Back to Journals » OncoTargets and Therapy » Volume 12
MiR-32-5p Regulates Radiosensitization, Migration And Invasion Of Colorectal Cancer Cells By Targeting TOB1 Gene
Authors Liang H, Tang Y, Zhang H, Zhang C
Received 28 August 2019
Accepted for publication 29 October 2019
Published 14 November 2019 Volume 2019:12 Pages 9651—9661
DOI https://doi.org/10.2147/OTT.S228995
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Hong Liang,* Yumei Tang,* Hui Zhang, Chao Zhang
Department of Gastrointestinal Surgery, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, School of Clinical Medicine, Henan University, Zhengzhou, Henan 450003, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chao Zhang
Department of Gastrointestinal Surgery, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, School of Clinical Medicine, Henan University, Zhengzhou 450003, People’s Republic of China
Email [email protected]
Background: MicroRNAs (miRNAs) play key roles in the development and progression of various cancers. However, the precise functions and regulation mechanisms of miRNAs in human tumors remain elusive.
Methods: Quantitative real time-PCR (qRT-PCR) was performed to detect the level of miR-32-5p in colorectal cancer tissues. The relationships between miR-32-5p level with clinicopathological characteristics and prognosis were analyzed. The miR-32-5p inhibitor was employed to knock down the expression of miR-32-5p. The overexpression plasmid and si-RNA targeting TOB1 were generated. Clone formation assays under radiant exposure were used to evaluate the radiosensitization. Transwell migration and invasion were employed to test the ability of cell migration and invasion. Luciferase reporter assays were used to confirm the regulation of miR-32-5p on the expression of TOB1. Rescue experiments were conducted to investigate the effects of TOB1 on the functions of miR-32-5p.
Results: In this study, we found that miR-32-5p was significantly upregulated in colorectal cancer tissues compared with adjacent normal tissues. The level of miR-32-5p was positively correlated with tumor differentiation and metastasis. Log-rank tests showed that high level of miR-32-5p was significantly correlated with poor overall survival and disease-free survival. Anti-miR-32-5p remarkably enhanced the radiosensitivity and inhibited migration and invasion of colorectal cancer cells. In addition, overexpression of TOB1 obviously increased the radiosensitivity and inhibited migration and invasion of colorectal cancer cells. Moreover, bioinformatics analysis and luciferase reporter assays demonstrated that miR-32-5p suppressed the expression of TOB1 through directly binding to the 3ʹ-UTR of TOB1 mRNA. Rescue experiments indicated that miR-32-5p regulated the radiosensitivity, migration and invasion of colorectal cancer cells through inhibiting TOB1 expression.
Conclusion: This study suggested that miR-32-5p may serve as a prognostic and therapeutic target for colorectal cancer, and downregulation of miR-32-5p enhanced the radiosensitivity and inhibited migration and invasion through promoting TOB1 expression.
Keywords: miR-32-5p, TOB1, radiosensitivity, migration and invasion, colorectal cancer
Introduction
Colorectal cancer is the third most common cancers across the world, as well as the second leading mortality of cancer-related death.1 Despite many advancements in diagnoses and surgery treatments for colorectal cancer, the morbidity and mortality of colorectal cancer are still in the rise. Distant metastasis is the main cause of cancer-related death.2 In addition, the decrease in radiosensitivity is the main factor of radiotherapy failure. However, the molecular mechanisms underlying metastasis and radiosensitivity remain unclear in colorectal cancer. Thereby, it is urgently needed to elucidate the molecular mechanisms underlying metastasis and radiosensitivity and discover novel molecular targets for early diagnoses and treatments of colorectal cancer.
Adjuvant radiotherapy and palliative radiotherapy are the main types of radiotherapy for colorectal cancer. The success of radiotherapy partly lies in a fundamental understanding of the mechanisms of radiotherapy. However, the mechanisms of radiotherapy resistance remain unclear. Therefore, it is imperative to investigate the radiotherapy resistance and radiosensitivity of colorectal cancer. The transducer of ERBB2, 1 (TOB1) is a member of the antiproliferative protein B-cell translocation gene (BTG)/transducer of erbB2 (TOB) family.3 It has been reported that TOB1 serves as a tumor suppressor to inhibit cell proliferation, migration and invasion in different types of human cancers.4 Previous studies also revealed that TOB1 enhances radiosensitivity through MAPK/ERK signaling pathway in lung cancer,5 JNK and p38 pathway in breast cancer.6 In this study, we investigated the role of TOB1 in the radiosensitivity of colorectal cancer.
Noncoding RNA has been the hot spot in the field of tumors in the recent decade. MicroRNAs (miRNAs) are a group of small noncoding RNA with 19–25 nucleotides in length.7 Generally, miRNAs exert their regulatory function by complete complementation or incomplete complementation to 3ʹ-untranslated region (3ʹUTR) of target mRNA, which results in mRNA degradation or inhibition of translation.8 Mounting evidence indicates that ectopic expression of miRNAs involves in various tumor biological processes, such as cancer cell growth, proliferation, radiosensitivity, migration and invasion.9 miR-32-5p was reported to be highly expressed in hepatocellular carcinoma tissues and positively correlated with poor prognosis.10 Zhang et al revealed that downregulation of miR-32-5p increases the chemosensitivity of prostate cancer through promoting KLF4 expression.11 However, the roles of miR-32-5p on radiosensitivity have not been explored. In this study, we found that miR-32-5p was highly expressed in colorectal cancer tissues and positively correlated with clinicopathological features and poor prognosis. Mechanismly, downregulation of miR-32-5p enhanced the radiosensitivity and inhibited migration and invasion through promoting TOB1 expression.
Materials And Methods
Human colorectal cancer strain SW480 was purchased from the Type Culture Collection of the Chinese Academy of Science (Shanghai, China) and cultured in RPMI 1640 medium with 10% FBS (Gibco, Australia) and 1% penicillin-streptomycin, at 37°C in a humidified atmosphere containing 5% CO2.
Transfection And Oligonucleotides And Plasmids
To regulate the expression of TOB1, overexpression plasmids and siRNA targeting TOB1 were designed and synthesized. The anti-miRNA and negative control of has-miR-32-5p were purchased from RiBoBio (Guangzhou, China). SW480 cells in the logarithmic growth phase were seeded in 6-well plates. After culturing for 24 hrs, the plasmid and oligonucleotides were transfected into cells with LipofectamineTM 2000 (Invitrogen, USA) according to the manufacturer’s instructions.
Patients And Clinical Specimens
54 pairs of fresh frozen samples of colorectal cancer tissues and matched adjacent normal tissues were collected from patients with primary colorectal cancer in Henan Provence People’s Hospital from 2013 to 2015. The samples were snap-frozen into RNA keeper Tissue Stabilizer (Vazyme Biotech Co., Ltd, Jiangsu, China) and then stored at 4°C overnight and transferred to −80°C refrigerator. No patients have received radiotherapy or chemotherapy before surgery. The project was approved by Institutional Review Boards of Henan Provence People’s Hospital. Written informed consent was obtained before the patients enrolled in the research.
Radiant Exposure And Clone Formation Assay
A Siemens 6 MV X-ray linear accelerator was conducted to deliver a single dose of ionizing radiation with the source-skin distance 100 cm and at a dose rate of 2 Gy/min at room temperature. The gradient of the irradiation dose is 0, 2, 4, 6 and 8 Gy. At 14 days after the first irradiation, the cells were fixed with 75% ethanol and then stained with 0.1% crystalline purple. Finally, the cells were counted. The survival fraction was calculated with the number of colonies/the number of seeded cells.
Transwell Migration And Invasion Assays
2×104 cells of each group with 200 μL serum-free medium were seeded in the upper chamber without (migration) or with (invasion) Matrigel (BD Bioscience, USA). The lower chamber was added with 600 μL RPMI medium with 10% FBS. After incubating for 24 hrs, the upper chambers were fixed with 4% polymethanol for 30 mins and then stained with 0.1% crystal violet for 30 mins. The cells that migrated or invaded to the reverse side of the upper chambers were photographed. Five random fields were selected to calculate cells that migrated or invaded.
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from colorectal cancer and tissues using TRIzol (TaKaRa, Shiga, Japan) according to the manufacturer’s instructions. TOB1 and GAPDH mRNA were reversely transcribed into cDNA with a PrimeScriptTM RT Master Mix reagent kit (TaKaRa). miR-32-5p and U6 were reversely transcribed into cDNA by the PrimeScriptTM RT reagent kit (TaKaRa). RNA expression was quantified by qRT-PCR with SYBR Premix Ex Taq™ (TaKaRa) on a ViiA 7 Fast Real-Time PCR system (Applied Biosystems, NY, USA). GAPDH or U6 were adapted as internal controls. The primers for TOB1 are as follows: F: 5ʹ- CCCAGGTTTTTATGCCCATAAG-3ʹ, R: 5ʹ-GTGGCAGTGGTAAAGGTTAAAG-3ʹ. The primers for GAPDH are as follows: F: 5ʹ-CACCATTGGCAATGAGCGGTTC-3ʹ, R: 5ʹ-AGGTCTTTGCGGATGTCCACGT-3ʹ. The primers for miR-32-5p and U6 were purchased from RiBoBio (Guangzhou, China). The ΔΔCt method was employed to assay relative expression levels.
Western Blot
Total protein was separated and extracted from colorectal cancer cells using radioimmunoprecipitation assay (RIPA) buffer with 1% protease inhibitor phenylmethanesulfony fluoride (Beyotime Biotechnology, Jiangsu, China). Protein concentrations were measured with a BCA protein assay kit (Beyotime Biotechnology, Jiangsu, China) according to the manufacturer’s instructions. SDS-PAGE was performed with 30 μg of total protein. Then, the protein was transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, MA, USA). Thereafter, the membranes were blocked in 5% non-fat milk at room temperature for 1.5 hrs, followed by incubation with appropriate primary antibody at 4°C overnight. After washing with TBST buffer, the secondary antibodies were incubated at room temperature for 1.5 hrs. Protein bands were visualized by ECL chemiluminescent reagent (Millipore). The following antibodies were used: TOB1 (1:1000, Abcam, MA, USA) and GAPDH (1:5000; Cell Signaling Technology, USA).
Luciferase Reporter Assay
The luciferase reporter plasmids (pGL3-TOB1 3ʹ-UTR sequence/WT and mutant sequence/Mutation) were synthesized by GenePharma Co. (Shanghai, China). The luciferase reporter plasmids were co-transfected into SW480 cells with anti-miR-NC or anti-miR-32-5p. After 36 hrs, the firefly luciferase and renilla luciferase activity were detected. The efficacy was calculated with the ratio of firefly luciferase/renilla luciferase activity.
Statistical Analysis
The SPSS 22.0 software was conducted for statistical analyses. Data were calculated by the χ2 or Fisher's exact test. Paired and unpaired continuous variables were compared by Student’s t-test or the Mann–Whitney U-test. The survival curves were drawn using the Kaplan–Meier method and were analyzed by log-rank tests. p<0.05 was considered statistically significant.
Results
MiR-32-5p Is Upregulated In Colorectal Cancer Tissues And Closely Correlated With Clinicopathological Features
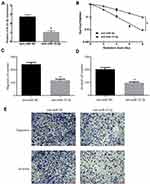
A previous study showed that the level of miR-32-5p was upregulated in prostate cancer and highly expressed miR-32-5p induced radioresistance through targeting DAB2IP.12 To investigate whether miR-32-5p was aberrantly expressed in colorectal cancer, we tested the expression of miR-32-5p in 54 pairs of fresh frozen samples of colorectal cancer tissues and matched adjacent normal tissues using qRT-PCR technology. The results showed that miR-32-5p was upregulated in most (85.19%, 46/54) colorectal cancer tissues (Figure 1A). Further analysis indicated that miR-32-5p was significantly higher in colorectal cancer tissues than adjacent normal tissues (Figure 1B). Next, the correlation between the level of miR-32-5p and clinicopathological features was analyzed in 54 colorectal cancer tissues. The results indicated that the level of miR-32-5p was positively correlated with cell differentiation and metastasis. The level of miR-32-5p was significantly higher in colorectal cancer tissues with moderate and poor colorectal differentiation (G2+G3) than with well-differentiation (G1) (Figure 1C) and higher in colorectal cancer tissues with metastasis (M1) than with no-metastasis (M0) (Figure 1D). Based on the median expression of miR-32-5p, these samples were divided into two groups, high miR-32-5p group and low miR-32-5p group. Kaplan–Meier Plotter analysis showed that colorectal cancer patients with high miR-32-5p had a shorter overall survival (Figure 1E) and disease-free survival (Figure 1F) than those with low expression of miR-32-5p (p<0.05). These results suggest that miR-32-5p is a highly expressed miRNA in colorectal cancer tissues and may serve as an appropriate diagnostic and prognostic marker for colorectal cancer.
Effects Of miR-32-5p On Radiosensitization, Migration And Invasion Of Colorectal Cancer Cells
To test the roles of miR-32-5p in colorectal cancer cells, anti-miR-32-5p was designed and synthesized to decrease the expression of miR-32-5p. qRT-PCR results showed that anti-miR-32-5p significantly decreased the expression of miR-32-5p in SW480 cells (Figure 2A). To detect the role of miR-32-5p on radiosensitization of colorectal cancer cells, clone formation assay was performed under different radiation doses. The results showed that knockdown of miR-32-5p significantly decreased the survival fraction (Figure 2B), indicating that knockdown of miR-32-5p enhanced the radiosensitization of colorectal cancer cells.
To test the roles of miR-32-5p on migration and invasion, transwell migration and invasion assays were conducted. Knockdown of miR-32-5p significantly decreased the migrated cell numbers (Figure 2C and E) and invaded cell numbers (Figure 2D and E). These results demonstrated that anti-miR-32-5p decreased the ability of migration and invasion of colorectal cancer cells.
Effects Of TOB1 On Radiosensitization, Migration And Invasion Of Colorectal Cancer Cells
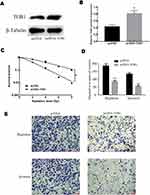
To test the roles of TOB1 in colorectal cancer cells, an overexpression plasmid of TOB1 (pcDNA-TOB1) was designed and synthesized. Western bolt assays showed that pcDNA-TOB1 obviously increased the expression of TOB1 protein in SW480 cells (Figure 3A). Similarly, qRT-PCR results indicated pcDNA-TOB1 significantly increased the expression of TOB1 mRNA in SW480 cells (Figure 3B). To detect the role of TOB1 in colorectal cancer cell, clone formation assay under different radiation doses, transwell migration and invasion assays were conducted. Clone formation assay showed that overexpression of TOB1 significantly decreased the survival fraction under different irradiation doses (Figure 3C), indicating that overexpression of TOB1 enhanced the radiosensitization of colorectal cancer cells. Transwell migration and invasion assays indicated that overexpression of TOB1 significantly decreased the migrated cell numbers and invaded cell numbers (Figure 3D and E). Taken together, these results demonstrated that overexpression of TOB1 enhanced the radiosensitization and decreased the ability of migration and invasion of colorectal cancer cells.
TOB1 Is A Target Of miR-32-5p
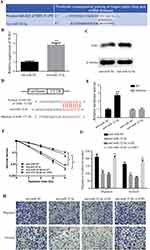
To determine how TOB1 is regulated in colorectal cancer, a bioinformatical analysis was used to seek for its potential upstream miRNAs. According to TargetScan, PITA and RNA22 database, miR-32-5p was found to share the binding sites on 3ʹUTR of TOB1 mRNA (Figure 4A). The expression level of TOB1 mRNA was decreased when transfected with anti-miR-32-5p into SW480 cells (Figure 4B). Similarly, knockdown of miR-32-5p in MGC-803 cells increased the expression of TOB1 protein (Figure 4C). To further determine the negative regulation of miR-32-5p on TOB1 expression, 3ʹUTR of wild type TOB1 mRNA (WT) and mutant 3ʹUTR of TOB1 mRNA on the binding sites with miR-32-5p (Mutation) were cloned into luciferase vectors (Figure 4D). Then, Luc-TOB1-WT or Luc-TOB1-Mutation were transfected into SW480 cells with anti-miR-32-5p or anti-miR-NC. Luciferase reporter assays showed that the luciferase activity was markedly increased by anti-miR-32-5p in SW480 cells with Luc-TOB1-WT, but not Luc-TOB1-Mutation (Figure 4E). Accordingly, our results determined that miR-32-5p suppressed TOB1 expression through directly binding to 3ʹUTR of TOB1 mRNA.
TOB1 Reverses The Ability Of miR-32-5p On Radiosensitization, Migration And Invasion Of Colorectal Cancer Cells
To test the effects of TOB1 on the roles of miR-32-5p in colorectal cancer, siRNA targeting TOB1 was generated and rescue experiments were performed. Clone formation assay showed that knockdown of miR-32-5p significantly decreased the survival fraction under different irradiation doses (Figure 4F), while si-TOB1 increased the survival fraction on the base of knockdown of miR-32-5p (Figure 4F), indicating that TOB1 reverses the ability of miR-32-5p to enhance the radiosensitization of colorectal cancer cells. Transwell migration and invasion assays indicated knockdown of miR-32-5p significantly decreased the migrated cell numbers and invaded cell numbers (Figure 4G and H), while si-TOB1 increased the migrated cell numbers and invaded cell numbers on the base of knockdown of miR-32-5p (Figure 4G and H), implying that TOB1 reverses the ability of miR-32-5p to promote migration and invasion of colorectal cancer cell. Taken together, knockdown of TOB1 reverses the ability of anti-miR-32-5p on enhancing radiosensitization and suppressing migration and invasion of colorectal cancer cells.
Discussion
Up to now, large amounts of miRNAs have been found to play key roles in the development and progression of various cancers.13 However, the mechanisms of miRNAs in radiosensitization and metastasis of colorectal cancer have not been verified. In this study, we found that miR-32-5p was obviously upregulated in colorectal cancer tissues and positively correlated with clinicopathological features and survival. Mechanistically, knockdown of miR-32-5p enhanced the radiosensitization and suppressed metastasis of colorectal cancer through directly binding to the 3ʹ-UTR of TOB1 mRNA.
miRNAs have been widely identified to be involved in the development and progression of various cancers, such as cell cycle, proliferation, apoptosis, migration, invasion, radiosensitization and drug-resistance. Qi et al determined that miR-142-3p was overexpressed in nasopharyngeal carcinoma tissues, and knockdown of miR-142-3p significantly suppressed cell proliferation and growth in vitro and in vivo.14 Xu et al found that miR-1296 expression was remarkably reduced in hepatocellular carcinoma tissues and was closely correlated with recurrence and metastasis.15 Meanwhile, they tested that miR-1296 could inhibit epithelial–mesenchymal transition (EMT) and migration and invasion in vitro and in vivo. Also, it has been covered that miR-320a played an indispensable role in cell proliferation, migration, invasion, apoptosis and chemosensitivity in a multiple of cancers, such as liver cancer,16 salivary adenoid cystic carcinoma,16 colorectal cancer,17 myeloma18 and gastric cancer. In this study, we found that miR-32-5p was significantly upregulated in colorectal cancer tissues and positively correlated with cell differentiation and metastasis. Furthermore, high miR-32-5p predicted a poor overall survival and disease-free survival in colorectal cancer patients. Taken together, these results suggest that miR-32-5p acts as an oncogenic factor in the development of colorectal cancer, and may well be a promising biomarker for diagnosing, a predictor for survival and a target for treatment.
Radiotherapy was one of the major methods for auxiliary treatment of tumor. The radiosensitivity is one of the main factors that affect its efficacy. Therefore, the exploration of the radiosensitivity mechanism of cancer cells is of great significance. Multiple studies have revealed that miRNAs are involved in cell radiosensitivity. Cheng et al have reported that miR-449b-5p was relatively downregulated in cervical cancer tissues, enhanced radiosensitivity and suppressed cell proliferation, migration and invasion.19 In addition, miR-365 was found to be dysregulated in non-small cell lung cancer and enhance the radiosensitivity by inhibiting CDC25A expression.20 What’s more, Liao et al have reported that miR-32-5p was upregulated in prostate cancer tissues and induced autophagy and radioresistance.12 In this research, we demonstrated that knockdown of miR-32-5p cell decreased the survival fraction in a dose-dependent manner, indicating that suppressed miR-32-5p significantly enhances radiosensitivity of colorectal cancer cells. Moreover, invasion and metastasis of cancer cells are the main cause of colorectal cancer-related death. Results from Transwell migration and invasion experiments showed that downregulated miR-32-5p decreased the ability of migration and invasion of colorectal cancer. In summary, knockdown of miR-3-5p enhanced radiosensitivity and the ability of migration and invasion of colorectal cancer.
As a cancer-suppressing gene, TOB1 was located in human chromosome 17q21. TOB1 was reported to suppress proliferation, promote apoptosis and increase radiosensitivity of tumor cells through multiple signaling pathways. TOB1 protein was composed of 345 amino acids, and half of N terminus was heterogeneous to products of anti-proliferation genes. The C terminus of TOB1 was correlated with protein–protein interactions, being of great importance to the regulation in organisms. It has been reported that overexpression of TOB1 significantly inhibited cell growth, induced apoptosis and radiosensitivity of breast cancer cells through regulating JNK and p38 phosphorylation.6 In addition, Sun et al demonstrated that overexpressed TOB1 eliminated cell cycle G2/M block induced by ionizing radiation, reduced cell growth and enhanced radiosensitivity of non-small cell lung cancer cells through regulating MAPK/ERK signal pathway.5 In the current study, results showed that overexpression of TOB1 markedly increased the radiosensitivity of colorectal cancer SW 480 cells. Furthermore, upregulated TOB1 inhibited migration and invasion of colorectal cancer. Taken together, these results demonstrated that miR-32-5p acted as a tumor suppressor in colorectal cancer and enhanced radiosensitivity and the ability of migration and invasion of colorectal cancer cells.
At present, it is widely accepted that miRNAs negatively regulate target genes’ expression through complete complementation or incomplete complementation with 3ʹUTR of mRNA, which leads to mRNA degradation or inhibition of translation, thereby participating in multiple cell biological processes.21 Yu et al found that miR-182 could potentiate TGF β -induce Lipofectamined EMT and promoted invasion of breast cancer cells and osteoclastogenesis for bone metastasis.9 In terms of mechanism, they determined that miR-182 could directly bind to the 3ʹ-UTR of SMAD7 mRNA, an important protein in the TGFβ downstream pathway. It has been reported that increased miR-195 suppresses the expression of coactivator-associated arginine methyltransferase1 (CARM1) through directly binding to the 3ʹ-UTR of CARM1 mRNA.22 In this study, bioinformatics analysis showed that TOB1 shared the binding sites of miR-32-5p, and knockdown of miR-32-5p decreased the expression of TOB1 mRNA and protein. Luciferase reporter assays demonstrated that miR-32-5p negatively regulated TOB1 expression through directly binding to the 3ʹ-UTR of TOB1 mRNA. Furthermore, to explore the effects of TOB1 on the role of miR-32-5p on radiosensitivity, metastasis and invasion in colorectal cancer, rescue experiments were conducted. Knockdown of miR-32-5p enhanced the radiosensitivity and suppressed metastasis and invasion of colorectal cancer cells, while si-TOB1 reversed the ability of decreased miR-32-5p on the radiosensitivity, metastasis and invasion of colorectal cancer cells. In a word, knockdown of miR-32-5p enhanced the radiosensitivity and suppressed metastasis and invasion of colorectal cancer cells through negatively regulating TOB1 expression.
Conclusion
In conclusion, miR-32-5p is obviously upregulated in colorectal cancer tissues and positively correlated with clinicopathological features and poor prognosis. Furthermore, we demonstrated that miR-32-5p regulated the radiosensitivity and suppressed metastasis and invasion of colorectal cancer cells through negatively regulating TOB1 expression. Our findings identify the role of miR-32-5p, which may provide a new theoretical basis for radiosensitivity and offer an effective biomarker for diagnosis and prognosis and a promising target for therapy in colorectal cancer.
Abbreviations
miRNA, microRNA; qRT-PCR, real-time quantitative polymerase chain reaction; TOB1, transducer of ERBB2, 1; BTG, B-cell translocation gene; 3ʹUTR, 3′-untranslated region; MREs, microRNA response elements; FBS, fetal bovine serum; PVDF, polyvinylidene fluoride.
Acknowledgements
This work was supported by Henan Province 2019 Overseas Research Project of Health Science and Technology Talents (HWYX2019116), Henan Medical Science and Technology Research Project (No. 201303132, No. 201303136, No. 2018020432).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
3. Li D, Xiao L, Ge Y, et al. High expression of Tob1 indicates poor survival outcome and promotes tumour progression via a Wnt positive feedback loop in colon cancer. Mol Cancer. 2018;17(1):159. doi:10.1186/s12943-018-0907-9
4. Guo H, Ji F, Zhao X, et al. MicroRNA-371a-3p promotes progression of gastric cancer by targeting TOB1. Cancer Lett. 2019;443(18):179–188. doi:10.1016/j.canlet.2018.11.021
5. Sun KK, Zhong N, Yang Y, Zhao L, Jiao Y. Enhanced radiosensitivity of NSCLC cells by transducer of erbB2.1 (TOB1) through modulation of the MAPK/ERK pathway. Oncol Rep. 2013;29(6):2385–2391. doi:10.3892/or.2013.2403
6. Wu D, Zhou W, Wang S, Zhou Z, Wang S, Chen L. Tob1 enhances radiosensitivity of breast cancer cells involving the JNK and p38 pathways. Cell Biol Int. 2015;39(12):1425–1430. doi:10.1002/cbin.10545
7. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi:10.1016/j.cell.2009.01.002
8. Tang Z, Fang Y, Du R. MicroRNA-107 induces cell cycle arrests by directly targeting cyclin E1 in ovarian cancer. Biochem Biophys Res Commun. 2019;512(2):331–337. doi:10.1016/j.bbrc.2019.03.009
9. Yu J, Lei R, Zhuang X, et al. MicroRNA-182 targets SMAD7 to potentiate TGFbeta-induced epithelial-mesenchymal transition and metastasis of cancer cells. Nat Commun. 2016;7(13):884. doi:10.1038/ncomms13884
10. Fu X, Liu M, Qu S, et al. Exosomal microRNA-32-5p induces multidrug resistance in hepatocellular carcinoma via the PI3K/Akt pathway. J Exp Clin Cancer Res. 2018;37(1):52. doi:10.1186/s13046-018-0677-7
11. Zhang L, Li X, Chao Y, et al. KLF4, a miR-32-5p targeted gene, promotes cisplatin-induced apoptosis by upregulating BIK expression in prostate cancer. Cell Commun Signal. 2018;16(1):53. doi:10.1186/s12964-018-0270-x
12. Liao H, Xiao Y, Hu Y, Xiao Y, Yin Z, Liu L. microRNA-32 induces radioresistance by targeting DAB2IP and regulating autophagy in prostate cancer cells. Oncol Lett. 2015;10(4):2055–2062. doi:10.3892/ol.2015.3551
13. Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–874. doi:10.1038/nrg3074
14. Qi X, Li J, Zhou C, Lv C, Tian M. MiR-142-3p suppresses SOCS6 expression and promotes cell proliferation in nasopharyngeal carcinoma. Cell Physiol Biochem. 2015;36(5):1743–1752. doi:10.1159/000430147
15. Xu Q, Liu X, Liu Z, et al. MicroRNA-1296 inhibits metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by targeting SRPK1-mediated PI3K/AKT pathway. Mol Cancer. 2017;16(1):103. doi:10.1186/s12943-017-0675-y
16. Lu C, Liao Z, Cai M, Zhang G. MicroRNA-320a downregulation mediates human liver cancer cell proliferation through the Wnt/beta-catenin signaling pathway. Oncol Lett. 2017;13(2):573–578. doi:10.3892/ol.2016.5479
17. Zhao H, Dong T, Zhou H, et al. miR-320a suppresses colorectal cancer progression by targeting Rac1. Carcinogenesis. 2014;35(4):886–895. doi:10.1093/carcin/bgt378
18. Lu Y, Wu D, Wang J, Li Y, Chai X, Kang Q. miR-320a regulates cell proliferation and apoptosis in multiple myeloma by targeting pre-B-cell leukemia transcription factor 3. Biochem Biophys Res Commun. 2016;473(4):1315–1320. doi:10.1016/j.bbrc.2016.04.069
19. Cheng L, Shi X, Huo D, Zhao Y, Zhang H. MiR-449b-5p regulates cell proliferation, migration and radioresistance in cervical cancer by interacting with the transcription suppressor FOXP1. Eur J Pharmacol. 2019;856(172):399. doi:10.1016/j.ejphar.2019.05.028
20. Li H, Jiang M, Cui M, et al. MiR-365 enhances the radiosensitivity of non-small cell lung cancer cells through targeting CDC25A. Biochem Biophys Res Commun. 2019;512(2):392–398. doi:10.1016/j.bbrc.2019.03.082
21. Yu S, Lu Z, Liu C, et al. miRNA-96 suppresses KRAS and functions as a tumor suppressor gene in pancreatic cancer. Cancer Res. 2010;70(14):6015–6025. doi:10.1158/0008-5472.CAN-09-4531
22. Zheng L, Chen J, Zhou Z, He Z. miR-195 enhances the radiosensitivity of colorectal cancer cells by suppressing CARM1. Onco Targets Ther. 2017;10(8):1027–1038. doi:10.2147/OTT
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.