Back to Journals » Cancer Management and Research » Volume 12
MiR-218-5p Suppresses the Progression of Retinoblastoma Through Targeting NACC1 and Inhibiting the AKT/mTOR Signaling Pathway
Received 15 January 2020
Accepted for publication 17 June 2020
Published 6 August 2020 Volume 2020:12 Pages 6959—6967
DOI https://doi.org/10.2147/CMAR.S246142
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Li Li, Hua Yu, Qian Ren
Department of Ophthalmology, The First Hospital of Shijiazhuang, Shijiazhuang, Hebei, People’s Republic of China
Correspondence: Qian Ren
Department of Ophthalmology, The First Hospital of Shijiazhuang, No. 36 Fanxi Road, Shijiazhuang, Hebei, People’s Republic of China
Email [email protected]
Introduction: MicroRNA-218-5p (miR-218-5p) was involved in the progression of multiple tumors as a tumor suppressor miRNA. Its specific role on human retinoblastoma (RB) cells remains unknown.
Methods: We constructed the miR-218-5p overexpression and knockdown cells to detect their role on RB cell line WERI-Rb-1, and we analyzed its binding sites on TargetScan. CCK8 and clonogenic assays were performed to detect cell viability. Flow cytometry was used for the detection of cell apoptosis.
Results: Our results showed that the miR-218-5p inhibitor enhanced cell viability and blocked the apoptosis in RB cells. The AKT/mTOR signaling pathway was also inhibited by the miR-218-5p inhibitor. MiR-218-5p mimics lead to diametrically opposite results. Nucleus accumbens-associated 1 (NAC1) encoded by the NACC1 gene is involved in the regulation of many biological functions, including gene transcription, protein degradation of ubiquitin pathway, cell viability, and apoptosis. In this research, dataset analysis suggested that NACC1 might be a downstream target of miR-218-5p. Then, qPCR and Western blot analysis proved that miR-218-5p inhibited the expression of NACC1 in RB cells. NACC1 could promote cell viability and inhibit the apoptosis by activating the AKT/mTOR signaling pathway. MiR-218-5p mimics blocked the enhancement of cell growth induced by NACC1 overexpression as well as the activation of the AKT/mTOR signaling pathway in RB cells.
Discussion: MiR-218-5p inhibited cell growth by targeting NACC1 and suppressing the AKT/mTOR signaling pathway. MiR-218-5p/NACC1/AKT/mTOR might be a new target axis for the clinical treatment strategy.
Keywords: retinoblastoma, miR-218-5p, NACC1, apoptosis, signaling pathway
Introduction
Retinoblastoma (RB) is the most common intraocular malignant tumor in infants and young children, and its incidence has increased in recent years.1 RB has attracted widespread attention in the medical community due to its unique genetic laws, multi-directional differentiation potential, and high rate of degeneration.
MicroRNA-218-5p (miR-218-5p) is an important tumor suppressor miRNA. Its abnormal expression can be observed in multiple solid tumors and precancerous lesions, including lung cancer,2 breast cancer,3 gallbladder cancer,4 colorectal cancer5 and gastric cancer.6 However, its role in RB has not been reported.
Nucleus accumbens-associated 1 (NAC1) is a Broad-complex, Tramtrack, and Bric-a-brac/poxvirus and zinc finger (BTB/POZ) domain protein, encoded by the NACC1 gene. Earlier research has found that NACC1 is highly expressed in ovarian cancer.7 Highly expressed NACC1 is closely related to cell viability, migration, and tumor recurrence. Many studies have now found that NACC1 is highly expressed in various tumors, such as cervical cancer,8 endometrial cancer,9 ovarian cancer,10 and melanoma.11 NACC1 not only regulates the tumorigenesis and development but also affects the therapeutic effect of chemotherapeutic drugs, causing a chemical resistance.12–15 NACC1 knockdown inhibited the cisplatin-induced autophagy, resulting in increased cisplatin cytotoxicity.13 NAC1 mediates cisplatin resistance by altering the High-mobility group box 1 (HMGB1)-mediated autophagic response in ovarian cancer.12 In another report, NAC1 contributes to paclitaxel resistance in ovarian cancer through inactivating the growth arrest and DNA damage-inducible 45 (GADD45) pathway.9 In the present research, we found that miR-218-5p inhibited the expression of NAC1, and we studied their function in the RB cell line.
Materials and Methods
Cell Line and Transfection
Human RB cell line WERI-Rb-1 was purchased from the Cell Resource Center, Shanghai Institute for Biological Sciences, the Chinese Academy of Sciences. WERI-Rb-1 cells were maintained in DMEM with 10% fetal bovine serum at 37°C with 5% CO2. Cells were transfected with miR-218-5p mimics or inhibitors, or NACC1 expression plasmid (NACC1 overexpression, NACC1-OE), or shRNA (NACC1 knockdown, NACC1-KD) in a 6-well plate during the exponential growth phase. Untreated WERI-Rb-1 was used as the negative control (NC) using lipofectamine 2000 (Thermo Fisher Scientific, USA). MiR-218-5p mimics, miR-218-5p inhibitor, NACC1 expression plasmid (pcDNA3.1-NACC1) and NACC1-siRNA were all purchased from Ribobio (RiboBio Co. Ltd., China). The following sequences were used in this study, miR-218-5p mimics (5ʹ-ACAUGGUUAGAUCAAGCACAA-3ʹ), miR-218-5p inhibitor (5ʹ-CCAUGUGGUUGCGAGGUAUGA-3ʹ) and NACC1-siRNA (5ʹ-CAGTGAAACGGGCAAGATCGAGC-3ʹ).
Cell Viability Assay
CCK8 and clonogenic assays were performed to detect the viability of WERI-Rb-1 cells. For CCK8 assay, cells (1000 cells/well) were seeded into the 96-well plate after transfection. Ten microlitres of CCK8 reagent (Promega Corporation) was added into the target well every 24 hours. The optical density (OD) value was measured in the spectrophotometer.
For clonogenic assay, cells (1000 cells/well) were seeded into the 6-well plate after transfection. After being allowed to culture for 2 weeks, plates were fixed with 4% paraformaldehyde for 15 minutes, stained with Giemsa for 15 minutes, and then observed under a microscope. Colony numbers in 5 random fields under each well were counted for further statistical analysis.
Quantitative Real-Time RT-PCR Assay (qPCR)
After the transfection for 24 hours, total RNA was extracted using TRIzol reagent (Invitrogen, USA) and reverse transcribed to cDNA using a HiFiScript cDNA Synthesis Kit (CoWin Biotech Co. Ltd., China). MiRNA was extracted using an miRNA Purification Kit (CoWin Biotech Co. Ltd., China) and reverse transcribed to cDNA using an miRNA cDNA Synthesis Kit (CoWin Biotech Co. Ltd., China). UltraSYBR Mixture and miRNA qPCR Assay Kit (CoWin Biotech Co. Ltd., China) were used for qPCR according to the manufacturer’s protocol. The following primers were used in this study, miR-218-5p (5ʹ-TTGTGCTTGATCTAACCATGT-3ʹ), NACC1-Forward (5ʹ-GCCTGTACTGTGACGTGTCA-3ʹ) and NACC1-Reverse (5ʹ-CGTGGAGAACTTGGCCATCT-3ʹ). The relative expressions of genes were calculated using the 2−ΔΔCt method.
Western Blot
After the transfection for 48 hours, total protein was extracted from cells using a RIPA buffer. Protein concentration was determined by the BCA method. Protein (20 μg) from each group was loaded on the lane and separated with SDS-PAGE. Then, the protein was transferred to a PVDF membrane. After blocking in 5% non-fat milk for 1 hour, the membrane was incubated with primary antibodies at room temperature for 1 hour followed by secondary antibodies at room temperature for 1 hour. The cECL Western Blot Kit (CoWin Biotech Co. Ltd., China) was used for substrate luminescence. GDPDH was used as the internal reference gene. ImageJ software was used for analysis of protein levels.
Flow Cytometry Analysis
After transfection for 24 hours, the cell concentration was adjusted to 1 × 106/mL with Annexin V binding buffer. Apoptosis was detected using an Annexin V-FITC-PI Apoptosis Detection Kit (Sigma, USA) according to the manufacturer’s protocol. Flowjo software was used for the analysis of the percentage of apoptotic cells. The Annexin channel and PI channel were set up in the horizontal and vertical axes, respectively, and the double negative cell group was selected. In the double-negative cell gate, set the horizontal axis and the vertical axis to FCS and SSC, respectively, and reverse select the fragment gate to get all cells. The horizontal axis and the vertical axis were set as Annexin channel and PI channel, respectively, and the cross gate is drawn to determine the cell proportion.
Statistical Analyses
All data were presented as Mean ± Standard Deviation. Statistical significance between two groups was determined using one-way ANOVA. All experiments were performed 3 times independently. P<0.05 was statistically significant.
Results
MiR-218-5p Inhibited the Viability in RB Cells
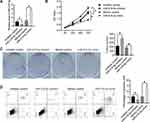
To investigate the effect of miR-218-5p on RB, we transfected miR-218-5p inhibitor (miR-218-5p specific siRNA) or mimics into the RB cell line WERI-Rb-1 with the nonspecific siRNA or mimics as negative control. From the result of qPCR, miR-218-5p level was significantly suppressed by miR-218-5p inhibitor compared with inhibitor control and enhanced by miR-218-5p mimics compared with mimics control (Figure 1A). Then, the viability of each group was detected by CCK8 and clonogenic assay. As shown in Figure 1B, the OD value of WERI-Rb-1 cells increased markedly after the transfection of miR-218-5p inhibitor for 72 hours and these decreased after the transfection of miR-218-5p mimics for 72 hours. Clonal formation experiment offered consistent results. The colony number of miR-218-5p inhibitor increased significantly compared with the inhibitor control group, while the colony number of miR-218-5p mimics decreased significantly compared with the mimics control group (Figure 1C). These results proved that miR-218-5p inhibited cell viability of WERI-Rb-1.
MiR-218-5p Induced the Apoptosis in RB Cells
After the transfection for 24 hours, cell apoptosis was analyzed using flow cytometry. As shown in Figure 1D, the percentage of apoptotic cells declined after the transfection of miR-218-5p inhibitor compared with the inhibitor control group and rose after the transfection of miR-218-5p mimics compared with the mimics control group. This data indicated that miR-218-5p induced the apoptosis of WERI-Rb-1 cells.
MiR-218-5p Inhibited the Activation of the AKT/mTOR Signaling Pathway
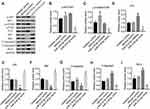
Previous studies have shown that miR-218 regulated cell viability and apoptosis by targeting the PI3K/AKT/mTOR signaling pathway in several human tumors, including hepatocellular carcinoma,16 esophageal squamous cell carcinoma,17 colon cancer,18 oral cancer,19 prostate cancer4 and cervical cancer.20 To confirm the mechanism by which miR-218 inhibited the growth of WERI-Rb-1 cells, we detected the critical protein of the AKT/mTOR signaling pathway. As shown in Figure 2, the miR-218-5p inhibitor significantly increased the phosphorylation of p-AKT/AKT and p-mTOR and the level of P70; the miR-218-5p inhibitor also reduced the levels of p53, Bax, and C-Caspasse3 but enhanced the levels of T-caspase-3 and Bcl-2. MiR-218-5p mimics the opposite effect. The results prove that miR-218-5p suppressed cell growth by inhibiting the activation of the AKT/mTOR signaling pathway in RB cells.
MiR-218-5p Inhibited the Expression of NACC1 via Binding to the 3ʹ-Untranslated Region (3ʹUTR) of NACC1
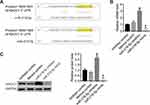
To further investigate the mechanism of miR-218-5p function, we analyzed the binding site of miR-218-5p on the TargetScan dataset.21 The results showed that there are two binding sites between the 3ʹ region of NACC1 and miR-218-5p (Figure 3A). Then, we detected the effect of miR-218-5p on the regulation of NACC1 expression. As shown in Figure 3B and C, the expression of NACC1 increased significantly after the transfection of the miR-218-5p inhibitor and decreased significantly after the transfection of miR-218-5p mimics, thereby suggesting that miR-218-5p inhibited NACC1 expression in WERI-Rb-1 cells. We hypothesized that miR-218-5p could inhibit the growth of tumor cells through targeting NACC1 in RB cells.
NACC1 Promoted Cell Viability and Inhibited the Apoptosis in RB Cells
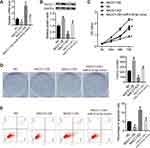
To study the specific effect of NACC1 on RB cells, we constructed NACC1 knockdown and overexpressed cell lines by transfection of shRNA or overexpression plasma in WERI-Rb-1 cells. The results of qPCR and Western blot proved that NACC1 expression was suppressed by miR-218-5p inhibitor and enhanced by miR-218-5p mimics (Figure 4A and B). Then, cell viability was determined by CCK8 and clonogenic assay. The OD value of WERI-Rb-1 cells increased in the NACC1-OE group and declined in the NACC1-KD group compared with the NC group (Figure 4C). Similar results were observed in clonogenic assay. The colony number of NACC1-OE group increased, but colony number of the NACC1-KD group decreased compared with NC cells (Figure 4D). Cell apoptosis was analyzed using flow cytometry. As shown in Figure 4E, the percentage of apoptotic cells reduced after the overexpression of NACC1, but increased after the knockdown of NACC1. This data proved that NACC1 could promote cell viability and inhibit the apoptosis in RB cells.
NACC1 Activated the AKT/mTOR Signaling Pathway in RB Cells
Since NACC1 was the target of miR-218-5p, we further investigated its effect on AKT/mTOR signaling pathway. From the results of Western blot, we found that NACC1 overexpression could activate the phosphorylation of p-AKT/AKT and p-mTOR and increase the level of P70, T-caspase-3, and Bcl-2, while decreasing the level of p53, Bax, and C-Caspasse3. NACC1 knockdown showed the opposite effect. Our results suggested that NACC1 promoted cell growth by activating the AKT/mTOR signaling pathway in RB cells.
MiR-218-5p/NACC1/AKT/mTOR Inhibited Cell Growth in Human RB
Then, we verified the function axis of miR-218-5p/NACC1/AKT/mTOR by overexpression of both NACC1 and miR-218-5p in WERI-Rb-1 cells (NACC1-OE+ miR-218-5p mimics). As shown in Figure 4, overexpression of miR-218-5p significantly blocked the increase of cell viability induced by NACC1 overexpression as well as the inhibition of cell apoptosis induced by NACC1 overexpression. In addition, the activation of the AKT/mTOR signaling pathway induced by NACC1-OE was inhibited by miR-218-5p mimics (Figure 5).
Discussion
MiRNAs are single-stranded small non-coding RNAs with a length of 21 to 24 nucleotides.22 They bind to the 3ʹ-untranslated region (3ʹ-UTR) of the target gene mRNA completely or incompletely to promote the degradation of the mRNA and/or inhibit the translation process.23 Numerous studies have shown that miRNAs are involved in tumorigenesis and development through regulation of multiple target genes and signaling pathways.24 In recent years, it has been found that miR-218-5p can participate in tumor progression through multiple signaling pathways. MiR-218-5p was found to exert an anti-tumor effect through binding to EGFR in non-small cell lung cancer both in vitro and in vivo.20 MiR-218-5p can bind to the 3ʹ-UTR of PRKCE, reduce PRKCE expression, and promote sensitivity to gemcitabine in gallbladder cancer.4 In addition, miR-218-5p was also significantly downregulated in liver cancer,16 prostate cancer,18 gastric cancer17 and gastrointestinal stromal tumor.19
In order to further investigate the role of miR-218-5p in the pathogenesis of RB and its effect on the biological behavior of RB cells, the synthetic miR-218-5p mimics and inhibitors were transfected into RB WERI-Rb-1 cells. From the results of CCK8 and clonogenic assays, we found that miR-218-5p inhibited the viability in WERI-Rb-1 cells. Flow cytometry analysis showed that miR-218-5p promoted apoptosis in WERI-Rb-1 cells. Therefore, we speculated that miR-218-5p might play an anti-cancer role by regulating the viability and apoptosis of RB cells. To clarify the mechanism by which miR-218-5p inhibited tumor progression, the crucial protein levels of the AKT/mTOR signaling pathway were detected by Western blot. MiR-218-5p could inhibit the phosphorylation of AKT and mTOR and also regulate the downstream protein expression involved in the viability and apoptosis in WERI-Rb-1 cells.
MiRNAs can affect the occurrence and development of tumors by targeting the oncogenes or tumor suppressor genes. At present, miR-218-5p has been found to have multiple target genes, which distribute in different signaling pathways.2,4,17,25-27 Through the analysis of the online target gene prediction software TargetScan, we found that NACC1 may be the downstream target of miR-218-5p. qPCR and Western blot assays proved that miR-218-5p inhibited the expression of NACC1 in RB cells. We further constructed NACC1 knockdown and overexpression cell lines. Cell functional analysis proved that NACC1 promoted viability and inhibited apoptosis in RB cells. In addition, miR-218-5p mimics could block the cell growth induced by NACC1 overexpression, indicating a functional axis of miR-218-5p/NACC1/AKT/mTOR in RB progression.
The protein encoded by NACC1 is a member of the BTB/POZ family.28 The highly conserved BTB/POZ homeodomain is necessary for the formation of the NACC1 dimer complex, which is involved in a variety of biological functions, including gene transcription regulation, ubiquitin pathway protein degradation, cell morphology, proliferation, and apoptosis.29,30 In recent years, it has been found that NACC1 is overexpressed in a variety of tumors, those such as ovarian cancer,31 cervical cancer,8 endometrial cancer32 and breast cancer.9 In the present research, we proved that NACC1 promoted growth and metastasis in human RB cells. According to our results, mir-218-5p can inhibit the expression of NACC1, and then leads to the inhibition of Akt/mTOR signaling pathways activity, which can decrease the P70 level and the activation of caspase-3, and finally lead to the apoptosis and inhibition of cell activity. In conclusion, we proved that miR-218-5p inhibited cell viability and promoted apoptosis through targeting the NACC1 and AKT/mTOR signaling pathways. MiR-218-5p/NACC1/AKT/mTOR might be a new target axis for the clinical treatment strategy.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dada R, Basant Kumar S, Chawla B, Bisht S, Khan S. Oxidative stress induced damage to paternal genome and impact of meditation and yoga - can it reduce incidence of childhood cancer? Asian Pac J Cancer Prev. 2016;17(9):4517.
2. Zhu K, Ding H, Wang W, et al. Tumor-suppressive miR-218-5p inhibits cancer cell proliferation and migration via EGFR in non-small cell lung cancer. Oncotarget. 2014;7(19):28075–28085. doi:10.18632/oncotarget.8576
3. Taipaleenmäki H, Farina NH, van Wijnen AJ, et al. Antagonizing miR-218-5p attenuates Wnt signaling and reduces metastatic bone disease of triple negative breast cancer cells. Oncotarget. 2016;7(48):79032–79046. doi:10.18632/oncotarget.12593
4. Wang H, Zhan M, Xu S-W, et al. MiR-218-5p restores sensitivity to gemcitabine through PRKCE/MDR1 axis in gallbladder cancer. Cell Death Dis. 2017;8(5):e2770. doi:10.1038/cddis.2017.178
5. Liu M, Yin K, Guo X, et al. Diphthamide biosynthesis 1 is a novel oncogene in colorectal cancer cells and is regulated by MiR-218-5p. Cell Physiol Biochem. 2017;44:505–514.
6. Shi Y, Chen G-B, Huang Q-W, et al. miR218-5p regulates the proliferation of gastric cancer cells by targeting TFF1 in an Erk1/2-dependent manner. Biochim Biophys Acta. 2015;1852(5):970–979. doi:10.1016/j.bbadis.2015.01.016
7. Nakayama K, Nakayama N, Davidson B, et al. A BTB/POZ protein, NAC-1, is related to tumor recurrence and is essential for tumor growth and survival. Proc Natl Acad Sci U S A. 2007;103(49):18739–18744. doi:10.1073/pnas.0604083103
8. Yeasmin S, Nakayama K, Rahman MT, et al. Biological and clinical significance of NAC1 expression in cervical carcinomas: a comparative study between squamous cell carcinomas and adenocarcinomas/adenosquamous carcinomas. Human Pathol. 2012;43;4:0–519.
9. Ishikawa M, Nakayama K, Yeasmin S, et al.NAC1, a potential stem cell pluripotency factor expression in normal endometrium, endometrial hyperplasia and endometrial carcinoma. Int J Oncol. 2010;36(5):1091–103.
10. Nakayama N, Sakashita G, Nariai Y, et al. Cancer-related transcription regulator protein NAC1 forms a protein complex with CARM1 for ovarian cancer progression. Oncotarget. 2018;9(47):28408–28420. doi:10.18632/oncotarget.25400
11. Jiao H, Jiang S, Wang H, Li Y, Zhang W. Upregulation of LINC00963 facilitates melanoma progression through miR-608/NACC1 pathway and predicts poor prognosis. Biochem Biophys Res Commun. 2018; 504(1):34–39.
12. Zhang Y, Cheng Y, Ren X, et al. NAC1 modulates sensitivity of ovarian cancer cells to cisplatin by altering the HMGB1-mediated autophagic response. Oncogene. 2012;31(8):1055–1064. doi:10.1038/onc.2011.290
13. Zhang Y, Cheng Y, Ren X, Yang JM. Abstract C63: identification of NAC1 as a novel regulator of autophagy: its implication in cisplatin resistance. Cancer Res. 2011;71(18 Supplement):C63.
14. Nakayama K, Rahman M, Rahman MT, et al. Nucleus accumbens-1/GADD45GIP1 axis mediates cisplatin resistance through cellular senescence in ovarian cancer. Oncol Lett. 2017;13(6):4713–4719.
15. Jinawath N, Vasoontara C, Yap KL, et al. NAC-1, a potential stem cell pluripotency factor, contributes to paclitaxel resistance in ovarian cancer through inactivating Gadd45 pathway. Oncogene. 2009;28(18):1941–1948.
16. Fu WM, Tang L-P, Zhu X, et al. MiR-218-targeting-Bmi-1 mediates the suppressive effect of 1,6,7-trihydroxyxanthone on liver cancer cells. Apoptosis. 2015;20(1):75–82. doi:10.1007/s10495-014-1047-3
17. Deng M, Zeng C, Lu X, et al. miR-218 suppresses gastric cancer cell cycle progression through the CDK6/Cyclin D1/E2F1 axis in a feedback loop. Cancer Lett. 2017;403(p):175–185. doi:10.1016/j.canlet.2017.06.006
18. Peng P, Chen T, Wang Q, et al. Decreased miR-218-5p levels as a serum biomarker in bone metastasis of prostate cancer.Oncol Res Treat. 2018;42(4):165–181.
19. Fan R, Zhong J, Zheng S, et al. MicroRNA-218 inhibits gastrointestinal stromal tumor cell and invasion by targeting KIT. Tumor Biol. 2014;35(5):4209–4217. doi:10.1007/s13277-013-1551-z
20. Zhu K, Ding H, Wang W, et al. Tumor-suppressive miR-218-5p inhibits cancer cell proliferation and migration via EGFR in non-small cell lung cancer. Oncotarget. 2016;7(19):28075–28085.
21. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are MicroRNA targets. Cell. 2005;120(1):0–20.
22. Sun JL, Wen B, Sun H, Chen G, Songqi HE. Research progress of relevance between miRNAs and hepatocellular carcinoma. Chin Pharmacol Bull. 2017;33(4):445–449.
23. Rouleau S, Glouzon JP, Brumwell A, Bisaillon M, Perreault JP. 3′ UTR G-quadruplexes regulate miRNA binding. Rna. 2017;23(8):1172.
24. Beijnum JRV, Giovannetti E, Poel D, et al. miRNAs: microManagers of anti-cancer combination therapies. Angiogenesis. 2017;20(2):269–285. doi:10.1007/s10456-017-9545-x
25. Tang S, Wang D, Zhang Q, Li L. miR-218 suppresses gastric cancer cell proliferation and invasion via regulation of angiopoietin-2. Exp Ther Med. 2016;12(6):3837–3842.
26. Na L, Wang L, Tan G, et al. MicroRNA-218 inhibits proliferation and invasion in ovarian cancer by targeting Runx2. Oncotarget. 2017;8(53):91530–91541. doi:10.18632/oncotarget.21069
27. Chen P, Zhao Y, Li Y. MiR-218 inhibits migration and invasion of lung cancer cell by regulating Robo1 expression. Chin J Lung Cancer. 2017;20(7):452–458.
28. Fedele M, Benvenuto G, Pero R, et al. A novel member of the BTB/POZ family, PATZ, associates with the RNF4 RING finger protein and acts as a transcriptional repressor. J Biol Chem. 2000;275(11):7894–7901.
29. Xie Q. Arabidopsis NAC1 transduces auxin signal downstream of TIR1 to promote lateral root development. Genes Dev. 2000;14(23):3024–3036. doi:10.1101/gad.852200
30. Yan R, He J, Wu W, et al. Nac1 promotes self-renewal of embryonic stem cells through direct transcriptional regulation of c-Myc. Oncotarget. 2017;8(29):47607–47618. doi:10.18632/oncotarget.17744
31. Nakayama K, Rahman MT, Rahman M, et al. Biological role and prognostic significance of NAC1 in ovarian cancer. Gynecol Oncol. 2010;119(3):469–478. doi:10.1016/j.ygyno.2010.08.031
32. Ishibashi M, Nakayama K, Yeasmin S, et al. Expression of a BTB/POZ protein, NAC1, is essential for the proliferation of normal cyclic endometrial glandular cells and is up-regulated by estrogen. Clin Cancer Res. 2009;15(3):804–811. doi:10.1158/1078-0432.CCR-08-2134
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.