Back to Journals » Cancer Management and Research » Volume 12
MiR-29b-3p Reverses Cisplatin Resistance by Targeting COL1A1 in Non-Small-Cell Lung Cancer A549/DDP Cells
Received 19 January 2020
Accepted for publication 16 March 2020
Published 16 April 2020 Volume 2020:12 Pages 2559—2566
DOI https://doi.org/10.2147/CMAR.S246625
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Rui Jia,1,2 Changli Wang1
1Department of Lung Cancer, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer; Key Laboratory of Cancer Prevention and Therapy, Tianjin; Tianjin’s Clinical Research Center for Cancer, Tianjin 300000, People’s Republic of China; 2Department of Thoracic Surgery, The First Hospital of Qinhuangdao, Qinhuangdao, Hebei 066000, People’s Republic of China
Correspondence: Changli Wang
Department of Lung Cancer, Tianjin Medical University Cancer Institute and Hospital; National Clinical Research Center for Cancer; Key Laboratory of Cancer Prevention and Therapy, Tianjin, Tianjin’s Clinical Research Center for Cancer, West Huan-Hu Road, Ti Yuan Bei, Hexi District, Tianjin 300000, People’s Republic of China
Tel +86-13930355265
Email [email protected]
Objective: To investigate the expression of miR-29b-3p in tissues and cells of non-small-cell lung cancer (NSCLC) and its effect on cisplatin resistance in NSCLC cells and its mechanism.
Methods: The mRNA expression of miR-29b-3p and COL1A1 in NSCLC tissue, cell line A549 and cisplatin-resistant cell line A549/DDP was detected by RT-qPCR. MiR-29b-3p Mimics was transfected into A549/DDP cells, and the cell viability, proliferation, apoptosis and related protein expression were detected by CCK-8, flow cytometry and Western blot. Also, luciferase reporter gene assay was used to verify the targeting relationship between miR-29b-3p and COL1A1. Moreover, COL1A1 overexpression lentivirus and miR-29b-3p mimics (Mimics+COL1A1) were simultaneously transfected into A549/DDP cells, and then the cell viability and related protein expression were measured.
Results: In NSCLC tissue and its cell line, miR-29b-3p was downregulated and COL1A1 was upregulated (P< 0.05). After A549/DDP cell was transfected by mimics, its cell viability and proliferation rate decreased, apoptosis rate and the expression of tumor suppressor gene PTEN and apoptosis-related protein BAX were increased (P< 0.05), which could be reversed by Mimics+COL1A1 co-transfection. Luciferase reporter gene assay indicated that COL1A1 was the target gene of miR-29b-3p.
Conclusion: All in all, miR-29b-3p can reverse the cisplatin resistance of A549/DDP cells by inhibiting the expression of COL1A1 gene and increasing the expression of PTEN and BAX.
Keywords: MiR-29b-3p, non-small-cell lung cancer, COL1A1, cisplatin, drug resistance
Introduction
Lung cancer is the most common malignant tumor, and its morbidity and mortality are the highest among all malignant tumors.1 Non-small-cell lung cancer (NSCLC), a type of lung cancer, accounts for about 85% of all newly diagnosed lung cancer cases.2 At present, there are many treatments for NSCLC, such as surgery, radiotherapy and chemotherapy, immunotherapy. As the early symptoms of NSCLC are not obvious and relatively insidious, most of the patients are diagnosed with advanced tumor stage and lose the opportunity of radical operation. Chemotherapy is one of the main methods for the treatment of advanced NSCLC. However, due to drug resistance, the efficacy of chemotherapeutic drugs is greatly reduced in clinic.3,4 Currently, cisplatin (DDP) is one of the most effective and widely used anti-tumor drugs. It has been used to treat a variety of solid malignant tumors, including head and neck, lung, ovary, leukemia, breast cancer, brain cancer, kidney cancer, testicular cancer, colorectal cancer, bladder cancer, etc., all of which have shown better efficacy.5 However, long-term use of cisplatin can lead to drug resistance of tumor cells and weaken its clinical efficacy.6 NSCLC patients are sensitive to cisplatin at the beginning and later develop cisplatin resistant, which leads to the failure of chemotherapy.7,8 Therefore, understanding the molecular mechanism of cisplatin resistance in NSCLC to identify potential therapeutic targets can improve the prognosis of patients with NSCLC.
MicroRNAs (miRNAs) is a non-coding regulatory factor, which contains 21–25 nucleotides and is a key regulator of post-transcriptional gene expression. Multiple studies have demonstrated that miRNAs play a key role in tumor proliferation, metastasis and drug resistance.9,10 Some studies have shown that miR-29b-3p has anti-cancer effects in a variety of tumors, and its expression decreases in pancreatic cancer, colorectal cancer and oral cancer.11–13 Inoue et al reported that mir-29b-3p can inhibit the activity of colon cancer cells mutated by KRAS by inhibiting NF-κB signaling pathway.14 Wang et al demonstrated that mir-29b can inhibit the growth and metastasis of colorectal cancer by downregulating the expression of Tiam1 and inhibiting epithelial-mesenchymal transformation.13 Wang et al11 found that mir-29b can inhibit the proliferation of pancreatic cancer cells by targeting DNMT3b. He et al showed that miRNA-29b-3p can inhibit the migration and invasion of OSCC cells through il32/Akt signaling pathway.12 In contrast, type I collagenα 1 (COL1A1) is an extracellular matrix protein, which is highly expressed in breast cancer, colorectal cancer, etc. It is related to tissue hypoxia and oxidative stress response, which may help cells adapt to oxidative stress and hypoxia, thus promoting tumor cell invasion and metastasis.15–17 Under hypoxia condition, COL1A1 was upregulated in mRNA and/or protein level, which mainly enhanced the transcription and extracellular deposition of COL1A1 through TGF-b pathway.18,19 Moreover, at present, the expression of miR-29b-3p and COL1A1 in NSCLC and whether they are involved in cisplatin resistance of NSCLC are unknown. Therefore, this study investigated the expression of miR-29b-3p and COL1A1 in NSCLC and its effect on cisplatin resistance and its mechanism.
Materials and Methods
Tissue Specimens Collection
Tumor tissues and corresponding paracancerous tissues with normal histology were selected from patients who underwent surgery resection for NSCLC in Tianjin medical university cancer institute and hospital from January 2014 to December 2016. Twenty cases in normal group and tumor group, respectively. All tissues were resected and immediately put into liquid nitrogen and then stored in refrigerator at −80°C. This experiment was approved by Tianjin medical university cancer institute and hospital ethics review board. All patients did not receive radiotherapy, chemotherapy and other related treatments before surgery and signed an informed consent form.
Main Reagents
Human normal pulmonary epithelial cells (BESA-2B) and human lung adenocarcinoma cell line A549 were purchased from Shanghai Cell Bank of Chinese Academy of Medical Sciences (CAMS), while cisplatin-resistant variant A549/DDP cells were purchased from Cancer Hospital of Chinese Academy of Medical Sciences and Peking Union Medical College. LipofectamineTM2000 was purchased from Invitrogen Company of the United States. DMEM medium, fetal bovine serum and penicillin-streptomycin mixture were purchased from Gibco Company, while FITC/PE apoptosis detection kit, Cell Counting Kit-8 (CCK-8), trypan blue, anhydrous ethanol and PBS were purchased from Jiangsu Kaiji Biotechnology Co., Ltd. The dual-luciferase reporter gene assay kit was purchased from Beyotime Company, China. BestarTM qPCR RT Kit and Bestar® SybrGreen qPCR Master Mix were purchased from DBI Company in Germany.
Cell Culture and Transfection
All cryopreserved BESA-2B, A549 and A549/DDP cells were resuscitated and cultured in DMEM medium containing 10% fetal bovine serum, penicillin and streptomycin. Then, they were placed in an incubator with temperature 37°C, CO2 concentration 5% and humidity 100%. Moreover, DDP was added to the DMEM medium of A549/DDP cells to adjust its final concentration to 1 μg/mL. When the cells in logarithmic growth phase, subculture and follow-up experiments were carried out. According to the experimental design, A549/DDP cells were divided into empty transfection group (Blank group), mimic transfection negative control group (NC group), miR-29b-3p mimic transfection group (Mimics group), negative overexpression lentivirus and miR-29b-3p mimic co-transfection group (Mimics+con group), and COL1A1 overexpression lentivirus and miR-29b-3p mimic co-transfection group (Mimics+COL1A1 group). A549/DPP cells in logarithmic growth phase were inoculated into 96-well plates with 1.5×104 cells per well and a total volume of 100 μL per well, and cultured for 24 hrs in an incubator at 37°C. 10 μL OPTI-MEM culture medium was used to dilute Mimic, 25 μL OPTI-MEM medium was used to dilute 0.25 μL LipofectamineTM 2000 reagent, and after 5 mins, the two diluted solutions were mixed and shaken gently, then let the mixture stand for 20 mins. The mixture of NC mimics, mir-29b-3p, Mimics+con and Mimics+COL1A1 group was prepared with this system.
Cisplatin Sensitivity and Proliferation Rate of Cells Detected by CCK8
A549 and A549/DPP cells in logarithmic growth phase were inoculated into 96-well plate containing a total volume of 200 μL and 1×105 cells per well, and the wells were randomly divided into 7 Groups containing 0, 1, 10, 20, 40, 80, 160 μM Cisplatin, respectively. After the cells of each group were cultured in an incubator at 37°C with 5% CO2 for 24 hrs, 10 μL CCK8 reagent was added to each well and incubated in the dark incubator for 1.5 hrs. After the well had colour reaction, the optical density (OD) was detected by ELISA at 450 nm wavelength.
Moreover, A549/DPP cells in logarithmic growth phase were inoculated into 6-well plates with a total volume of 2 mL per well and 1.5×106 cells per well, and then cultured for 24 hrs in an incubator at 37°C. After that, they were divided into Blank, NC and Mimics groups. The corresponding mixture was added to each group of cells, and then the complete medium was added. After adding CCK8 reagent and incubating in the incubator without light for 1.5 hrs, the optical density was detected by ELISA at the wavelength of 450 nm.
Furthermore, the cells in Blank, NC and Mimics groups were cultured in 5% CO2 incubator at 37°C for 0, 24, 48, 72 hrs; then, CCK8 reagent was added and incubated in the dark incubator for 1.5 hrs. The optical density was detected by ELISA at 450 nm wavelength, and the cell proliferation rate was calculated.
Detection of Apoptosis by Flow Cytometry
A549/DPP cells in logarithmic growth phase were inoculated in 6-well plates having 1.5×106 cells and a total volume of 2 mL per well, and cultured in an incubator at 37°C for 24 hrs. Then, they were divided into blank, NC and Mimics groups. The adherent cells of each group were digested by trypsin without EDTA and washed with PBS twice (centrifuged at 2000 rpm for 5 mins) to collect 1~5×105 cells; 500 μL Binding Buffer was added to suspend the cells. After mixing with 5 μL Annexin V-FITC, 5 μL Propidium Iodide was added and mixed well, and then let the mixture stand in dark at room temperature for 5–15 mins. Finally, flow cytometry was used to detect cell apoptosis.
Detection of mRNA Expression by RT-qPCR
The mRNA expression of miR-29b-3p and COL1A1 in NSCLC tissue and its adjacent normal tissues, also mRNA expression of miR-29b-3p, COL1A1 and PTEN in BESA-2B group, A549 group and A549/DDP group were measured by qRT-PCR. The tissues and logarithmic growing cells of each group were collected and the total RNA was extracted by Trizol and reverse transcribed into cDNA at 37°C for 15 mins, 98°C for 5 mins and then keeping at 4°C. Then, qPCR reaction was carried out, and parallel experiments of three target genes and three internal reference genes were set up in each sample. The PCR reaction system was 20 μL. The reaction conditions were as follows: at 95°C for 2 mins, 95°C for 10 s, 60°C for 34 s, 72°C for 30 s and lasting 45 cycles. Ct method (2−ΔΔCT) was used for relative quantitative analysis. The primer sequences were: COL1A1-F: CGGAGCAGACGGGAGTTTC, COL1A1-R:CCATTCTTTCCAGGGGGACC; PTEN-F: TTTGAGAGTTGAGCCGCTGT, PTEN-R: ATGCTTTGAATCCAAAAACCTTACT; miR-29b-3p-F: TTGTGACTAAAGTTTACCACGAT.
Detection of Protein Expression by Western Blot
The cells in logarithmic growth phase were lysed by RIPA lysis buffer to collect total protein, which were quantified according to BCA protein quantitative kit. The extracted total protein was added with 2×SDS protein loading buffer (according to the ratio of 1:1) and boiled at 100°C for 5 min, and then stored at −20°C for later use. A 30ug sample was slowly added to the well with a microinjector, and concentrated gel electrophoresis was carried out with 100 V for 20 mins and electrophoresis was performed in the separation gel with 140 V for 40 mins. The electrophoresis was terminated as soon as bromophenol blue ran out, the gel was taken out and transmembrane was performed at 350 mA for 70 mins. The membrane was soaked in the blocking solution at 37°C for 1 to 2 hrs or at 4°C overnight, after that the membrane was put into the primary antibody and sealed for 1 hr at 37°C or overnight at 4°C, PBST was used to rinse the membrane for 5 mins (repeated 4 times). Then, the membrane was put into the second antibody at 37°C for 1 hr. After that, the membrane was washed by PBST for 5 mins (repeated 5 times), and chemiluminescence (ECL) was used to visualize the membrane. The protein expression levels of Bax (ab32503, abcam), Bcl-2 (ab185002, abcam), β-catenin (ab16051, abcam), COL1A1 (ab34710) and PTEN (ab32199, abcam) in each group were detected.
Luciferase Reporter Gene Assay
A549/DDP cells in logarithmic growth phase were inoculated into 96-well plates with a total volume of 100 μL and 1.5×104 cells per well, and then cultured for 24 hrs in an incubator at 37°C. Ten μL OPTI-MEM medium diluted mimic, 15 μL OPTI-MEM medium diluted target gene 3ʹUTR double reporter gene vector, 25 μL OPTI-MEM medium diluted 0.25 μL LipofectamineTM 2000 reagent, and after 5 mins, they were mixed and a total of 50 μL solution was gained, which was gently shook well, and left for 20 mins. This system was used to prepare the mixture of Mimic and wild-type vector, Mimic and mutant vector, Mimic NC and wild-type carrier, Mimic NC and mutant vector, respectively. Before adding the mixture of plasmid and mimics, 50 μL medium was sucked away from each well, and then the mixture of 50 μL was added, resulting in a total volume of 100 μL per well. Also, the wells were changed to 100 μL fresh medium after 8 hrs. Then, after the reporter gene cell lysis buffer was prepared, the 100 μL reporter gene cell lysis solution was added to fully lyse the cells. After centrifugation, the supernatant was taken to detect the activity of luciferase in each group of cells.
Statistical Analysis
The experiment data were expressed by x±SD and statistically analyzed by SPSS 19.0 software. T-test was used for comparison between the two groups, and one-way analysis of variance (ANOVA) was used for pairwise comparison between groups. P<0.05 was considered statistically significant.
Experimental Results
mRNA Expression of miR-29b-3p and COL1A1
qRT-PCR results are shown in Figure 1A, compared with non-tumor adjacent tissues, the mRNA expression of miR-29b-3p was significantly decreased and the mRNA expression of COL1A1 was significantly increased in NSCLC tissues. Also, at the cellular level, the mRNA expression of miR-29b-3p in A549/DDP cells was significantly lower than that in A549 cells, while the mRNA expression of COL1A1 in A549/DDP cells was significantly higher than that in A549 cells, as shown in Figure 1B. In addition, the mRNA expression of PTEN in A549/DDP cells was significantly lower than that in A549 and BESA-2B cells.
 |
Figure 1 mRNA expression of miR-29b-3p in tumor tissues and A549/DDP cells. *P<0.05, **P<0.01 |
Effects of miR-29b-3p on Cisplatin Sensitivity, Proliferation and Apoptosis of NSCLC Cells
CCK8 assay was used to detect the cell viability of A549 and A549/DDP cells in different DDP concentrations (0, 1, 10, 20, 40, 80, 160 μM). The results showed that the cell viability of A549 and A549/DDP cells decreased with the increase of DPP concentration. Also, in different DDP concentration, the cell viability of A549/DDP cells was higher than that of A549 cells, indicating that A549/DDP was less sensitive to cisplatin (Figure 2A). After transfection, the cell viability of A549/DDP cells in Mimics group was significantly lower than that in blank group and NC group, indicating that Mimics group was more sensitive to cisplatin, while there was no significant difference between blank group and NC group (Figure 2B). In addition, after being cultured at 37°C in 5% CO2 incubator for 0, 24, 48, 72 hrs, it was found that there was no significant difference in cell proliferation rate between blank group and NC group, while, the cell proliferation rate of Mimics groups was significantly lower than that of blank and NC groups (Figure 2C). The results of flow cytometry indicated that the apoptosis rate in Mimics group was significantly higher than that in blank group and NC group, and there was no significant difference between blank group and NC group (Figure 2D).
Target Verification of miR-29b-3p
The results of dual-luciferase reporter gene assay showed that COL1A1 contained the binding sequence of miR-29b-3p. Compared with NC group, Mimics group significantly inhibited the luciferase activity of COL1A1-WT carrier, while there was no significant difference between these two groups in COL1A1-MUT (Figure 3A). The results of cisplatin sensitivity (Figure 3B) suggested that the cell viability of Mimics group was significantly lower than that of blank group, indicating that Mimics group was more sensitive to cisplatin, while cell viability of Mimics+COL1A1 group was significantly higher than that of Mimics+con group, indicating that Mimics+COL1A1 group was less sensitive to cisplatin. Also, the increase of COL1A1 expression could reverse the decrease of cell viability induced by miR-29b-3p, and there was no significant difference between Mimics and Mimics+con groups. These results indicated that COL1A1 was the target gene of miR-29b-3p.
 |
Figure 3 Target verification of miR-29b-3p. (A) The results of dual-luciferase reporter gene assay; (B) Cell viability. *P<0.05; ***P<0.001. |
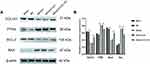
Effect of miR-29b-3p on Protein Expression of BAX, BCL-2, COL1A1 and PTEN
The results of Western blot shown in Figure 4A and B suggested that compared with blank and NC group, the protein expression of Bcl-2 and COL1A1 decreased and the protein expression of PTEN and Bax increased in Mimics group. Compared with Mimics+con group, the protein expression of COL1A1 and Bcl-2 increased and the protein expression of PTEN and Bax decreased in Mimics+COL1A1 group. Also, there was no significant difference between blank group and NC group, and there was no significant difference between Mimics group and Mimics+con group.
Discussion
Cisplatin is a cellular nonspecific drug with a broad spectrum of anti-tumor activity. It can react with DNA to form two-point or two-strand cross-connections in DNA resulting in DNA fragmentation and miscoding, thus it could inhibit DNA replication, transcription and cell mitosis.20,21 Moreover, the cisplatin resistance in tumor cells can be developed, due to long-term use of it, which greatly weakens its clinical efficacy. At present, multiple studies have shown that the abnormal expression of miRNA mediates the process of tumor chemotherapy resistance, which provides a certain reference value for increasing the sensitivity of tumor cells. Also, miR-29b-3p has a certain anticancer effect, it can inhibit the proliferation, invasion and migration of tumor cells and its expression level is decreased in pancreatic cancer, colorectal cancer, breast cancer, oral cancer, which make it have an opportunity to become a potential therapeutic target.11–13 In addition, Liu et al22 investigated whether and how lncRNA H19 regulated miR-29b-3p to affect the progression of lung adenocarcinoma through signal transduction and transcriptional activator 3 (STAT3), and found that the expression of miR-29b-3p was decreased in lung cancer tissues, and miR-29b-3p Mimics can reduce lung cancer cell viability, EMT process, even induce lung cancer cell apoptosis. In this study, RT-qPCR was used to detect the expression of miR-29b-3p in NSCLC tissues and corresponding paracancerous tissues, and it was found that miR-29b-3p expression was significantly low in NSCLC tissues. Also, at the cellular level, the expression of miR-29b-3p in human lung adenocarcinoma cell line A549 was lower than that in human normal lung epithelial cells (BESA-2B). Moreover, the mRNA expression of miR-29b-3p was significantly decreased in cisplatin-resistant variant cell line A549/DDP cells. Furthermore, it is unclear whether miR-29b-3p is involved in forming cisplatin resistance of NSCLC and the mechanism.
The results indicate that miR-29b-3p Mimics could significantly reduce the cell viability of A549/DDP cells, and greatly improve the sensitivity of tumor cells to cisplatin, which reversed the cisplatin resistance in NSCLC cells. Also, the proliferation rate of A549/DDP decreased significantly under the action of miR-29b-3p Mimics, suggesting that miR-29b-3p Mimics could inhibit the proliferation of NSCLC cells. Meanwhile, the result of flow cytometry showed that miR-29b-3p Mimics could promote the apoptosis of A549/DDP cells. In addition, previous studies16 found that COL1A1 can promote the development of tumor and increase the expression in pancreatic cancer. In this study, it was also found that the expression in NSCLC tissue and lung adenocarcinoma cell A549 was higher than that in the control group. In order to further study the mechanism of miR-29b-3p increasing cisplatin sensitivity of A549/DDP cells, luciferase reporter gene assay was used to verify whether COL1A1 was the target of miR-29b-3p. The results showed that miR-29b-3p had a significant down-regulation effect on the reported fluorescence of the COL1A1-wt vector, and there was no significant change in the reported fluorescence of the mutant vector after the corresponding binding site mutation. In addition, when detecting the effect of transfection, it was found that the cell viability of Mimics group was significantly decreased, while that of COL1A1 cotransfection group was significantly increased, indicating that COL1A1 could reverse the increase of cisplatin sensitivity of NSCLC cells induced by Mimics. Therefore, COL1A1 is the target gene of miR-29b-3p. PTEN is a tumor suppressor gene located on chromosome 10q23.3. Down-regulated PTEN can promote the growth and invasion of NSCLC cells.23,24 It is reported that PTEN tumor suppressor function loss can lead to abnormal activation of PI3K Signal network, thus promoting the occurrence and development of prostate cancer.25 In addition, the decrease of PTEN is related to the poor survival outcome of renal cancer patients, PTEN plays a role in the occurrence and development of renal cancer.26 Apoptosis-related gene Bax interacts with Bcl-2 to form a heterodimer or with itself to form a homodimer, and the increase in Bax homodimer often leads to apoptosis.27 Bax upregulated and Bcl-2 downregulated can promote the apoptosis of melanoma skin cancer cells.28 El Sisi et al reported that imatinib could increase the expression of Bax and decrease the expression of Bcl-2 by targeting Bax/Bcl-2, thus enhancing its antitumor activity.29 In this study, it was demonstrated that the mRNA expression of PTEN in A549 cells and A549/DDP cells decreased. Moreover, Western blot assay showed that miR-29b-3p Mimics could up-regulate the protein expression of apoptosis-related gene Bax and tumor suppressor gene PTEN, and down-regulate the protein expression of Bcl-2 and COL1A1. When the cells were cotransfected with miR-29b-3p Mimics and COL1A1, the above changes caused by miR-29b-3q mimics could be reversed, which could further confirm that COL1A1 is the target gene of miR-29b-3p.
To sum up, in NSCLC tissue and its cell lines, the expression of miR-29b-3p was down-regulated and the expression of COL1A1 was upregulated. MiR-29b-3p can reverse the cisplatin resistance of NSCLC cells by targeting COL1A1. This conclusion further improves the mechanism of cisplatin resistance in NSCLC, and up-regulation of miR-29b-3p may be of some value in a new scheme for reversing cisplatin resistance in NSCLC.
Ethics and Consent Statement
The study was approved by Tianjin Medical University, Cancer Institute and Hospital Ethics Review Board.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cai Z, Liu Q. Understanding the Global Cancer Statistics 2018: implications for cancer control. Sci China Life Sci. 2019. doi:10.1007/s11427-019-9816-1
2. Xiao H, Liu Y, Liang P, et al. TP53TG1 enhances cisplatin sensitivity of non-small cell lung cancer cells through regulating miR-18a/PTEN axis. Cell Biosci. 2018;8(1):23. doi:10.1186/s13578-018-0221-7
3. Tan XL, Moyer AM, Fridley BL, et al. Genetic variation predicting cisplatin cytotoxicity associated with overall survival in lung cancer patients receiving platinum-based chemotherapy. Clin Cancer Res. 2011;17(17):5801–5811. doi:10.1158/1078-0432.CCR-11-1133
4. Chen FF, Lv X, Zhao QF, et al. Inhibitor of DNA binding 3 reverses cisplatin resistance in human lung adenocarcinoma cells by regulating the PI3K/Akt pathway. Oncol Lett. 2018;16(2):1634–1640. doi:10.3892/ol.2018.8849
5. Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi:10.1016/j.ejphar.2014.07.025
6. Galluzzi L, Senovilla L, Vitale I, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31(15):1869–1883. doi:10.1038/onc.2011.384
7. Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355(10):983–991. doi:10.1056/NEJMoa060570
8. Wang Z, Liu L, Guo X, Guo C, Wang W. microRNA-1236-3p regulates DDP resistance in lung cancer cells. Open Med (Wars). 2018;14(1):41–51. doi:10.1515/med-2019-0007
9. Qiu T, Zhou L, Wang T, et al. miR-503 regulates the resistance of non-small cell lung cancer cells to cisplatin by targeting Bcl-2. Int J Mol Med. 2013;32(3):593–598. doi:10.3892/ijmm.2013.1439
10. Kovalchuk O, Filkowski J, Meservy J, et al. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol Cancer Ther. 2008;7(7):2152–2159. doi:10.1158/1535-7163.MCT-08-0021
11. Wang LH, Huang J, Wu CR, et al. Downregulation of miR29b targets DNMT3b to suppress cellular apoptosis and enhance proliferation in pancreatic cancer. Mol Med Rep. 2018;17(2):2113–2120.
12. He J, Ye W, Kou N, et al. MicroRNA-29b-3p suppresses oral squamous cell carcinoma cell migration and invasion via IL32/AKT signalling pathway. J Cell Mol Med. 2020;24(1):841–849. doi:10.1111/jcmm.v24.1
13. Wang B, Li W, Liu H, et al. miR-29b suppresses tumor growth and metastasis in colorectal cancer via downregulating Tiam1 expression and inhibiting epithelial-mesenchymal transition. Cell Death Dis. 2014;5(7):e1335. doi:10.1038/cddis.2014.304
14. Inoue A, Mizushima T, Wu X, et al. A miR-29b byproduct sequence exhibits potent tumor-suppressive activities via inhibition of NF-kappaB signaling in KRAS-mutant colon cancer cells. Mol Cancer Ther. 2018;17(5):977–987. doi:10.1158/1535-7163.MCT-17-0850
15. Oleksiewicz U, Liloglou T, Tasopoulou K-M, et al. COL1A1, PRPF40A, and UCP2 correlate with hypoxia markers in non-small cell lung cancer. J Cancer Res Clin Oncol. 2017;143(7):1133–1141. doi:10.1007/s00432-017-2381-y
16. Shintani Y, Hollingsworth MA, Wheelock MJ, Johnson KR. Collagen I promotes metastasis in pancreatic cancer by activating c-Jun NH(2)-terminal kinase 1 and up-regulating N-cadherin expression. Cancer Res. 2006;66(24):11745–11753. doi:10.1158/0008-5472.CAN-06-2322
17. Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30(1):1–22. doi:10.1146/annurev-immunol-100311-102839
18. Falanga V, Zhou L, Yufit T. Low oxygen tension stimulates collagen synthesis and COL1A1 transcription through the action of TGF-beta1. J Cell Physiol. 2002;191(1):42–50. doi:10.1002/jcp.10065
19. Lai MC, Chang CM, Sun HS. Hypoxia induces autophagy through translational up-regulation of lysosomal proteins in human colon cancer cells. PLoS One. 2016;11(4):e153627. doi:10.1371/journal.pone.0153627
20. Riddell IA. Cisplatin and oxaliplatin: our current understanding of their actions. Met Ions Life Sci. 2018;18.
21. Qi MM, Ge F, Chen XJ, Tang C, Ma J. MiR-124 changes the sensitivity of lung cancer cells to cisplatin through targeting STAT3. Eur Rev Med Pharmacol Sci. 2019;23(12):5242–5250. doi:10.26355/eurrev_201906_18190
22. Liu L, Liu L, Lu S. lncRNA H19 promotes viability and epithelial-mesenchymal transition of lung adenocarcinoma cells by targeting miR-29b-3p and modifying STAT3. Int J Oncol. 2019;54(3):929–941. doi:10.3892/ijo.2019.4695
23. Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K, Yang GH. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC). Clin Chim Acta. 2010;411(11–12):846–852. doi:10.1016/j.cca.2010.02.074
24. Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133(3):403–414. doi:10.1016/j.cell.2008.04.013
25. Wise HM, Hermida MA, Leslie NR. Prostate cancer, PI3K, PTEN and prognosis. Clin Sci (Lond). 2017;131(3):197–210. doi:10.1042/CS20160026
26. Que WC, Qiu HQ, Cheng Y, Liu MB, Wu CY. PTEN in kidney cancer: A review and meta-analysis. Clin Chim Acta. 2018;480:92–98. doi:10.1016/j.cca.2018.01.031
27. Chen L, Li Q, Wang J, et al. MiR-29b-3p promotes chondrocyte apoptosis and facilitates the occurrence and development of osteoarthritis by targeting PGRN. J Cell Mol Med. 2017;21(12):3347–3359. doi:10.1111/jcmm.2017.21.issue-12
28. Won YS, Seo KI. Sanggenol L promotes apoptotic cell death in melanoma skin cancer cells through activation of caspase cascades and apoptosis-inducing factor. Food Chem Toxicol. 2020;138:111221. doi:10.1016/j.fct.2020.111221
29. El-Sisi AE, Sokkar SS, Ibrahim HA, Hamed MF, Abu-Risha SE. Targeting MDR-1 gene expression, BAX/BCL2, caspase-3 and Ki-67 by nano-encapsulated imatinib and hesperidin to enhance anticancer activity and ameliorate cardiotoxicity. Fundam Clin Pharmacol. 2020. doi:10.1111/fcp.12549
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.