Shun Wang reports grants from Natural Science Foundation of Shandong Province, grants from National Natural Science Foundation, during the conduct of the study. In addition, Dr Shun Wang has a patent miR-328-3p pending to 2020110016209. The authors declare that they have no other conflicts of interest.
References
1. Saam T, Hetterich H, Hoffmann V, et al. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol. 2013;62:1081–1091. doi:10.1016/j.jacc.2013.06.015
2. Amani H, Mostafavi E, Alebouyeh MR. Would colloidal gold nanocarriers present an effective diagnosis or treatment for ischemic stroke? Int J Nanomed. 2019;14:8013–8031. doi:10.2147/IJN.S210035
3. Yu L, Zhang W, Huang C, et al. FoxO4 promotes myocardial ischemia-reperfusion injury: the role of oxidative stress-induced apoptosis. Am J Transl Res. 2018;10:2890–2900.
4. Chen CY, Chen RJ, Lee GA. Two-vessel occlusion mouse model of cerebral ischemia-reperfusion. J Vis Exp. 2019;145:e59078.
5. Dewdney B, Trollope A, Moxon J, Thomas Manapurathe D, Biros E, Golledge J. Circulating microRNAs as biomarkers for acute ischemic stroke: a systematic review. J Stroke Cerebrovasc Dis. 2018;27:522–530. doi:10.1016/j.jstrokecerebrovasdis.2017.09.058
6. Elghoroury EA, ElDine HG, Kamel SA. Evaluation of miRNA-21 and miRNA let-7 as prognostic markers in patients with breast cancer. Clin Breast Cancer. 2018;18(4):e721–e726. doi:10.1016/j.clbc.2017.11.022
7. Vijayan M, Reddy PH. Peripheral biomarkers of stroke: focus on circulatory microRNAs. Biochim Biophys Acta. 2016;1862(10):1984–1993. doi:10.1016/j.bbadis.2016.08.003
8. Liang Y-Z, Li -J-J-H, Xiao H-B, He Y, Zhang L, Yan Y-X. Identification of stress-related microRNA biomarkers in type 2 diabetes mellitus: a systematic review and meta-analysis. J Diabetes. 2020;12(9):633–644. doi:10.1111/1753-0407.12643
9. Wang Y, Ma Z, Kan P, Zhang B. The diagnostic value of serum miRNA-221-3p, miRNA-382-5p, and miRNA-4271 in ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26:1055–1060. doi:10.1016/j.jstrokecerebrovasdis.2016.12.019
10. Huang R, Ma J, Niu B, et al. MiR-34b protects against focal cerebral ischemia-reperfusion (I/R) injury in rat by targeting keap1. J Stroke Cerebrovasc Dis. 2019;28(1):1–9. doi:10.1016/j.jstrokecerebrovasdis.2018.08.023
11. Hu Y, Rao SS, Wang ZX, et al. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics. 2018;8:169–184. doi:10.7150/thno.21234
12. Deng Z, Rong Y, Teng Y, et al. Exosomes miR-126a released from MDSC induced by DOX treatment promotes lung metastasis. Oncogene. 2017;36:639–651. doi:10.1038/onc.2016.229
13. Shen H, Yao X, Li H, et al. Role of exosomes derived from miR-133b modified MSCs in an experimental rat model of intracerebral hemorrhage. J Mol Neurosci. 2018;64:421–430. doi:10.1007/s12031-018-1041-2
14. Wang S, Jiang J, Qu C, Wang C, Sun Z. Predictive value of serum pregnancy-associated plasma protein A for patients with ischemic cerebrovascular disease. J Clin Lab Anal. 2017;31(5):e22091. doi:10.1002/jcla.22091
15. Wang S, Wang L, Zhang X, et al. Profile of serum pregnancy-associated plasma protein A after sustained subcutaneous low molecular weight heparin administration in patients with cerebrovascular diseases. Clin Chem. 2011;57(3):526–527. doi:10.1373/clinchem.2010.152702
16. Martinez B, Peplow P. MicroRNAs as diagnostic markers and therapeutic targets for traumatic brain injury. Neural Regen Res. 2017;12(11):1749–1761. doi:10.4103/1673-5374.219025
17. Yan S, Du L, Jiang X. Evaluation of serum exosomal lncRNAs as diagnostic and prognostic biomarkers for esophageal squamous cell carcinoma. Cancer Manag Res. 2020;12:9753–9763. doi:10.2147/CMAR.S250971
18. Wang X, Shen B, Sun D, Cui X. Aspirin ameliorates cerebral infarction through regulation of TLR4/NF-κB-mediated endoplasmic reticulum stress in mouse model. Mol Med Rep. 2018;17:479–487. doi:10.3892/mmr.2017.7879
19. Shin TH, Lee DY, Basith S, et al. Metabolome changes in cerebral ischemia. Cells. 2020;9(7):1630. doi:10.3390/cells9071630
20. Wang J, Huang Q, Ding J, Wang X. Elevated serum levels of brain-derived neurotrophic factor and miR-124 in acute ischemic stroke patients and the molecular mechanism. 3 Biotech. 2019;9(11):386. doi:10.1007/s13205-019-1914-2
21. Chen R, Chen S, Liao J, Chen X, Xu X. MiR-145 facilitates proliferation and migration of endothelial progenitor cells and recanalization of arterial thrombosis in cerebral infarction mice via JNK signal pathway. Int J Clin Exp Pathol. 2015;8:13770–13776.
22. Jiang Q, Shen D, Wang Y, Huang D, Wang Z. miR-24 reduces serum lipid levels and inhibits brain tissue cells apoptosis of rats with cerebral infarction. Turk Neurosurg. 2020;30:483–490. doi:10.5137/1019-5149.JTN.23127-18.2
23. Liu L, Wang NH, Zhang Q, Li SY, Gu WJ, Wu Y. Micro-ribonucleic acids participate in electroacupuncture intervention-induced improvement of ischemic stroke. Zhen Ci Yan Jiu. 2019;44:686–692. doi:10.13702/j.1000-0607.180643
24. Lublinsky S, Major S, Kola V, et al. Early blood-brain barrier dysfunction predicts neurological outcome following aneurysmal subarachnoid hemorrhage. EBioMedicine. 2019;43:460–472. doi:10.1016/j.ebiom.2019.04.054
25. Doll DN, Rellick SL, Barr TL, Ren X, Simpkins JW. Rapid mitochondrial dysfunction mediates TNF-alpha-induced neurotoxicity. J Neurochem. 2015;132(4):443–451. doi:10.1111/jnc.13008
26. Del Giudice M, Gangestad SW. Rethinking IL-6 and CRP: why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun. 2018;70:61–75. doi:10.1016/j.bbi.2018.02.013
27. Chen Y, Huang W, Li Z, et al. The effect of acupuncture on the expression of inflammatory factors TNF-α, IL-6, IL-1 and CRP in cerebral infarction: a protocol of systematic review and meta-analysis. Medicine (Baltimore). 2019;98:e15408. doi:10.1097/MD.0000000000015408
28. Wang L, Xu L. Combined value of serum miR-124, TNF-α and IL-1β for vulnerable carotid plaque in acute cerebral infarction. J Coll Physicians Surg Pak. 2020;30:385–388.
29. Maestrini I, Ducroquet A, Moulin S, Leys D, Cordonnier C, Bordet R. Blood biomarkers in the early stage of cerebral ischemia. Rev Neurol (Paris). 2016;172(3):198–219. doi:10.1016/j.neurol.2016.02.003
30. Lu Y, Thavarajah T, Gu W, Cai J, Xu Q. Impact of miRNA in Atherosclerosis. Arterioscler Thromb Vasc Biol. 2018;38(9):e159–e170. doi:10.1161/ATVBAHA.118.310227
31. Zhang L, Dong L, Liu X, et al. α-Melanocyte-stimulating hormone protects retinal vascular endothelial cells from oxidative stress and apoptosis in a rat model of diabetes. PLoS One. 2014;9:e93433. doi:10.1371/journal.pone.0093433
32. Chuang PY, Dai Y, Liu R, et al. Alteration of forkhead box O (foxo4) acetylation mediates apoptosis of podocytes in diabetes mellitus. PLoS One. 2011;6:e23566. doi:10.1371/journal.pone.0023566
33. Nakayoshi T, Sasaki K, Kajimoto H, et al. FOXO4-knockdown suppresses oxidative stress-induced apoptosis of early pro-angiogenic cells and augments their neovascularization capacities in ischemic limbs. PLoS One. 2014;9:e92626. doi:10.1371/journal.pone.0092626
34. Zhu M, Goetsch SC, Wang Z, et al. FoxO4 promotes early inflammatory response upon myocardial infarction via endothelial Arg1. Circ Res. 2015;117:967–977. doi:10.1161/CIRCRESAHA.115.306919
35. Qin X, Guo J. MicroRNA-328-3p protects vascular endothelial cells against oxidized low-density lipoprotein induced injury via targeting forkhead box protein O4 (FOXO4) in atherosclerosis. Med Sci Monit. 2020;26:e921877.
36. Fu X, Ouyang Y, Mo J, Li R, Fu L, Peng S. Upregulation of microRNA-328-3p by hepatitis B virus contributes to THLE-2 cell injury by downregulating FOXO4. J Transl Med. 2020;18(1):143. doi:10.1186/s12967-020-02299-8
37. Chang Y, Huang W, Sun Q, Li S, Yan Z. MicroRNA-634 alters nerve apoptosis via the PI3K/Akt pathway in cerebral infarction. Int J Mol Med. 2018;42:2145–2154. doi:10.3892/ijmm.2018.3777
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.