Back to Journals » OncoTargets and Therapy » Volume 12
miR-214-3p inhibits epithelial-to-mesenchymal transition and metastasis of endometrial cancer cells by targeting TWIST1
Authors Fang YY, Tan MR, Zhou J, Liang L, Liu XY, Zhao K, Bao EC
Received 21 July 2018
Accepted for publication 5 December 2018
Published 18 November 2019 Volume 2019:12 Pages 9449—9458
DOI https://doi.org/10.2147/OTT.S181037
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Yuan-Yuan Fang,1,* Ming-Rong Tan,2,* Jian Zhou,1 Li Liang,1 Xiao-Yun Liu,3 Kun Zhao,4 Er-Chen Bao5
1Department of Gynaecology, Xu Zhou Maternal and Child Health Care Hospital, Xuzhou 221009, China; 2Department of Operation Room, Xiangyang No 1 People’s Hospital, Hubei University of Medicine, Xiangyang 441000, China; 3Central Laboratory, The Affiliated Hospital of Xu Zhou Medical University, Xuzhou 221009, China; 4Department of Laboratory, Xu Zhou Maternal and Child Health Care Hospital, Xuzhou 221009, China; 5Department of Gynaecology, Xinyi People’s Hospital, Xinyi 221400, China
*These authors contributed equally to this work
Correspondence: Yuan-Yuan Fang
Department of Gynaecology, Xu Zhou Maternal and Child Health Care Hospital, No 46, Heping Road, Xuzhou 221009, Jiangsu, China
Tel +86 139 5218 6059
Email [email protected]
Background: Substantive studies have described the ectopic microRNAs as a determinant of the pathogenesis of endometrial cancer (EC). miR-214-3p has been reported to be significantly downregulated in EC tissues, and its overexpression has been shown to inhibit the proliferation, migration, and invasion of EC cells. Our study sought to explore the molecular mechanism underlying the inhibitory effect of miR-214-3p on metastasis of EC cells.
Methods: The expressions of miR-214-3p and TWIST1 in EC tissues and cells were detected by quantitative real-time PCR. Cell migration, invasion, and epithelial-to-mesenchymal transition (EMT) were measured by transwell and Western blot analyses, respectively. The interaction between miR-214-3p and TWIST1 was confirmed by luciferase reporter assay. Xenograft tumor assay was performed to verify the role and underlying mechanism of miR-214-3p in EC in vivo.
Results: miR-214-3p was downregulated and TWIST1 was upregulated in EC tissues and cells. miR-214-3p was negatively correlated with TWIST1 expression in EC tissues. Overexpression of miR-214-3p suppressed migration, invasion, and EMT in EC cells. TWIST1 was identified as a target of miR-214-3p in EC cells, and its overexpression significantly restored the inhibitory effects of miR-214-3p on cell migration, invasion, and EMT while its knockdown remarkably abolished miR-214-3p inhibitor-mediated promotion of progression of EC cells. Additionally, addition of miR-214-3p inhibited tumor growth by regulating EMT in vivo.
Conclusion: miR-214-3p suppressed the EMT and metastasis of EC cells by targeting TWIST1, providing a novel biomarker for treatment of EC.
Keywords: miR-214-3p, TWIST1, EMT, metastasis, endometrial cancer
Introduction
Endometrial cancer (EC) is one of the most frequently diagnosed gynecologic tumors with increasing incidence in the developed countries.1 There were approximately 61,380 new diagnosed cases of EC, with an estimated 10,920 deaths in the USA in 2017.2 Despite great advances in therapeutic approaches, the prognosis of EC is still dismal and the 5-year survival rate remains, <40%.3 The poor prognosis in EC patients is mainly attributable to recurrence and distant metastasis.4 Therefore, it is essential to probe novel and specific therapeutic targets for treatment of EC.
Epithelial-to-mesenchymal transition (EMT) is a crucial event which plays a pivotal role in the progression of various cancers, including EC.5,6 A previous study has suggested that EMT is characterized by the decreased expressions of epithelial markers, such as E-cadherin, and the increased expressions of mesenchymal markers, including vimentin, N-cadherin, and fibronectin.7 TWIST1, a highly conserved helix-loop-helix transcription regulator, plays a crucial role in cancer progression and development.8 Former works have demonstrated that TWIST1 is overexpressed in a variety of solid cancers9,1° and functions as a crucial inducer of EMT in invasive EC cells.11,12 Nevertheless, the regulatory mechanism that allows TWIST1 to regulate EMT-related metastasis in EC remains far from addressed.
A recent study has indicated microRNAs (miRNAs) as emerging and critical regulators of EMT-related tumor metastasis.13 miRNAs are a class of evolutionarily conserved, single-stranded noncoding RNAs with a length of 22 nucleotides which repress gene expression by specific base-pairing interaction with the 3′ UTR of their target mRNAs, causing either mRNA degradation or translational repression. A recent work has reported that miRNAs play central regulatory roles in diverse basic cellular processes, such as cell proliferation, migration, invasion, and EMT.14 Moreover, the available evidence has revealed the importance of abnormally expressed miRNAs in the pathogenesis and development of human cancers, including EC.15 For example, miR-34a is suggested to inhibit the proliferation, migration, invasion, and EMT-associated phenotypes by downregulating Notch1 in EC cells.16 In addition, overexpression of miR-381 suppresses proliferation and invasion of EC cells by targeting IGF-1R through blocking Akt and ERK signaling pathways.17 miR-214, located at the chromosomal region 1q24.3, is a vertebrate-specific family of miRNA precursors which is involved in the regulation of initiation, growth, and progression of cancer.18 Notably, a previous study has reported that miR-214-3p is downregulated in EC tissues and its overexpression impedes the proliferation, migration, and invasion of EC cells.19 Recently, multiple miRNAs have been reported to regulate EMT process by targeting TWIST1 in several tumors.2° However, there is no direct evidence in support of the interaction between miR-214-3p and TWIST1 in EC cells.
In the present study, we aimed to explore the functional role of miR-214-3p in regulating EMT and metastasis in EC cells and the underlying mechanism.
Materials and methods
EC patients and samples
Twenty-two paired EC tissues and corresponding adjacent normal samples were collected from patients who underwent hysterectomy at the Department of Gynaecology of Xu Zhou Maternal and Child Health Care Hospital between February 2016 and February 2017. The patients had not received radiotherapy or chemotherapy, and were aged between 35 and 59 years with a mean age of 48.3±6.4 years. According to the clinicopathological characteristics, the patients were classified as follows: seven, 10, and five cases presented with poorly, moderately, and well-differentiated EC, respectively. Moreover, 19 cases were free of distant metastasis, and three showed distant metastasis. Four, seven, six, and five cases were at stages I, II, III, and IV, respectively. All samples were snap frozen in liquid nitrogen immediately after surgical removal, followed by storage at –80°C until RNA was extracted. This study was approved by the ethics committee of Xu Zhou Maternal and Child Health Care Hospital, and written informed consent was obtained from all patients before participating in this study.
Cell culture and transfection
Human EC cell lines (HEC-1-A, HEC-1-B, and RL95-2) and human embryonic kidney cell line HEK293 were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Human endometrial epithelial cells (hEECs) were obtained from PriCells (Wuhan, China). These cells were cultured in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (Thermo Fisher Scientific) and 1% penicillin/streptomycin (Sigma Aldrich, St Louis, MO, USA) at 37°C in a humidified atmosphere with 5% CO2. The cells were allowed to grow to 70%–80% confluence before further treatment. miR-214-3p mimic, miRNA negative control (miR-NC), miR-214-3p inhibitor, inhibitor negative control (anti-miR-NC), small interfering RNA (siRNA) against TWIST1 (si-TWIST1), siRNA negative control (si-NC), pcDNA empty control, and pcDNA-TWIST1 (TWIST1) were synthesized by GenePharma Inc. (Shanghai, China) and transiently transfected into HEC-1-A and RL95-2 cells using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s protocols.
Quantitative real-time PCR(qRT-PCR)
Total RNA from tissues or cultured cells was isolated with TRIzol RNA Isolation Reagent (Thermo Fisher Scientific, Waltham, MA, USA). For the detection of TWIST1 mRNA expression, 1 μg of total RNA was reverse transcribed into cDNA using a PrimeScript RT reagent kit (Thermo Fisher Scientific), and TWIST1 mRNA expression was detected using SYBR-Green Premix Ex Taq (Takara, Kusatsu, Japan). For the detection of miR-214-3p level, cDNA was synthesized using TaqMan reverse transcription kit (Applied Biosystems, Foster City, CA, USA) and qRT-PCR was performed with a TaqMan MicroRNA Assay kit (Applied Biosystems). All PCRs were carried out under an ABI 7500 Real-time PCR system (Applied Biosystems). The expressions of miR-214 and TWIST1 mRNA were normalized to U6 and GAPDH, respectively. The fold changes of gene expression were calculated using the comparative 2−ΔΔCT methods.21,22
Transwell assay
Cell migration and invasion were evaluated using transwell chambers (Corning Incorporated, Corning, NY, USA) following the manufacturer’s instructions. Briefly, HEC-1-A and RL95-2 cells were transfected with miR-214-3p mimic, miR-NC, miR-214-3p mimic + pcDNA, miR-214-3p + TWIST1, miR-214-3p inhibitor, anti-miR-NC, miR-214-3p inhibitor + si-TWIST1, or miR-214-3p inhibitor + si-NC and collected at 48 hours posttransfection. Subsequently, 5×104 transfected HEC-1-A and RL95-2 cells resuspended in 200 μL serum-free medium were seeded into the upper chamber of transwell chamber precoated with Matrigel (BD Biosciences, San Jose, CA, USA), while the lower chamber contained 600 μL complete medium supplemented with 10% FBS as a chemoattractant. After incubation for 24 hours, cells remaining on the upper chamber were wiped off by cotton swabs. Cells that had invaded to the lower chamber were fixed with methanol (Sigma Aldrich), stained with 0.1% crystal violet (Sigma Aldrich), and counted in five random microscopic fields at ×400 magnification under a CKX41 inverted microscope (SZ61; Olympus, Tokyo, Japan). For migration assay, the experimental methods were performed in the same way as the invasion assay, except that the inserts was not precoated with Matrigel.
Western blot
The protein from tissues and cells was extracted using RIPA buffer (Sigma Aldrich) and then was quantified using the protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). The protein samples (50 μg/lane) were separated by SDS-PAGE and then electrotransferred onto nitrocellulose membrane (Millipore, Bedford, MA, USA). After blocking with 5% nonfat dry milk for 2 hours in Tris-buffered saline-Tween 20 (Sigma Aldrich) at room temperature, the membranes were incubated with the primary antibodies against N-cadherin (ab18203; Abcam, Cambridge, UK), E-cadherin (ab76055; Abcam), and TWIST1 (ab50581; Abcam) or β-actin (ab8227; Abcam) overnight at 4°C. Thereafter, the membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (ab6789; Abcam). Protein bands were detected by the enhanced chemiluminescence system (PSC Biotech Pte., Ltd., Singapore, Singapore).
Luciferase reporter assay
The promising binding sites of miR-214-3p and 3′ UTR of TWIST1 were predicted by TargetScan online (http://www.targetscan.org/vert_72/). The full-length wild-type 3′ UTR of TWIST1 was amplified from genomic DNA with primers separately synthesized by Sangon Biotech, Co., Ltd. (Shanghai, China) and inserted into the pMIR-Report vector (Promega, Madison, WI, USA), namely TWIST1-WT. The plasmids with mutant-type TWIST1 3′ UTR (TWIST1-MUT) were generated using Quik Change Multi Site-Directed Mutagenesis kit (Agilent Technologies, Santa Clara, CA, USA). HEK293 cells were seeded into 24-well plates at a density of 1×105 cells per well. When grown to 80% confluence, cells were co-transfected with miR-214-3p mimic, miR-214-3p inhibitor, or matched controls, and constructed luciferase reporter plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Following 48 hours of transfection, luciferase activity was detected by using the Dual-Luciferase Reporter System (Promega) and normalized to Renilla luciferase activity.
Construction of lentiviral vectors carrying miR-214-3p
The synthesized oligonucleotide fragments of miR-214-3p or negative control were cloned into Age I and EcoR I sites of pGCsil-GFP vector to construct lentivirus-mediated miR-214-3p vector (Lv-miR-214-3p) or lentiviral negative control (Lv-NC). pGCsil-miR-214-3p-GFP, pHelper 1.0 Vector (packaging plasmid), and pHelper 2.0 vector (envelop plasmid) were then transfected into HEK293T cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. The recombinant virus particles in the supernatant were collected after 48 hours through ultracentrifugation (2 hours at 50,000× g) and then filtered with a 0.45 μm filter to remove cellular debris. The viral titer was measured with a Centricon-plus-20 (Millipore).
Subcutaneous xenograft model
All animal procedures were approved by the Research Ethics Committee of Xu Zhou Maternal and Child Health Care Hospital and performed according to the guide for the Care and Use of Laboratory Animals. Four-week-old athymic BALB/c nude mice (15–20 g) were purchased from Shanghai Experimental Animal Center of the Chinese Academy of Sciences (Shanghai, China) and maintained under a specific pathogen-free environment with an alternating 12-hour light/dark cycle at 25°C±2°C. HEC-1-A cells stably transfected with Lv-miR-214-3p or Lv-NC (5.0×106 cells per mouse) were suspended in 100 μL medium and subcutaneously injected into the right-side flanks of the mice. The growth of tumors was monitored every 7 days by a digital caliper, and the volume of xenograft tumors was calculated based on the equation: length × width2×1/2. The mice were euthanized on the 28th day after injection, and the tumors were stripped, weighed, and subjected to gene expression analysis.
Statistical analyses
All results are displayed as mean ± SD from three independent experiments. The differences were analyzed using the Student’s t-test between two groups or one-way ANOVA among three or more groups by GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA). The difference was considered to be statistically significant when P-value was, <0.05.
Results
miR-214-3p was less expressed in EC tissues and cells
To determine the biological role of miR-214-3p in progression of EC, we initially examined the expression of miR- 214-3p in 22 paired EC tissues and corresponding adjacent normal tissues. qRT-PCR analysis showed that miR-214-3p expression was abnormally downregulated in 22 EC tissues compared with that in pair-matched normal tissues (Figure 1A). Moreover, the expression of miR-214-3p was also detected in EC cells (HEC-1-A, HEC-1-B, and RL95-2), as well as hEECs. As shown in Figure 1B, miR-214-3p expression was also strikingly lower in EC cells (HEC-1-A, HEC-1-B, and RL95-2) than that in hEECs. These results suggested the downregulation of miR-214-3p in EC tissues and cells. HEC-1-A and RL95-2 cells with lower expression of miR-214-3p were used for further experiments.
miR-214-3p inhibited metastasis and EMT of EC cells
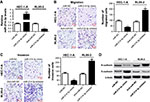
Loss-of-function and gain-of-function experiments were conducted to assess the biological role of miR-214-3p in metastasis of EC cells. HEC-1-A cells were transfected with miR-214-3p mimic or miR-NC, and RL95-2 cells were introduced with miR-214-3p inhibitor or anti-miR-NC. As expected, miR-214-3p expression was effectively elevated in HEC-1-A cells transfected with miR-214-3p mimic but remarkably decreased in RL95-2 cells transfected with miR-214-3p inhibitor compared with their corresponding control, respectively (Figure 2A). Transwell assays proved that overexpression of miR-214-3p effectively blocked the migration and invasive abilities in HEC-1-A cells with respect to miR-NC group (Figure 2B and C). On the contrary, knockdown of miR-214-3p played an opposite effect in RL95-2 cells (Figure 2B and C). In addition, the effects of miR-214-3p on EMT of EC cells were further explored by Western blot. The results manifested that addition of miR-214-3p triggered a substantial decline of N-cadherin level and a significant elevation of E-cadherin expression in HEC-1-A cells, while inhibition of miR-214-3p induced the inverse change in RL95-2 cells (Figure 2D). Collectively, these findings revealed that miR-214-3p inhibited EMT and metastasis of EC cells.
TWIST1 was a target of miR-214-3p in EC cells
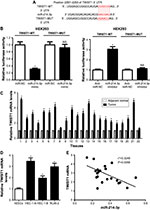
To address the molecular mechanism of miR-214-3p in EC cells, we predicted the potential regulatory targets of miR- 214-3p using bioinformatic analyses. The results showed the potential binding sites between miR-214-3p and 3′ UTR of TWIST1 by TargetScan online (Figure 3A). To confirm the prediction, we constructed the luciferase reporter plasmids containing the WT or MUT binding sites in the 3′ UTR of TWIST1. As shown in Figure 3B, miR-214-3p overexpression considerably suppressed the luciferase activity in HEK293 cells transfected with TWIST1-WT in comparison with miR-NC group, but the efficacy was lost in response to TWIST-MUT group (Figure 3B). Conversely, miR-214-3p inhibitor caused an opposite effect on luciferase activity (Figure 3B). In addition, the expression of TWIST1 mRNA was exceptionally enhanced in EC tissues and cell lines compared to their corresponding controls (Figure 3C and D). Moreover, miR-214-3p abundance was negatively correlated with TWIST1 mRNA expression in EC tissues (r2=0.3249, P=0.0056) (Figure 3E). Collectively, these data implied that miR-214-3p suppressed TWIST1 expression via binding to its 3′ UTR in EC cells.
miR-214-3p repressed metastasis and EMT of EC cells by targeting TWIST1
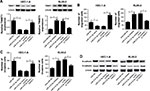
To gain insight into the mechanism by which miR-214-3p inhibited EMT and metastasis of EC cells, rescue experiments were performed in EC cells. HEC-1-A cells were co-transfected with miR-214-3p mimic and TWIST1 or pcDNA, and RL95-2 cells were co-transfected with miR- 214-3p inhibitor and si-TWIST1 or si-NC. As a result, miR-214-3p overexpression inhibited TWIST1 protein level in HEC-1A cells and its knockdown enhanced TWIST1 protein level in RL95-2 cells, which was reversed by restoration and interference of TWIST1, respectively (Figure 4A). Introduction of TWIST1 remarkably reversed the repression of cell migration and invasion mediated by miR-214-3p mimic in HEC-1-A cells, while TWIST1 silencing notably undermined miR-214-3p inhibitor-induced promotion of migration and invasion potentials in RL95-2 cells (Figure 4B and C). Moreover, restoration of TWIST1 strikingly abolished miR-214-3p overexpression-induced decrease of N-cadherin level and increase of E-cadherin level in HEC-1-A cells (Figure 4D). On the opposite, interference of TWIST1 greatly overturned the increase of N-cadherin protein level and the decrease of E-cadherin protein level mediated by miR-214-3p inhibitor in RL95-2 cells (Figure 4D). Together, these results indicated that miR-214-3p inhibited EMT and metastasis of EC cells by targeting TWIST1.
miR-214-3p inhibited tumor growth and EMT in vivo
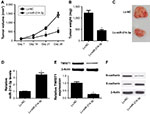
To further investigate whether miR-214-3p could suppress tumor growth and EMT in vivo, the xenograft mouse model of EC was established by subcutaneously injecting with HEC- 1-A cells stably transfected with Lv-miR-214-3p or Lv-NC. As shown in Figure 5A, tumor volume was dramatically impeded in Lv-miR-214-3p group compared with that in control group. Moreover, the tumor weight in the Lv-miR- 214-3p group was also obviously reduced in comparison to control group (Figure 5B and C). Additionally, miR-214-3p expression was evidently enhanced after injection with Lv-miR-214-3p in the xenografted tumors (Figure 5D). However, miR-214-3p overexpression effectively reduced the protein levels of TWIST1 and N-cadherin while successfully increasing E-cadherin level in the xenografted tumors (Figure 5E and F). Taken together, these results suggested that miR-214-3p inhibited tumor growth and EMT in EC in vivo.
Discussion
Emerging evidence has indicated that multiple miRNAs act as master regulators of EMT and metastasis in many types of tumors.23 Herein, we showed that miR-214-3p was less expressed in EC tissues and cells and exerted an inhibitory role in EMT and metastasis in EC cells in vitro and in vivo. Additionally, mechanistic analysis demonstrated that TWIST1, as a direct target of miR-214-3p, was involved in miR-214-3p-mediated suppression of EMT and metastasis in EC cells.
miRNAs have been reported to be implicated in cell proliferation, apoptosis, invasion, and migration in human cancers by serving as either oncogenes or tumor suppressors.24 Recent studies have indicated that miR-124 was downregulated and played a tumor suppressive role in many malignant tumors. For example, miR-214 acted as a tumor suppressor via inhibiting proliferation, migration, and invasion of cervical cancer cells through targeting ARL2.25 Upregulation of miR-214 inhibited cell proliferation, colony formation, and invasive capacity in esophageal squamous cell carcinoma through regulating CDC25B.26 Moreover, restoration of miR-214 inhibited cell proliferation, migration, and invasion and promoted apoptosis in bladder cancer cells by downregulating oncogene PDRG1.27 Such works also demonstrated that miR-214 was upregulated and contributed to tumor progression by serving as an oncogene. For instance, miR- 214 functioned as an oncogene in breast cancer by promoting cell invasion through the downregulation of p53.28 In gastric cancer, knockdown of miR-214 hindered cell proliferation, migration, and invasion by upregulating PTEN.29 In the present study, miR-214-3p expression was significantly reduced in EC tissues and cells vs the corresponding controls, which is in accordance with the previous studies.19,3° Moreover, functional analyses revealed that ectopic expression of miR-214-3p inhibited cell migration, invasion, and EMT in EC cells, while miR-214-3p inhibitor exhibited the opposite effects, indicating the tumor suppressive role of miR-214-3p in EC cells, which is also in agreement with the previous study.19
TWIST1, a transcriptional repressor of E-cadherin, has been shown to contribute to cancer metastasis by induction of EMT in human cancers.29 More recently, a large body of literature is focusing on the role of TWIST1 in tumor invasion and metastasis. For example, overexpression of TWIST1 induced cell morphological changes and facilitated cell migration and invasion in cancer cells.31,32 TWIST1 promoted breast cancer invasion and metastasis by silencing FOXA1 expression.33 Additionally, TWIST1-mediated modulation of EMT contributed to the migration and invasion of cervical cancer cells.34 Therefore, TWIST1 was suggested to exert oncogenic activities. Several miRNAs, such as miR-106,11 miR-548c,35 and miR-543,36 have been reported to regulate EMT and metastasis in EC cells by targeting TWIST1. In our study, we identified TWIST1 as a target of miR-214-3p in EC cells. We found that TWIST1 was upregulated in EC tissues and cells with respect to their respective controls, consistently with the previous studies. Moreover, miR- 214-3p was negatively correlated with TWIST1 mRNA expressions in EC tissues. Rescue experiments manifested that TWIST1 overexpression significantly restored the inhibitory effects of miR-214-3p mimic on cell migration, invasion, and EMT while TWIST1 knockdown remarkably abolished miR-214-3p inhibitor-mediated promotion of cell migration, invasion, and EMT in EC cells. In vivo experiments demonstrated that miR-214-3p inhibited tumor growth and EMT in EC.
In summary, this study demonstrated that miR-214-3p was downregulated in EC tissues and cells. Moreover, miR- 214-3p overexpression inhibited the metastasis and EMT of EC cells, whereas its knockdown promoted the progression of EC. In addition, TWIST1 was directly targeted by miR- 214-3p and it reversed the regulatory effect of miR-214-3p. miR-214-3p lowered the tumor growth and EMT in vivo. Collectively, miR-214-3p protected against metastasis and EMT of EC cells by targeting TWIST1, which suggests that miR-214-3p can serve as a promising therapeutic target for EC.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
3. Dedes KJ, Wetterskog D, Ashworth A, Kaye SB, Reis-Filho JS. Emerging therapeutic targets in endometrial cancer. Nat Rev Clin Oncol. 2011; 8(5):261–271.
4. Sakuragi N, Hareyama H, Todo Y, et al. Prognostic significance of serous and clear cell adenocarcinoma in surgically staged endometrial carcinoma. Acta Obstet Gynecol Scand. 2000;79(4):311–316.
5. Pecina-Slaus N, Cicvara-Pecina T, Kafka A. Epithelial-to-mesenchymal transition: possible role in meningiomas. Front Biosci (Elite Ed). 2012; 4:889–896.
6. Dong P, Kaneuchi M, Konno Y, Watari H, Sudo S, Sakuragi N. Emerging therapeutic biomarkers in endometrial cancer. BioMed Research International. 2013;2013(1):1–11.
7. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428.
8. Zhao Z, Rahman MA, Chen ZG, Shin DM. Multiple biological functions of Twist1 in various cancers. Oncotarget. 2017;8(12):20380–20393.
9. Kyo S, Sakaguchi J, Ohno S, et al. High Twist expression is involved in infiltrative endometrial cancer and affects patient survival. Hum Pathol. 2006;37(4):431–438.
10. Rosivatz E, Becker I, Specht K, et al. Differential expression of the epithelial-mesenchymal transition regulators snail, SIP1, and twist in gastric cancer. Am J Pathol. 2002;161(5):1881–1891.
11. Dong P, Kaneuchi M, Watari H, Sudo S, Sakuragi N. MicroRNA-106b modulates epithelial-mesenchymal transition by targeting TWIST1 in invasive endometrial cancer cell lines. Mol Carcinog. 2014;53(5): 349–359.
12. Liu W, Zhang B, Xu N, Wang MJ, Liu Q. miR-326 regulates EMT and metastasis of endometrial cancer through targeting TWIST1. Eur Rev Med Pharmacol Sci. 2017;21(17):3787–3793.
13. Yan J, Gumireddy K, Li A, Huang Q. Regulation of mesenchymal phenotype by MicroRNAs in cancer. Curr Cancer Drug Targets. 2013; 13(9):930–934.
14. Iorio MV, Croce CM. microRNA involvement in human cancer. Carcinogenesis. 2012;33(6):1126–1133.
15. Castilla MÁ, Moreno-Bueno G, Romero-Pérez L, et al. Micro-RNA signature of the epithelial-mesenchymal transition in endometrial carcinosarcoma. J Pathol. 2011;223(1):72–80.
16. Wang Z, Wang W, Huang K, Wang Y, Li J, Yang X. MicroRNA-34a inhibits cells proliferation and invasion by downregulating Notch1 in endometrial cancer. Oncotarget. 2017;8(67):111258–111270.
17. Tu C, Wang F, Wan J. MicroRNA-381 inhibits cell proliferation and invasion in endometrial carcinoma by targeting the IGF-1R. Mol Med Rep. 2018;17(3):4090–4098.
18. Penna E, Orso F, Taverna D. miR-214 as a key hub that controls cancer networks: small player, multiple functions. J Invest Dermatol. 2015; 135(4):960–969.
19. Wang J, Zhao X, Guo Z, Ma X, Song Y, Guo Y. Regulation of NEAT1/miR-214-3p on the growth, migration and invasion of endometrial carcinoma cells. Arch Gynecol Obstet. 2017;295(6):1469–1475.
20. Abba M, Patil N, Leupold J, Allgayer H. MicroRNA regulation of epithelial to mesenchymal transition. J Clin Med. 2016;5(1):8.
21. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408.
22. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108.
23. Kim J, Yao F, Xiao Z, Sun Y, Ma L. MicroRNAs and metastasis: small RNAs play big roles. Cancer Metastasis Rev. 2018;37(1):5–15.
24. Gu J, Wang Y, Wu X. MicroRNA in the pathogenesis and prognosis of esophageal cancer. Curr Pharm Des. 2013;19(7):1292–1300.
25. Peng R, Men J, Ma R, et al. miR-214 down-regulates ARL2 and suppresses growth and invasion of cervical cancer cells. Biochem Biophys Res Commun. 2017;484(3):623–630.
26. Wang M, Wang L, Zhang M, Li X, Zhu Z, Wang H. MiR-214 inhibits the proliferation and invasion of esophageal squamous cell carcinoma cells by targeting CDC25B. Biomed Pharmacother. 2017;95:1678–1683.
27. Wang J, Zhang X, Wang L, et al. MicroRNA-214 suppresses oncogenesis and exerts impact on prognosis by targeting PDRG1 in bladder cancer. PLoS One. 2015;10(2):e0118086.
28. Wang F, Lv P, Liu X, Zhu M, Qiu X. microRNA-214 enhances the invasion ability of breast cancer cells by targeting p53. Int J Mol Med. 2015;35(5):1395–1402.
29. Yang TS, Yang XH, Wang XD, Wang YL, Zhou B, Song ZS. MiR-214 regulate gastric cancer cell proliferation, migration and invasion by targeting PTEN. Cancer Cell Int. 2013;13(1):68.
30. Ramón LA, Braza-Boïls A, Gilabert J, et al. microRNAs related to angiogenesis are dysregulated in endometrioid endometrial cancer. Hum Reprod. 2012;27(10):3036–3045.
31. Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7): 927–939.
32. Qin Q, Xu Y, He T, Qin C, Xu J. Normal and disease-related biological functions of Twist1 and underlying molecular mechanisms. Cell Res. 2012;22(1):90–106.
33. Xu Y, Qin L, Sun T, et al. Twist1 promotes breast cancer invasion and metastasis by silencing Foxa1 expression. Oncogene. 2017;36(8): 1157–1166.
34. Wang D, Li Q, Li K, Xiao P, Yin R. Twist-related protein 1-mediated regulation of mesenchymal change contributes to the migration and invasion of cervical cancer cells. Oncol Lett. 2015;10(5):3107–3112.
35. Sun X, Cui M, Zhang A, et al. MiR-548c impairs migration and invasion of endometrial and ovarian cancer cells via downregulation of Twist. J Exp Clin Cancer Res. 2016;35(1):10.
36. Bing L, Hong C, Li-Xin S, Wei G. MicroRNA-543 suppresses endometrial cancer oncogenicity via targeting FAK and TWIST1 expression. Arch Gynecol Obstet. 2014;290(3):533–541.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.