Back to Journals » Cancer Management and Research » Volume 12
MiR-27a-3p Targeting GSK3β Promotes Triple-Negative Breast Cancer Proliferation and Migration Through Wnt/β-Catenin Pathway
Authors Wu R, Zhao B, Ren X, Wu S, Liu M, Wang Z, Liu W
Received 25 March 2020
Accepted for publication 30 June 2020
Published 24 July 2020 Volume 2020:12 Pages 6241—6249
DOI https://doi.org/10.2147/CMAR.S255419
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Ruizhen Wu, Bingqing Zhao, Xunxin Ren, Shiheng Wu, Mingzao Liu, Zipeng Wang, Wei Liu
The First Affiliated Hospital, School of Clinical Medicine, Guangdong Pharmaceutical University, Guangzhou, People’s Republic of China
Correspondence: Wei Liu
The First Affiliated Hospital, School of Clinical Medicine, Guangdong Pharmaceutical University, No. 520 Waihuandong Road, University Town, Guangzhou 510006, People’s Republic of China
Tel/ Fax +86-20-39943021
Email [email protected]
Background: Dysregulation of microRNAs (miRNAs) was found to play crucial roles in varieties of cancers, which affect tumor proliferation and migration. MiR-27a-3p has been identified as a tumor-related miRNA in liver cancer, lung cancer, and colorectal cancer. However, the function of miR-27a-3p in triple-negative breast cancer (TNBC) and its possible molecular mechanisms have still not been elucidated.
Methods: QRT-PCR technique was used to detect the expression of miR-27a-3p in TNBC and normal breast cell lines or the effects of miR-27a-3p knockdown and overexpression in TNBC cell lines. Proliferation and migration were measured by CCK-8 method, colony formation, wound healing, and Transwell assays, respectively. Furthermore, we used a dual-luciferase reporter gene assay and Western blot analysis to identify GSK3β as a target of miR-27a-3p.
Results: In this study, we found that miR-27a-3p expression was significantly elevated in TNBC cell lines. Database analysis suggested that TNBC patients with a high expression of miR-27a-3p have poorer overall survival possibilities. Overexpression of miR-27a-3p promotes TNBC cells proliferation, colony formation, and cell migration in vitro. Nevertheless, dual-luciferase reporter result showed that miR-27a-3p directly targeted the 3ʹ-UTR regions of GSK3β mRNA and negatively regulated its expression. Lastly, we demonstrated that miR-27a-3p inactivates Wnt/β-catenin signaling pathway via targeting GSK3β.
Conclusion: These results indicate that expression of miR-27a-3p was highly expressed in TNBC and promoted tumor progression through attenuating GSK3β and may have a potential molecular-targeted strategy for TNBC therapy.
Keywords: triple-negative breast cancer, miR-27a-3p, GSK3β, Wnt/β-catenin pathway
Introduction
Breast cancer is one of the most common malignant tumors in women and the sixth leading cause of cancer-related death worldwide.1 In 2017, breast cancer will be diagnosed in 12% of women in the United States over the course of their lifetimes and 9.6% of all deaths from breast cancer worldwide.2 Triple-negative breast cancer (TNBC) accounts for 15% to 20% of newly diagnosed breast cancer cases and is known for its aggressive biological behavior and poor patient outcomes compared with hormone receptor-positive breast cancer.3 TNBC is defined as a subtype of breast cancer that does not express estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2).4 Metastasis, recurrence, and high susceptibility to drug resistance are the main factors related to poor prognosis in patients with TNBC.5 Currently, due to the devoid of early detection biomarkers and clear therapeutic targets, TNBC patients are often diagnosed late with a high histological grade and do not benefit from hormonal or targeted therapies.6 Therefore, effective therapeutic approaches for TNBC are essential to improve outcomes for patients with this malignancy.
MicroRNAs (miRNAs) are small non-coding RNAs, 19–25 nucleotides in length, and these RNAs can bind to the 3ʹuntranslated regions (3ʹUTRs) of their target mRNA to regulate gene expression at the posttranscriptional stages.7,8 MiRNAs exert a wide variety of fundamental biological processes in TNBC development including cell proliferation, invasion, and metastasis.9 Some of miRNAs are emerging as potential targets for diagnostic and therapeutic interventions in TNBC.10,11 Previous studies have shown that the expression of several miRNAs was dysregulated during the carcinogenesis and progression of TNBC, such as miR-335, miR-873, miR-483-3p, and miR-124.12–15 Abnormal expression of miRNAs affects the oncogenic behaviors of TNBC cells through play oncogenes or tumor suppressor roles.16 Therefore, it is important to understand abnormally expressed miRNAs in TNBC progression to realize potential therapeutic targets. The miR-27a-3p has been widely implicated in the tumorigenesis of a variety of cancers, the expression and function of miR-27a-3p in TNBC remain poorly understood.
The canonical Wnt signal pathway, also known as the Wnt/β-catenin or the β-catenin/T-cell factor (TCF) pathway, controls many biological processes via signal transduction, including cell proliferation, cell fate determination, and organ formation.17 The key regulatory factor within Wnt pathway is GSK3β.18,19 A large number of studies have reported that GSK3β proteins play important roles during embryo development.20–22 Notably, recent studies show that GSK3β act as oncogenes regulating cancer development in several types of cancers.18,21 In this study, we investigated the effect of GSK3β on triple-negative breast cancer for the first time.
In our research, we confirmed that miR-27a-3p was overexpressed in TNBC cell lines. Elevated miR-27a-3p expression predicted a poor prognosis in TNBC patients. In vitro, miR-27a-3p overexpression promoted cell growth and migration. Furthermore, we demonstrated that miR-27a-3p promoted cell proliferation and migration by directly targeting GSK3β and inactivated Wnt/β-catenin signaling in TNBC. Together, these results implicated that miR-27a-3p interference may be a potential method for TNBC therapy.
Materials and Methods
Cell Culture
Breast cancer cell lines (BT-549, MDA-MB-231, MDA-MB-468, MDA-MB-453) and immortalized human breast epithelial cell lines (MCF-10A, DU4475) were all obtained from the American Type Culture Collection (ATCC). BT-549, MDA-MB-231, MDA-MB-468, and MDA-MB-453 were cultured in recommended medium supplemented with 10% fetal bovine serum (FBS, Gibco, USA) in a humidified incubator at 37°C with 5% CO2. MCF-10A and DU4475 were cultured in complex including Mammary Epithelial Cell Growth Medium DMEM (Gibco, USA), 5% Horse serum (Gibco, USA), 20ng/mL EGF (Sigma, USA), 0.5μg/mL hydrocortisone (Thermo Fisher Scientific, USA), 10μg/mL insulin (Thermo Fisher Scientific, USA), 100ng/mL cholera toxin (Sigma, USA) in a humidified incubator at 37°C with 5% CO2.
Cell Transfection with miRNA Mimics or Inhibitors
MiRNA NC, miR-27a-3p mimic, and miR-27a-3p inhibitor were purchased and biosynthesized from Ribobio (Guangzhou, China). The cells were seeded into 6-well plates and transfected with 100 nM oligonucleotides (miRNA NC, miR-27a-3p mimic, and miR-27a-3p inhibitor) using Lipofectamine 3000 reagent (Invitrogen, USA) in recommended concentrations according to the manufacturer’s instructions.
RNA Isolation and Quantitative Reverse Transcription PCR (qRT-PCR)
Total RNA from cells was extracted using Trizol Reagent (Takara, Japan) and quantified with NanoDrop 2000 (Thermo Fisher Scientific, USA). Complementary DNA (cDNA) was synthesized using PrimeScript™ RT Master Mix (Takara, Japan). Quantification of miRNA expression was performed using the Mir-X™ miRNA qRT-PCR TB Green™ Kit (Takara, Japan) on a Light Cycler® 480 II (Roche, Applied Science). Primers were synthesized by Ribobio (Guangzhou, China) and its sequences as follows: U6 snRNA forward, 5′-ATTGGAACGATACAGAGAAGATT-3′ and reverse, 5′-GGAACGCTTCACGAATTTG-3; miR-27a-3p, forward, 5ʹ-TGCGGTTCACAGTGGCTAAG-3ʹ and reverse, 5ʹ-CTCAACTGGTGTCGTGGA-3ʹ. MiR-27a-3p expression level was normalized to the U6 small nuclear RNA level and calculated using the 2-ΔΔCt method.
Cell Proliferation Assay
BT-549 and MDA-MB-231 cells were seeded into 96-well plates at a density of 5000 cells per well and transfected with 100 nM oligonucleotides (miRNA NC, miR-27a-3p mimic, and miR-27a-3p inhibitor). Cells were incubated at the cell culture condition for 0, 24, 48, and 72 h. Cell Counting kit-8 (CCK-8; Sigma, USA) assay was performed according to the manufacturer’s protocol. Finally, the absorbance of each sample was determined at a wavelength of 450 nM using microplate reader (Bio-Rad, USA). Each experiment was repeated three times.
Colony Formation Assay
BT-549 and MDA-MB-231 cells (1×103 cells/well) were seeded in 6-well plate with treatment of 100 nM oligonucleotides (miRNA NC, miR-27a-3p mimic and inhibitor) transfection and were then maintained in cell culture conditions for 14 days. Colonies were fixed using 4% paraformaldehyde, stained with 0.1% crystal violet. Cell colonies images were randomly taken by Nikon microscope. Cell colony formation was counted and analyzed.
Transwell Migration Assay
Cell migration assay was performed using Transwell chamber membranes (BD Biosciences, USA) in accordance with manufacturer’s methods. Briefly, BT-549 and MDA-MB-231cellswere seeded in the upper chamber of the Transwell migration system after being transfected with 100 nM oligonucleotides (miRNA NC, miR-27a-3p mimic and inhibitor) and the low chambers were filled with culture medium as chemoattractant and then stay for 2 days, migrated cells on the lower chambers were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Cells were counted in five random fields under a light microscope. Each experiment was duplicated three times.
Wound Healing Assay
Cells were grown to 90% confluence on 6-well culture plates and were transfected with 100 nM miRNA NC or miR-27a-3p mimic and inhibitor. We Used a 10μL sterile pipette tip drew a straight line vertically at the center of each well. Images were captured immediately after wounding for indicated time points, and the healing was monitored by microscopy.
MiRNA Target Prediction and Luciferase Activity Assay
The miRNA target prediction and analysis were performed with the algorithms from miRWalk (http://mirwalk.umm.uni-heidelberg.de/), TargetScan (http://www.targetscan.org/vert_72/), PITA (http://genie.weizmann.ac.il/pubs/mir07/mir07_data.html) and RNA22 (https://cm.jefferson.edu/rna22/). We cloned the wild-type (WT) GSK3β-3ʹ untranslated region (UTR) or the GSK3β-3ʹUTR with various miRNA-binding site mutations (Mut) into pGL3-Basic-Vector. Luciferase reporter assays were performed using a dual-specific luciferase assay kit (Promega, USA) by following manufacturer’s protocol. The reporters were transfected into cells using Lipofectamine 3000 (Invitrogen, USA). The ratio of F-luc/R-luc was calculated to analyze its expression level. Experiments were performed in triplicates.
Western Blotting Analysis
Total cell lysates were prepared with a detergent lysis buffer. Western blots were performed as instructions. In short, cellular proteins were separated by 10% SDS-PAGE, transferred to polyvinylidene difluoride membranes, and were probed with antibodies against GSK3β (1:2000, Abcam), β-Catenin, p-β-Catenin (1:2000, Abcam), C-Myc (1:2000, Sigma) and Vimentin (1:1000, CST). Horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse antibody (1:1000, Abcam) were used as a secondary antibody. GAPDH protein levels were determined by using the specific antibody (1:5000, Abcam) as a loading control for cells, ECL reagent (Tanon, China) was used to visualize the protein bands.
Statistical Analysis
All data were displayed from three independent experiments in triplicate. The data represent the mean ± SD unless otherwise indicated. Data were analyzed by two-tailed unpaired Student’s t-test between two groups and by One-Way ANOVA followed by Bonferroni test for multiple comparisons. Significance was considered P < 0.05. Statistical analyses were performed using GraphPad PRISM (version 8.0; Graph Pad Software).
Results
Upregulation of miR-27a-3p in TNBC Cells
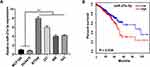
MiR-27a-3p is upregulated in TNBC cell lines. To determine the expression patterns of miR-27a-3p in TNBC cells, we analyzed the expression of miR-27a-3p in four TNBC cell lines: BT-549 (BT549), MDA-MB-231 (231), MDA-MB-468 (468), MDA-MB-453 (453), and normal human breast epithelial cell lines MCF-10A and DU4475 by Western blotting analysis. QRT-PCR results confirmed that the expression level of miR-27a-3p in all four TNBC cell lines was significantly increased than that in MCF-10A and DU4475 (P<0.05; Figure 1A). Furthermore, analysis of TCGA survival data revealed that patients with high miR-27a-3p expression were associated with poor overall survival (Figure 1B). In total, miR-27a-3p might be associated with TNBC progression.
Effects of miR-27a-3p Expression on Cell Proliferation and Migration of TNBC
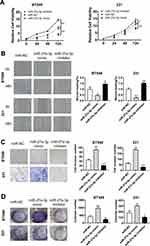
We confirmed the upregulation of miR-27a-3p expression in TNBC cells compared with normal human breast epithelial cells and supposed that miR-27a-3p may play an oncogenic role in TNBC. Therefore, we further explored the effects of miR-27a-3p on proliferation and migration of TNBC cells, we transfected miRNA NC (miR-NC), miR-27a-3p mimic, and miR-27a-3p inhibitor into the BT549 and 231 cell lines. The CCK-8 assay was used to measure cell proliferation and results showed that BT549 and 231 cell lines obviously increased the cell proliferation in miR-27a-3p mimic group compared to the miR-NC group. Meanwhile the proliferation of BT549 cells and 231 cell lines after being transfected with miR-27a-3p inhibitor was inversely suppressed compared with the miR-NC group (Figure 2A). Then, we further investigated the effects of miR-27a-3p on cell migration by wound healing assay and Transwell. Wound healing assay displayed that miR-27a-3p mimic obviously promoted wound closure, compared with miR-NC in BT549 and 231 cell lines (Figure 2B). Transwell migration assays showed that miR-27a-3p mimic greatly promoted migration in BT549 cells, compared with that of the miR-NC, while inhibiting miR-27a-3p expression decreased the cell’s migration ability compared to the miR-NC groups (Figure 2C). Similar trends were also observed in 231 cells. In addition, colony formation assay provided that the number of cell colonies in the miR-27a-3p mimic group was significantly higher than that in the miR-NC group, but the miR-27a-3p inhibitor groups presented the converse results (Figure 2D). All these results indicated that miR-27a-3p can promote cell proliferation and migration in TNBC cells.
GSK3β is a Direct Target of miR-27a-3p in TNBC Cells
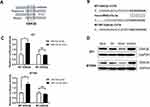
To identify novel miR-27a-3p target genes, we queried the different published prediction databases, including miRDB, miRWalk, PITA, TargetScan. Intriguingly, we identified a novel potential candidate as GSK3β, which was predicted in on four databases (Figure 3A). To support our hypothesis that miR-27a-3p directly regulates GSK3β expression through its 3ʹ-untranslated region (UTR), we generated luciferase reporter plasmids, which harbored either WT or mutated-type (MT) miR-27a-3p binding sites within the 3ʹ-UTR of GSK3β (Figure 3B). The dual-luciferase reporter assay showed that miR-27a-3p inhibitor can notably augment luciferase activity in cells with WT-GSK3β transfection, but did not affect those with MT-GSK3β transfection (Figure 3C). What’s more, miR-27a-3p inhibitor also significantly fortified luciferase activity with WT-GSK3β transfection rather than MT-GSK3β group in 231 and BT549 cell lines, suggesting that miR-27a-3p targets GSK3β via binding its 3ʹUTR. In addition, we overexpressed or inhibited miR-27a-3p in 231 and BT549 cell lines by using miR-27a-3p mimics or inhibitors. It is profoundly shown that GSK3β was consistently downregulated in miR-27a-3p overexpressing cells while upregulated in miR-27a-3p inhibition cells, suggesting GSK3β was a direct targeted gene of miR-27a-3p (Figure 3D). Taken together, we verified miR-27a-3p as a novel regulator of GSK3β through binding its transcript.
MiR-27a-3p Overexpression Inactivates the Wnt/β-Catenin Signaling Pathway
GSK3β has been reported to function as a negative regulator of Wnt/β-catenin pathway and phosphorylation of β-catenin is necessary for signal activation. Further, we detected the related genes of Wnt/β-catenin signaling pathway in BT549 and 231 cells after transfection with miR-NC, miR-27a-3p mimic, and miR-27a-3p inhibitor for 24 h by Western blotting, which was used to verify whether miR-27a-3p inactivate the pathway or not. Expectedly, it showed that the expression of C-Myc, Vimentin, and β-Catenin was totally increased and GSK3β, p-β-Catenin was decreased with miR-27a-3p mimic treatment in both 231 and BT-549 cells (Figure 4). Thus, these results indicated that miR-27a-3p regulates negatively Wnt/β-catenin signal activation in TNBC cells.
Discussion
TNBC is a commonsense health burden in Asia, as the pathogenesis process is very complex, which results in the understanding of TNBC progression remains limited.23 Despite advances in the treatment of TNBC in recent years, its prognosis has limitedly improved. In the past years, extensive studies have revealed miRNAs are critical regulators in TNBC progression.24,25 Aberrant expression of miRNAs may be used as prognosis prediction markers of therapeutic targets for TNBC.
It is well known that miRNAs can negatively regulate gene expression to perform various biological and pathological functions in posttranscriptional stage. Both GSK-3β and β-catenin are crucial regulators of Wnt/β-catenin signaling implicated in the progress of various tumors. Previous studies have pointed out that miR-27a-3p was recognized as a tumor-promoting miRNA in gastric cancer by targeting BTG2 or colorectal cancer by BTG1; it acts as a tumor suppressor in non-small lung cancer via targeting HOXB8.26,27 However, miR-27a-3p participates in the progression of TNBC remains unknown. In our current study, we revealed that miR-27a-3p overexpression profoundly promoted proliferation and migration in BT549 and 231 cell lines. Conversely, miR-27a-3p knockdown inhibited the proliferation and migration of 231 and BT549 cell lines. In addition, our data suggested that higher miR-27a-3p expression associated with shorter overall survival in patients with TNBC. Similarly, the relative expression of miR-27a-3p was higher in colon cancer patients compared to that in control ones.27 Thus, it is necessary to discover the target involved in the oncogenic role of miR-27a-3p in TNBC.
In the present study, we predicted the candidate target genes for miR-27a-3p using miRWalk, TargetScan, PITA, and RNA22 platform. A given miRNA can target numerous genes. The tumor suppressor GSK3β tremendously attracted our attention. Further investigation found that miR-27a-3p inversely regulated the expression of GSK3β protein in TNBC cells. Luciferase reporter assay demonstrated that miR-27a-3p directly regulated GSK3β expression via binding to 3ʹUTR. GSK3β functions as a tumor suppressor via regulating cell proliferation and cell cycle progression in colorectal cancer.28,29 Ectopic expression of GSK3β suppresses lung cancer cell proliferation, migration, and invasion.30 The absence of GSK3β breaks genomic stability to promote tumorigenesis and tumor progression.30 A previous study also showed that miR-27a-3p regulates proliferation and apoptosis of colon cancer cells by potentially targeting BTG1.27 Currently, the biological role of GSK3β in TNBC is still unexplored. In our study, we demonstrated that GSK3β functioned as a novel tumor-suppressor gene in TNBC cells. More importantly, GSK3β silencing partially abolished miR-27a-3p knockdown induced inhibitory effects on TNBC cell growth. Altogether, our work suggested that miR-27a-3p contributed to TNBC progression possibly by attenuating GSK3β.
The Wnt/β-catenin signaling pathway is involved in cancer development.31 The Wnt/β-catenin signaling pathway activation has been shown to promote cell proliferation, invasion, and metastasis in nasopharyngeal carcinoma.32,33 The activation has also been shown to contribute to the maintenance of cancer stem cells in hepatocellular carcinoma.34,35 Our results also showed that GSK3β suppression by miR-27a-3p triggered the Wnt/β-catenin signaling pathway leading to TNBC proliferation and migration, and its downstream mechanisms need further investigation.
Conclusion
We found that miR-27a-3p expression was elevated in TNBC cell lines and higher miR-27a-3p expression associated with prognosis in patients with TNBC. Furthermore, our research shows that miR-27a-3p promoted cell proliferation and migration by inhibiting expression of GSK3β and activating Wnt/β-catenin signaling pathway. These observations suggested that miR-27a-3p may be a potential target of TNBC treatment.
Disclosure
The authors declare that they have no competing interests.
References
1. Harbeck N, Penault-Llorca F, Cortes J, et al. Breast cancer. Nat Rev Dis Primers. 2019;5(1):67. doi:10.1038/s41572-019-0122-z
2. Wurstlein R, Hesse A, Konig A, et al. Tastbefund an der Brust: Auch bei Männern immer abklären. [Breast cancer in male]. MMW Fortschr Med. 2017;159(21–22):67–72. doi:10.1007/s15006-017-0395-7
3. Zhao S, Zuo WJ, Shao ZM, Jiang YZ. Molecular subtypes and precision treatment of triple-negative breast cancer. Ann Transl Med. 2020;8(7):499. doi:10.21037/atm.2020.03.194
4. Bareche Y, Buisseret L, Gruosso T, et al. Unraveling triple-negative breast cancer tumor microenvironment heterogeneity: towards an optimized treatment approach. J Natl Cancer Inst. 2019. doi:10.1093/jnci/djz208
5. Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–4434. doi:10.1158/1078-0432.CCR-06-3045
6. Bao C, Lu Y, Chen J, et al. Exploring specific prognostic biomarkers in triple-negative breast cancer. Cell Death Dis. 2019;10(11):807. doi:10.1038/s41419-019-2043-x
7. Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. doi:10.1038/nature02871
8. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi:10.1016/j.cell.2009.01.002
9. Vonkova B, Blahakova I, Hruban L, Janku P, Pospisilova S. MicroRNA-210 expression during childbirth and postpartum as a potential biomarker of acute fetal hypoxia. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2019;163(3):259–264. doi:10.5507/bp.2018.075
10. Aigner A. MicroRNAs (miRNAs) in cancer invasion and metastasis: therapeutic approaches based on metastasis-related miRNAs. J Mol Med. 2011;89(5):445–457. doi:10.1007/s00109-010-0716-0
11. Garrigos C, Molina-Pinelo S, Espinosa M, et al. MicroRNAs (miRNAs) as predictors of extreme response to tyrosine kinase inhibitors in renal cell cancer (RCC). J Clin Oncol. 2017;35.
12. Hao J, Lai MH, Liu CS. Expression of miR-335 in triple-negative breast cancer and its effect on chemosensitivity. J BUON. 2019;24(4):1526–1531.
13. Jia H, Sang MX, Liu F, Ai N, Geng CZ. miR-124 regulates EMT based on ZEB2 target to inhibit invasion and metastasis in triple-negative breast cancer. Pathol Res Pract. 2019;215(4):697–704. doi:10.1016/j.prp.2018.12.039
14. Menbari MN, Rahimi K, Ahmadi A, et al. miR-483-3p suppresses the proliferation and progression of human triple negative breast cancer cells by targeting the HDAC8 oncogene. J Cell Physiol. 2019.
15. Mokhlis HA, Kahraman N, Baydogan S, et al. MiR-873 is the master regulator of autophagy genes through a novel negative feedback mechanism mediated by Elongation factor 2 kinase (eEF-2K) and suppresses tumor growth and progression of triple negative breast cancer. Cancer Res. 2019;79(13).
16. Sugita BM, Zabala Y, Fonseca A, et al. The oncogenic role of miR-150-5p in triple-negative breast cancer. Cancer Res. 2017;77.
17. Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–999. doi:10.1016/j.cell.2017.05.016
18. Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: update on effectors and inhibitors. Cancer Treat Rev. 2018;62:50–60. doi:10.1016/j.ctrv.2017.11.002
19. Benajiba L, Alexe G, Su A, et al. Creatine kinase pathway inhibition alters GSK3 and WNT signaling in EVI1-positive AML. Leukemia. 2019;33(3):800–804. doi:10.1038/s41375-018-0291-x
20. Taelman VF, Dobrowolski R, Plouhinec JL, et al. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143(7):1136–1148. doi:10.1016/j.cell.2010.11.034
21. Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther. 2015;148:114–131. doi:10.1016/j.pharmthera.2014.11.016
22. Aparicio IM, Garcia-Herreros M, Fair T, Lonergan P. Identification and regulation of glycogen synthase kinase-3 during bovine embryo development. Reproduction. 2010;140(1):83–92. doi:10.1530/REP-10-0040
23. Shi Y, Jin J, Ji W, Guan X. Therapeutic landscape in mutational triple negative breast cancer. Mol Cancer. 2018;17(1):99. doi:10.1186/s12943-018-0850-9
24. Lee A, Djamgoz MBA. Triple negative breast cancer: emerging therapeutic modalities and novel combination therapies. Cancer Treat Rev. 2018;62:110–122. doi:10.1016/j.ctrv.2017.11.003
25. Naorem LD, Muthaiyan M, Venkatesan A. Identification of dysregulated miRNAs in triple negative breast cancer: a meta-analysis approach. J Cell Physiol. 2019;234(7):11768–11779. doi:10.1002/jcp.27839
26. Li L, Luo Z. Dysregulated miR-27a-3p promotes nasopharyngeal carcinoma cell proliferation and migration by targeting Mapk10. Oncol Rep. 2017;37(5):2679–2687. doi:10.3892/or.2017.5544
27. Su C, Huang DP, Liu JW, Liu WY, Cao YO. miR-27a-3p regulates proliferation and apoptosis of colon cancer cells by potentially targeting BTG1. Oncol Lett. 2019;18(3):2825–2834. doi:10.3892/ol.2019.10629
28. Zhao J, Ou B, Han D, et al. Tumor-derived CXCL5 promotes human colorectal cancer metastasis through activation of the ERK/Elk-1/Snail and AKT/GSK3beta/beta-catenin pathways. Mol Cancer. 2017;16(1):70. doi:10.1186/s12943-017-0629-4
29. Jung JH, Jung DB, Kim H, et al. Zinc finger protein 746 promotes colorectal cancer progression via c-Myc stability mediated by glycogen synthase kinase 3beta and F-box and WD repeat domain-containing 7. Oncogene. 2018;37(27):3715–3728. doi:10.1038/s41388-018-0225-0
30. Park SA, Lee JW, Herbst RS, Koo JS. GSK-3alpha is a novel target of CREB and CREB-GSK-3alpha signaling participates in cell viability in lung cancer. PLoS One. 2016;11(4):e0153075. doi:10.1371/journal.pone.0153075
31. Arend RC, Londono-Joshi AI, Straughn JM
32. Liu SL, Lin HX, Lin CY, et al. TIMELESS confers cisplatin resistance in nasopharyngeal carcinoma by activating the Wnt/beta-catenin signaling pathway and promoting the epithelial mesenchymal transition. Cancer Lett. 2017;402:117–130. doi:10.1016/j.canlet.2017.05.022
33. Cai CF, Ye GD, Shen DY, et al. Chibby suppresses aerobic glycolysis and proliferation of nasopharyngeal carcinoma via the Wnt/beta-catenin-Lin28/let7-PDK1 cascade. J Exp Clin Cancer Res. 2018;37(1):104. doi:10.1186/s13046-018-0769-4
34. Vilchez V, Turcios L, Marti F, Gedaly R. Targeting Wnt/beta-catenin pathway in hepatocellular carcinoma treatment. World J Gastroenterol. 2016;22(2):823–832. doi:10.3748/wjg.v22.i2.823
35. Chen J, Rajasekaran M, Hui KM. Atypical regulators of Wnt/beta-catenin signaling as potential therapeutic targets in hepatocellular carcinoma. Exp Biol Med (Maywood). 2017;242(11):1142–1149. doi:10.1177/1535370217705865
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.