Back to Journals » OncoTargets and Therapy » Volume 13
miR-214-3p Regulates Multi-Drug Resistance and Apoptosis in Retinoblastoma Cells by Targeting ABCB1 and XIAP
Authors Yang L, Zhang L, Lu L, Wang Y
Received 24 October 2019
Accepted for publication 17 December 2019
Published 28 January 2020 Volume 2020:13 Pages 803—811
DOI https://doi.org/10.2147/OTT.S235862
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
Lidong Yang, Liyou Zhang, Lu Lu, Yan Wang
Department of Ocular Fundus Disease, Cangzhou Eye Hospital, Cangzhou Central Hospital, Cangzhou 061001, People’s Republic of China
Correspondence: Lidong Yang; Lu Lu
Department of Ocular Fundus Disease, Cangzhou Eye Hospital, Cangzhou Central Hospital, #7-1 South Fuyang Avenue, Cangzhou 061001, People’s Republic of China
Email [email protected]; [email protected]
Background: MicroRNAs (miRNAs) have been shown to contribute to the initiation and progression of human cancer, including retinoblastoma. However, expression levels and potential roles of miRNAs in retinoblastoma remain largely unknown. In this study, we aimed to identify dysregulated miRNAs and explore their functional roles in the development of retinoblastoma.
Material and Methods: First, miRNA expression profiling in retinoblastoma tissues was performed via microarray analysis. To evaluate the involvement of miR-214-3p in multi-drug resistance, gain-of-function experiments were employed in vitro and in vivo. Bioinformatics analysis, luciferase reporter assay, qRT-PCR and Western blot were used to investigate the underlying mechanisms.
Results: Here, we identified 57 up-regulated and 34 down-regulated miRNAs. Among them, miR-214-3p was the most significantly decreased. We found that miR-214-3p level was positively correlated with clinical outcome and chemotherapy response. Overexpression of miR-214-3p significantly sensitized retinoblastoma cells to multiple chemodrugs and promoted cell apoptosis in vitro and in vivo. Further investigations revealed that miR-214-3p directly regulated ABCB1 and XIAP expression through interacting with the 3’ untranslated regions (3’UTRs). Pearson correlation analysis showed that miR-214-3p expression in retinoblastoma tissues was negatively correlated with ABCB1 and XIAP expression. We also observed that overexpression of ABCB1 or XIAP partly reversed the chemoresistance inhibition-induced by miR-214-3p overexpression.
Conclusion: Our data demonstrate that miR-214-3p functions as a tumor suppressor to inhibit the chemoresistance in retinoblastoma, suggesting that miR-214-3p might be potential diagnostic and therapeutic targets for retinoblastoma treatment.
Keywords: retinoblastoma, miR-214-3p, chemoresistance, ABCB1, XIAP
Introduction
MicroRNAs (miRNAs) are a class of small non-coding RNAs of 18–24 nucleotides. Increasing evidence has demonstrated that miRNAs are frequently dysregulated and involved in the initiation and progression of human cancer.1 Their expression levels can be used as biomarkers for diagnosis, prognosis and radiochemotherapy response.2 miRNAs can negatively regulate gene expression at the posttranscriptional level and thereby activate oncogenic pathways.3 Thus, understanding the expression profile and function of crucial miRNAs will help to elucidate tumor pathogenesis and develop novel therapeutic strategies for cancer treatment.
miR-214-3p was reported to be down-regulated in oral squamous cell carcinoma patients with poor prognosis and could be used as a useful biomarker.4 Phatak et al found that miR-214-3p enhanced sensitivity to cisplatin (DDP) of esophageal squamous cancer cells through targeting surviving.5 In endometrial carcinoma, breast cancer, lung cancer and hepatocellular carcinoma, miR-214-3p has been suggested to act as a tumor suppressor to inhibit tumorigenesis.6–9 However, several studies also showed that miR-214-3p expression level was significantly up-regulated in osteosarcoma and bladder cancer, and miR-214-3p overexpression promoted cell proliferation and metastasis.10,11 Taken together, these findings suggest a pivotal role of miR-214-3p in tumor pathogenesis, but its functions are complex in regard to different cancer types.
Retinoblastoma is the most common intraocular aggressive cancer of infants and children, and the mortality is approximately 70% in developing countries.12 Recently, abnormally expressed miRNAs were broadly implicated in retinoblastoma development.13–15 However, the biological role of miR-214-3p in retinoblastoma is still largely unclear. In this study, miRNA array analysis revealed that miR-214-3p was significantly down-regulated in retinoblastoma tissues. Furthermore, decreased miR-214-3p level was positively correlated with poor clinical outcome and chemotherapy response. Using gain-of-function assays in vitro and in vivo, we explored the biological functions of miR-214-3p and found that overexpression of miR-214-3p suppressed multi-drug resistance and promoted apoptosis of retinoblastoma cells. Taken together, our findings provide new insights into the chemotherapy of retinoblastoma and also suggest miR-214-3p as novel diagnostic biomarker and potential therapeutic target for retinoblastoma treatment.
Materials and Methods
Patients and Tissues
Fifty-six retinoblastoma tissues used in this study were obtained from retinoblastoma patients who underwent enucleation surgery in Cangzhou Central Hospital between 2013 and 2017. Fifteen age-matched normal retina tissues were donated by accidental death children. The Ethics Committee of the hospital granted approval of this study (CZCH-2017-0039) and written informed consent was obtained from each patient.
Cell Culture
Human retinoblastoma cell lines WERI-RB1, SO-RB50, Y79, and human retinal pigment epithelial cell line ARPE-19 were purchased from ATCC. Human embryonic kidney cell line HEK-293T cells were obtained from the Chinese Academy of Sciences Cell Bank (Shanghai, China). VCR-resistant SO-RB50 (SO-RB50/VCR) and CBP-resistant SO-RB50 (SO-RB50/CBP) cells were generated as previous study described.16
RNA Oligonucleotides and Cell Transfection
miR-214-3p mimics and miRNA negative control were synthesized by GenePharma (Shanghai, China). Full-length of ABCB1 and XIAP were cloned into pcDNA3.1 vector (Invitrogen) to generate ABCB1 and XIAP expression vectors, pcDNA-ABCB1 or pcDNA-XIAP. The wild type (wt) and mutant (mut) 3’UTR of ABCB1 and XIAP containing the predicted target sites of miR-214-3p were synthesized by GenePharma and then cloned into pmirGLO vector (Promega). The RNA oligonucleotides were transfected into cells using Lipofectamine 2000 (Invitrogen). At 48 hrs posttransfection, cells were harvested for next analysis.
qRT-PCR Assays
Total RNA was extracted from cells and tissues using TRIzol reagent (Invitrogen). cDNA of miRNA and mRNA was reverse transcribed using TaqMan miRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) and PrimeScript RT reagent Kit (TaKaRa, Dalian, China), respectively. All analyses were performed using the SYBR Premix Ex Taq II (TaKaRa) on an ABI PRISM 7500 Sequence Detection System (Applied Biosystems). The 2−ΔΔCt method was used to calculate relative expression.
Western Blot
Western blot analysis was performed according to a previous description.17 Primary antibodies included ABCB1, XIAP and β-actin (Cell Signaling Technology). Horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (1:5000, Beyotime) was applied as a secondary antibody. Signals were detected using chemiluminescence.
In vitro Drug Sensitivity Assay
Cells (5×103 cells/well) were seeded into 96-well plates. After cellular adhesion, cells were treated with different concentrations of vincristine (VCR, Solarbio, Beijing, China), carboplatin (CBP, Sigma), etoposide (VP-16), DDP (Sigma) and 5-fluorouracil (5-Fu, Sigma). After 48 hrs treatment, cell viability was assessed by MTT assay. The IC50 of each drug was evaluated and the resistance index was calculated as follows: Resistance index = IC50 (cell/resistant)/IC50 (cell/control).
Apoptosis Assay
Cells (4×105 cells/well) were seeded into 6-well plates. Forty-eight hours after the treatment of VCR or CBP, cells were collected and dual stained with 5 μL Annexin V and 5 μL propidium iodide for 30 min at room temperature. Flow cytometry was used to detect apoptosis (BD, San Diego, CA, USA).
Dual-Luciferase Activity Assay
HEK-293T cells (1×105 cells/well) were seeded into 24-well plates. After cellular adhesion, the wt or mut 3’UTR vector was transfected into HEK-293T cells together with miR-214-3p mimics or miRNA negative control. Luciferase activity was measured using the Dual Luciferase Reporter Assay System (Promega) after 48 hrs transfection.
miRNA Array Analysis
Total RNA was extracted and purified from 3 normal retina tissues and 3 retinoblastoma tissues using mirVana™ miRNA Isolation kit (Ambion; Thermo Fisher Scientific, Inc.). The samples were hybridized on Agilent human miRNA microarray v21 (Agilent Technologies, Santa Clara, CA, USA). Microarray data were normalized and analyzed using GeneSpring GX v.14.8 software (Agilent Technologies). The threshold set for differentially expressed miRNAs was P < 0.05 and fold-change ≥2.0.
Animal Experiments
Human miR-214-3p precursor was synthesized (GenePharma) and cloned into pSuper-GFP-Luc. SO-RB50 cell lines stably transfected with pSuper-GFP-Luc (pSuper) or pSuper-GFP-Luc-miR-214-3p (pSuper-miR-214-3p) were established by selection with G418 (Sigma) and then 5×106 cells were inoculated subcutaneously into 5-week-old female BALB/c mice (Shanghai, Chinese Academy of Sciences, China). Each experimental group included six mice. One week after inoculation, mice were injected intraperitoneally with VCR (1.0 mg/kg) or CBP (50 mg/kg) twice weekly. After 4 weeks treatment, the mice were sacrificed and the tumors were isolated and weighed. The animal experiments were approved by the Animal Ethics Committee of Cangzhou Central Hospital (CZCH-2018-0024). All experimental procedures and animal care were in accordance with the institutional ethics guidelines for animal experiments.
Statistical Analysis
All data are displayed as mean ± SD. Differences between two groups were assessed using Student’s t-test. A p value of <0.05 was considered to be statistically significant.
Results
miR-214-3p Is Down-Regulated in Retinoblastoma Tissues and Cell Lines
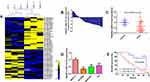
To identify dysregulated miRNAs involved in retinoblastoma development, miRNA array analysis was performed in 3 normal retina tissues and 3 retinoblastoma tissues. Our result showed that normal and tumor tissues are deeply different, with 57 up-regulated and 34 down-regulated miRNAs (fold change >2 and P < 0.05; Figure 1A). Of particular interest, miR-214-3p was the most significantly decreased in retinoblastoma tissues. qRT-PCR results showed that expression level of miR-214-3p was lower in most retinoblastoma tissues (43/56, 76.8%) when compared with normal retina tissues (Figure 1B and C). As illustrated in Figure 1D, miR-214-3p was significantly decreased in retinoblastoma cell lines SO-RB50, WERI-RB1, Y79 compared to that in retinal pigment epithelial cell line ARPE-19. Furthermore, Kaplan-Meier analysis revealed that high level of miR-214-3p was strongly correlated with favorable outcome in patients with retinoblastoma (P=0.004; Figure 1E). As shown in Table 1, in 56 retinoblastoma tissues, the level of miR-214-3p was lower in high-grade and chemotherapy nonresponse patients (P=0.002, P=0.014, respectively).
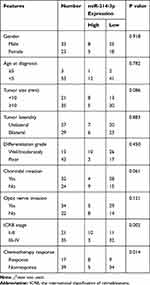 |
Table 1 Correlation of miR-214-3p Expression and Clinicopathological Features of Retinoblastoma Patients |
miR-214-3p Enhances the Sensitivity to Multi-Drugs in vitro
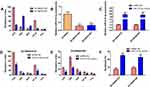
To explore the potential role of miR-214-3p in the chemotherapy response of retinoblastoma cells, two chemoresistant cell lines SO-RB50/VCR and SO-RB50/CBP were constructed by exposing SO-RB50 cells to increasing dose of VCR or CBP (Figure 2A). In both SO-RB50/VCR and SO-RB50/CBP cells, miR-214-3p expression level was down-regulated 3.95-fold and 3.24-fold compared with SO-RB50 cells, respectively (Figure 2B). Gain-of-function assays were then performed in SO-RB50/VCR and SO-RB50/CBP cells by transfecting with miR-214-3p mimics. miR-214-3p level was validated using qRT-PCR (Figure 2C). Compared with negative control, cells overexpressing miR-214-3p exhibited significantly increased sensitivity to multi-drugs (Figure 2D and E). Flow cytometry analysis showed a higher rate of apoptosis when incubated with gemcitabine for 48 h in SO-RB50/VCR and SO-RB50/CBP cells transfected with miR-214-3p mimics than that in cells transfected with negative control (Figure 2F).
miR-214-3p Enhances the Sensitivity to Multi-Drug in vivo
To further evaluate the effects of miR-214-3p on chemosensitivity in vivo, SO-RB50 cells stably overexpressing miR-214-3p (pSuper-miR-214-3p) were established and subcutaneously injected into nude mice and then treated with VCR or CBP. qRT-PCR results confirmed that miR-214-3p expression level was up-regulated 54.3-fold in stably expressing miR-214-3p cells (Supplementary Figure 1). In both VCR and CBP treatment, tumor growth of pSuper group was faster than that in the pSuper-miR-214-3p group (Figure 3A, B and D). The average weight of tumors in miR-214-3p overexpression group was significantly less than tumors derived from blank vector group (Figure 3C and E).
ABCB1 and XIAP are Direct Target Genes of miR-214-3p
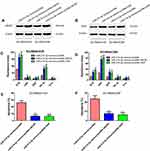
To understand the underlying mechanism through which miR-214-3p suppresses chemoresistance, bioinformatics analysis was performed to identify the potential targets of miR-214-3p using TargetScan. Of these putative targets, ABCB1 and XIAP attracted our attention as they have been implicated in chemoresistance previously (Figure 4A). ABCB1 and XIAP protein expression levels were significantly down-regulated in miR-214-3p mimics transfected SO-RB50/VCR and SO-RB50/CBP cells (Figure 4B). To confirm ABCB1 and XIAP as direct targets, wt or mut 3’UTR of ABCB1 and XIAP was co-transfected with miR-214-3p mimics or miRNA negative control into HEK-293T cells. Relative luciferase activity was significantly reduced when ABCB1-wt or XIAP-wt vector was co-transfected with the miR-214-3p mimics, but not with the miRNA mimic control (Figure 4C and D). In addition, ABCB1 and XIAP were up-regulated 2.33-fold and 2.70-fold (Figure 4E and 4F) and significant negatively correlated with miR-214-3p level in retinoblastoma tissues (R=−0.4491, P=0.0001; R=−0.5137, P<0.0001; Figure 4G and H).
ABCB1 and XIAP Rescue miR-214-3p-Suppressed Chemoresistance
To further validate the roles of ABCB1 and XIAP in chemoresistance inhibition-induced by miR-214-3p overexpression, pcDNA-ABCB1 or pcDNA-XIAP was co-transfected with miR-214-3p mimics or miRNA negative control into SO-RB50/VCR and SO-RB50/CBP cells. First, ABCB1 and XIAP protein levels were determined using Western blot. We observed an increased ABCB1 and XIAP expression after cells transfected with pcDNA-ABCB1 and pcDNA-XIAP, respectively (Figure 5A and B). MTT assay results showed that ABCB1 or XIAP overexpression attenuated the inhibitory effect of miR-214-3p on drug resistance (Figure 5C and D). In addition, the results of flow cytometry assays revealed that ABCB1 or XIAP overexpression significantly reversed cell apoptosis promotion of retinoblastoma cells induced by miR-214-3p overexpression (Figure 5E and F).
Discussion
In recent years, chemotherapy with CBP, VCR and VP-16 has become one of the major treatment methods for retinoblastoma and significantly improved patients’ prognosis and chance of eye preservation.18 However, patients with long-term chemotherapy often develop to drug resistance, leading to the increased incidence of treatment failure.19 Therefore, searching for new therapeutic targets and understanding the molecular mechanism of chemoresistance will help to minimize drug resistance and improve curative effect for retinoblastoma treatment.
miR-214-3p has been found to play important roles in several cancers, yet its function in retinoblastoma has not been reported. In this study, we observed that miR-214-3p was significantly down-regulated in both retinoblastoma tissues and cell lines, and patients with high expression level of miR-214-3p predicted better prognosis. Clinicopathological features analysis revealed that decreased miR-214-3p level was associated with ICRB stage and chemotherapy resistance. We also found that miR-214-3p overexpression significantly suppressed chemoresistance in vitro and in vivo. These data suggest that miR-214-3p acts as a tumor suppressor gene in retinoblastoma.
miRNAs always exert their functional roles through interacting with target genes. Based on bioinformatics analysis, ABCB1 and XIAP were predicted to be candidate targets of miR-214-3p. Further investigations confirmed that miR-214-3p could directly interact with the 3’UTR of ABCB1 and XIAP, and reduce their expression, which was consistent with Jin et al’s report of miR-214 inhibiting imatinib mesylate resistance in chronic myeloid leukemia by targeting ABCB1.20
ABCB1 and XIAP are two classical chemoresistance-related genes.21–23 ABCB1, also known as MDR1, encodes P-glycoprotein which is the best-studied member of the ABC family of transporters.24 ABCB1 is frequently up-regulated in drug-resistant cancer cells and functions as transporter of various drugs, such as carfilzomib, paclitaxel, methotrexate, doxorubicin and cisplatin.25–29 XIAP encodes a protein that belongs to a family of apoptotic suppressor proteins. XIAP has been initially identified for its role in blocking apoptosis upon inhibition of the activation of caspase-3, −7 and −9.30 Recent studies also suggest the regulatory roles of abnormally expressed XIAP in chemoresistance, inflammation and metastasis.31–33 In this study, we observed that ABCB1 or XIAP overexpression could significantly reverse chemoresistance and cell apoptosis-induced by miR-214-3p overexpression, indicating that miR-214-3p inhibits retinoblastoma progression at least partly through reducing ABCB1 and XIAP expression.
Conclusion
We show that miR-214-3p is down-regulated in retinoblastoma and high level of miR-214-3p is associated with better overall survival of retinoblastoma patients. miR-214-3p functions as a tumor suppressor to sensitize retinoblastoma cells to chemodrugs and promote apoptosis partly through targeting ABCB1 and XIAP. Our data provide new insight into underlying mechanisms of retinoblastoma chemoresistance and may contribute to develop new therapeutic strategies for cancer treatment.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–838. doi:10.1038/nature03702
2. Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–866. doi:10.1038/nrc1997
3. Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20(8):460–469. doi:10.1016/j.molmed.2014.06.005
4. Yoon AJ, Wang S, Shen J, et al. Prognostic value of miR-375 and miR-214-3p in early stage oral squamous cell carcinoma. Am J Transl Res. 2014;6(5):580–592.
5. Phatak P, Byrnes KA, Mansour D, et al. Overexpression of miR-214-3p in esophageal squamous cancer cells enhances sensitivity to cisplatin by targeting survivin directly and indirectly through CUG-BP1. Oncogene. 2016;35(16):2087–2097. doi:10.1038/onc.2015.271
6. Wang J, Zhao X, Guo Z, et al. Regulation of NEAT1/miR-214-3p on the growth, migration and invasion of endometrial carcinoma cells. Arch Gynecol Obstet. 2017;295(6):1469–1475. doi:10.1007/s00404-017-4365-1
7. Han LC, Wang H, Niu FL, et al. Effect miR-214-3p on proliferation and apoptosis of breast cancer cells by targeting survivin protein. Eur Rev Med Pharmacol Sci. 2019;23(17):7469–7474. doi:10.26355/eurrev_201909_18856
8. Yang Y, Li Z, Yuan H, et al. Reciprocal regulatory mechanism between miR-214-3p and FGFR1 in FGFR1-amplified lung cancer. Oncogenesis. 2019;8(9):50. doi:10.1038/s41389-019-0151-1
9. Li Y, Li Y, Chen Y, et al. MicroRNA-214-3p inhibits proliferation and cell cycle progression by targeting MELK in hepatocellular carcinoma and correlates cancer prognosis. Cancer Cell Int. 2017;17:102. doi:10.1186/s12935-017-0471-1
10. Cai H, Miao M, Wang Z. miR-214-3p promotes the proliferation, migration and invasion of osteosarcoma cells by targeting CADM1. Oncol Lett. 2018;16(2):2620–2628. doi:10.3892/ol.2018.8927
11. Yu H, Wang S, Zhu H, et al. LncRNA MT1JP functions as a tumor suppressor via regulating miR-214-3p expression in bladder cancer. J Cell Physiol. 2019;234:16160–16167. doi:10.1002/jcp.v234.9
12. Dimaras H, Kimani K, Dimba EA, et al. Retinoblastoma. Lancet. 2012;379(9824):1436–1446. doi:10.1016/S0140-6736(11)61137-9
13. Zhang Y, Zhu X, Zhu X, et al. MiR-613 suppresses retinoblastoma cell proliferation, invasion, and tumor formation by targeting E2F5. Tumour Biol. 2017;39(3):1010428317691674.
14. Li J, You X. MicroRNA-758 inhibits malignant progression of retinoblastoma by directly targeting PAX6. Oncol Rep. 2018;40(3):1777–1786. doi:10.3892/or.2018.6563
15. Zhang Y, Xue C, Zhu X, et al. Suppression of microRNA-125a-5p upregulates the TAZ-EGFR signaling pathway and promotes retinoblastoma proliferation. Cell Signal. 2016;28(8):850–860. doi:10.1016/j.cellsig.2016.04.002
16. Yang L, Zhang L, Lu L, et al. LncRNA UCA1 increases proliferation and multidrug resistance of retinoblastoma cells via down-regulating miR-513a-5p. Onco Targets Ther. 2019;12:8653–8662. doi:10.2147/OTT.S212352
17. Yang L, Zhang L, Lu L, et al. Long noncoding RNA SNHG16 sponges miR-182-5p and miR-128-3p to promote retinoblastoma cell migration and invasion by targeting LASP1. DNA Cell Biol. 2019.
18. Fabian ID, Johnson KP, Stacey AW, et al. Focal laser treatment in addition to chemotherapy for retinoblastoma. Cochrane Database Syst Rev. 2017;6:CD012366.
19. Shields CL, Say EA, Pointdujour-Lim R, et al. Rescue intra-arterial chemotherapy following retinoblastoma recurrence after initial intra-arterial chemotherapy. J Fr Ophtalmol. 2015;38(6):542–549. doi:10.1016/j.jfo.2015.03.004
20. Jin J, Yao J, Yue F, et al. Decreased expression of microRNA-214 contributes to imatinib mesylate resistance of chronic myeloid leukemia patients by upregulating ABCB1 gene expression. Exp Ther Med. 2018;16(3):1693–1700. doi:10.3892/etm.2018.6404
21. Zhang Z, Wang X, Wang S. Chemosensitization effects of simultaneous suppression of MDR1 and XIAP in multidrug resistant glioma cells. Med Oncol. 2008;25(4):367–373. doi:10.1007/s12032-008-9047-1
22. Li Y, Jian Z, Xia K, et al. XIAP is related to the chemoresistance and inhibited its expression by RNA interference sensitize pancreatic carcinoma cells to chemotherapeutics. Pancreas. 2006;32(3):288–296. doi:10.1097/01.mpa.0000218314.67111.fb
23. Stavrovskaya AA, Stromskaya TP. Transport proteins of the ABC family and multidrug resistance of tumor cells. Biochemistry (Mosc). 2008;73(5):592–604. doi:10.1134/S0006297908050118
24. Eckford PD, Sharom FJ. ABC efflux pump-based resistance to chemotherapy drugs. Chem Rev. 2009;109(7):2989–3011. doi:10.1021/cr9000226
25. Besse A, Stolze SC, Rasche L, et al. Carfilzomib resistance due to ABCB1/MDR1 overexpression is overcome by nelfinavir and lopinavir in multiple myeloma. Leukemia. 2018;32(2):391–401. doi:10.1038/leu.2017.212
26. Zhang H, Patel A, Wang YJ, et al. The BTK inhibitor ibrutinib (PCI-32765) overcomes paclitaxel resistance in ABCB1- and ABCC10-overexpressing cells and tumors. Mol Cancer Ther. 2017;16(6):1021–1030. doi:10.1158/1535-7163.MCT-16-0511
27. Han Z, Shi L. Long non-coding RNA LUCAT1 modulates methotrexate resistance in osteosarcoma via miR-200c/ABCB1 axis. Biochem Biophys Res Commun. 2018;495(1):947–953. doi:10.1016/j.bbrc.2017.11.121
28. Kun-Peng Z, Xiao-Long M, Chun-Lin Z. LncRNA FENDRR sensitizes doxorubicin-resistance of osteosarcoma cells through down-regulating ABCB1 and ABCC1. Oncotarget. 2017;8(42):71881–71893. doi:10.18632/oncotarget.v8i42
29. Cheng FH, Zhao ZS, Liu WD. Long non-coding RNA ROR regulated ABCB1 to induce cisplatin resistance in osteosarcoma by sponging miR-153-3p. Eur Rev Med Pharmacol Sci. 2019;23(17):7256–7265. doi:10.26355/eurrev_201909_18828
30. Deveraux QL, Reed JC. IAP family proteins–suppressors of apoptosis. Genes Dev. 1999;13(3):239–252. doi:10.1101/gad.13.3.239
31. Miyamoto M, Takano M, Iwaya K, et al. X-chromosome-linked inhibitor of apoptosis as a key factor for chemoresistance in clear cell carcinoma of the ovary. Br J Cancer. 2014;110(12):2881–2886. doi:10.1038/bjc.2014.255
32. Xia S, Fu TM, Wu H. Inflammation NODs to antagonists of RIP2-XIAP interaction. Mol Cell. 2018;69(4):535–536. doi:10.1016/j.molcel.2018.02.003
33. Yu Y, Jin H, Xu J, et al. XIAP overexpression promotes bladder cancer invasion in vitro and lung metastasis in vivo via enhancing nucleolin-mediated Rho-GDIβ mRNA stability. Int J Cancer. 2018;142(10):2040–2055. doi:10.1002/ijc.v142.10
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.