Back to Journals » Journal of Pain Research » Volume 15
MiR-204-5p Alleviates Neuropathic Pain by Targeting BRD4 in a Rat Chronic Constrictive Injury Model
Authors Guo X, Geng X, Chu Y, Gao J, Jiang L
Received 20 April 2022
Accepted for publication 8 August 2022
Published 18 August 2022 Volume 2022:15 Pages 2427—2435
DOI https://doi.org/10.2147/JPR.S371616
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qi Fang
Xiaona Guo,1,* Xia Geng,1,* Yunchao Chu,2 Jianfei Gao,1 Linkai Jiang1
1Pain Department, Dongying People’s Hospital, Dongying, Shandong, People’s Republic of China; 2Pain Department, Shengli Oilfield Central Hospital, Dongying, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Linkai Jiang, Pain Department, Dongying People’s Hospital, No. 317, Dongcheng Nanyi Road, Dongying, Shandong, 257091, People’s Republic of China, Tel +86 546-8901234, Email [email protected]
Purpose: The pathogenesis of neuropathic pain is complex, and previous studies have found that microRNAs are important regulators of neuropathic pain and are associated with the progression of neuropathic pain. This study aims to explore the level and role of miR-204-5p in the chronic constrictive injury (CCI) model of rats.
Patients and Methods: The CCI rat model was constructed to evaluate paw withdrawal threshold (PWT), paw withdrawal latency (PWL), the expressions of miR-204-5p, and the contents of inflammatory factors in the model. Overexpression of miR-204-5p in rat spinal cord was induced by intrathecal injection of miR-204-5p mimics. PWT and PWL were used to estimate mechanical and thermal pain thresholds. IL-6 and TNF-α were determined by ELISA. Luciferase reporter gene was conducted to verify the targeting relationship between miR-204-5p and BRD4.
Results: miR-204-5p was abnormally down-regulated in the CCI group. The thresholds of mechanical and thermal pain stimulation in the CCI group were lower, and the levels of inflammatory factors were higher than those in the sham group. Overexpression of miR-204-5p alleviated PWT, PWL and inflammatory factors. Besides, the luciferase reporter gene showed that BRD4 was a target gene of miR-204-5p.
Conclusion: These results suggested that miR-204-5p may alleviate neuropathic pain and inflammation through targeted regulation of BRD4 expression.
Keywords: neuropathic pain, miR-204-5p, chronic constrictive injury model, BRD4
Introduction
Neuropathic pain is a nervous system disease caused by primary pathological changes or dysfunction of central or peripheral nervous system, which is characterized by allodynia and hyperalgesia of somatosensory system.1,2 Infection, trauma, ischemia, inflammation and other factors can cause central and peripheral nerve damage, resulting in neuropathic pain.3 Neuropathic pain has become a global problem, affecting about 7% to 10% of the world’s population and incurring enormous socioeconomic costs.4 At present, neuropathic pain is a complex disease whose mechanism is still unclear. Therefore, a more accurate understanding of the molecular mechanisms underlying the pathogenesis of neuropathic pain is crucial for finding new and effective therapeutic targets.
MicroRNAs (miRNAs) are a new class of non-coding RNAs with about 22 nucleotides, and they can bind to the 3’-UTR of multiple mRNA targets.5,6 Today, miRNAs have been established as key micro-regulators of normal cellular homeostasis, and similarly, dysregulation of miRNA is associated with cancer, cardiovascular disease, inflammatory diseases, and neurodegenerative diseases.7 In the past few decades, the research of miRNA related to somatic pain has been developing rapidly, several studies have confirmed that neurological diseases can induce changes in miRNA expression profile. It was found that mouse sciatic nerve injury resulted in the down-regulation of miR-200b in postsynaptic neurons, which was closely related to the increase of DNMT3a.8 Another study analyzed hippocampal formation in CCI rats using the TaqMan low-density array, with 51 miRNA expressions significantly altered compared to contralateral sham surgery.9 MiR-204-5p was located in intron 6 of TRPM3 and was originally found to be abnormally expressed in melanoma.10 A study reported that miR-204-5p expression changes have been found in patients with neuropathic pain caused by spinal cord injury and is expected to be a potential marker of SCI.11 And another study has also been reported that miR-204 is abnormally expressed in rat spinal cord compression (SCC) model.12 However, at present, few studies have focused on analyzing the mechanism of miR-204-5p regulating the neuropathic pain. Furthermore, the miRNA.org database has predicted the relationship between miR-204-5p and bromodomain-containing protein 4 (BRD4). A previous study showed that BRD4 is associated with neuropathic pain. BRD4 has been reported to be involved in the regulation of various pathophysiological activities, such as inflammation and oxidative stress injury. Zhang et al found that the overexpression of BRD4 can aggravate vincristine induced neuropathic pain by promoting inflammation and oxidative stress damage.
According to the aforementioned studies, this study was designed to assess the functions of miR-204-5p in an animal model of neuropathic pain and to evaluate the potential mechanisms of the clinical application of miR-204-5p as a novel therapeutic target and tool.
Materials and Methods
Animals and Establishment of Animal Model
The experimental animals used in this study were adult male Sprague Dawley rats (190–220g) obtained from Shanghai Animal Experimental Center. Animals were placed in cages with temperatures of 20°C–25°C, humidity of 55%±5%, and adequate food and water. The animal experimental procedures involved in this study strictly followed the national institutes of health guidelines for the use and care of laboratory animals. This experiment was conducted with approval of the Animal Ethics Committee of Dongying People’s Hospital.
The CCI model was constructed based on the previously published articles.13 Briefly, the rats were anesthetized with proper dose of pentobarbital sodium according to body weight. The rat was fixed on the operating table in the right lateral position, the hair on the lateral thigh of the left hind leg was removed, and the skin was cut along the posterior edge of the femur at the midpoint of the thigh, with the incision length of about 2cm. After blunt muscle separation, the sciatic nerve was carefully separated and exposed with a nerve glass minute needle. The sciatic nerve was freed 7–8mm before the bifurcation, and ligated with 4-0 non-absorbable silk thread, each about 1mm apart. The degree of tightness should be appropriate to cause slight trembling of leg muscles. Finally, the surgical incision was sutured. Postoperative rats received daily intramuscular injections of penicillin to prevent wound infection. Rats that were postoperatively paralyzed or died were excluded. Rats in the sham operation group were referred to the modeling group except for the ligation.
Intrathecal Injection and Administration
Thirty rats were randomly divided into six groups with five rats in each group, namely sham-operated group, CCI group, CCI + LV-NC group, CCI + LV-miR-204-5p group, CCI + LV-miR-204-5p + oe-NC group and CCI + LV-miR-204-5p + oe-BRD4 group. Except for the sham-operated group, the other groups required an epidural catheter based on CCI, and the specific experimental method was referred to the published literature.14 In short, after anesthesia, the rats were placed in prone position and fixed on the operating table A PE-10 polyethylene catheter was inserted into the spinal cord subarachnoid space, followed by intrathecal implantation by injecting 2% lidocaine to paralyze bilateral hindlimbs. For in vivo transfection, lentiviral vector expressing miR-204-5p (LV-miR-204-5p) and negative control virus vector (LV-NC), and pcDNA3.1 negative control (oe-NC) and pcDNA3.1-BRD4 (oe-BRD4) were constructed by GenePharm Company, and their titers were determined. The above vectors were injected into rats at a dose of 1 μg via a microsyringe attached to an intrathecal catheter 72 hours before the initiation of CCI.
Pain Threshold Measurement
To eliminate external interference, this experiment should be carried out in a quiet environment. Paw withdrawal threshold (PWT) and paw withdrawal latency (PWL) measurements were performed on predetermined time point after the CCI model was established to assess the sensitivity of mechanical and thermal nociceptive stimuli. PWT was measured using a pain meter. The rats were placed in a transparent glass cage, and their feet were stimulated with a metal probe, and the minimum stimulus intensity (g), namely PWT, which produced the feet contraction was recorded. Each stimulus lasted for 5s, and the interval between two stimuli was 20s. The average value was taken for five consecutive tests. In addition, the rats were placed in plexiglass boxes with a radiant heat source focused on the surface of the hindlimb plantar. When the rats felt pain on the plantar, there was a foot contraction reflex, and the delay time (s) of paw retraction was recorded, which was PWL. Each rat was measured 5 times, and the interval of each measurement was 10min. The heating time was 10s, and the longest time was no more than 20s, so as not to burn the soles of the rats.
Cell Culture and Cell Transfection
HEK-293T cells were purchased from ATCC and were grown in DMEM, 10% FBS and 1% penicillin/streptomycin. Cells should be placed to the incubator with adjustable humidity and air/CO2 at 37°C. For cell transfection, mimic-NC, inhibitor-NC, miR-204-5p mimic and miR-204-5p inhibitor were purchased from GenePharm Company. Cells were inoculated into 6-well plates, and lentiviruses were transfected into cells according to the instructions of Lipofectamine 3000.
Isolation of RNA and RT-qPCR
Total RNA isolation from spinal cord tissues and cells were accomplished by TRIzol kit. After RNA quality and quantity were determined by Nanodrop 2000 spectrophotometer, cDNA was synthesized using PrimeScript RT kit. PCR reactions were performed using SYBR Premix Ex Taq TM II kit in a real-time PCR system to detect gene expression levels in cells and tissues according to manufacturer’s instructions. U6 and GAPDH were selected as the internal references of miR-204-5p and BRD4. Relative levels were determined using the 2−ΔΔCt method. The primer sequences are as follows: miR-204-5p: (forward primer): 5’-CCAGATCTGGAAGAAGATGGT-3’ and (reverse primer): 5’-GCGAATTCACAGTTGCCTACA-3’. BRD4: (forward primer): 5’-ACAACAAGCCTGGAGATGACA-3’ and (reverse primer): 5’-GTTTGGTACCGTGGAAACGC-3’. U6: (forward primer): 5’-CTCGCTTCGGCAGCACA-3 and (reverse primer): 5’-AACGCTTCACGAATTTGCGT-3’. GAPDH: (forward primer): 5’-TCATCTCTGCCCCCTCTGCT-3’ and (reverse primer): 5’-CGACGCCTGCTTCACCACCT-3’.
ELISA
The obtained spinal cord tissue was mixed with RIPA lysate containing protease inhibitor and homogenized at 4°C, then centrifuged and the supernatant was extracted and placed at 4°C for later use. Commercial ELISA kits were used to detect the concentrations of IL-6 and TNF-α. The absorbance value at 450nm was measured with a microplate reader.
Luciferase Reporter Gene
The target gene of miR-204-5p was predicted using the online database TargetScan 7.0, and then the results showed that BRD4 was the target gene. Luciferase reporter gene assay was conducted to verify the relationship of miR-204-5p and BRD4. Briefly, the 3’-URT of wild-type BRD4 (WT-BRD4) and mutant-type BRD4 (MUT-BRD4) was amplified and cloned into pmirGLO vector. Subsequently, WT-BRD4 or MUT-BRD4 was co-transfected into HEK-293T cells with miR-204-5p mimic or inhibitor using Lipofectamine 3000. Cells were collected and treated 48h later, and luciferase activity was detected using a dual-luciferase assay system. Renilla luciferase activity was deemed as the reference.
Data Analysis
Statistical analysis was conducted in SPSS 17.0. Data are expressed as mean ± standard deviation (SD). Student’s t-test and one-way ANOVA were used to analyze the statistical differences in two groups and multiple groups. The P < 0.05 are considered to be significantly different.
Results
MiR-204-5p Level Was Decreased in the CCI Group
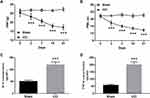
The level of miR-204-5p in spinal cord tissue of rats at 0, 3, 7, 14 and 21 days postoperatively was evaluated. PWT, PWL, IL-6 and TNF-α were detected after CCI surgery, respectively. Compared with sham operation group, the mechanical abnormal pain and thermal hyperalgesia in the CCI group were gradually increased after surgery (Figure 1A and B, P < 0.001). In addition, CCI-induced inflammatory response was also observed in rat models, mainly manifested by increased IL-6 and TNF-α levels (Figure 1C and D, P < 0.001). The above results indicate that the animal model is successfully constructed. Besides, it was observed that the miR-204-5p level in the CCI group showed a time-dependent decreasing trend (Figure 2, P < 0.05), while the miR-204-5p expression in the sham operation group did not change significantly with the extension of time.
 |
Figure 2 MiR-204-5p level was down-regulated in chronic constriction injury (CCI) rat model. One-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001 vs sham group. |
Overexpression of miR-204-5p Alleviates Mechanical Pain Stimulation, Thermal Pain Stimulation and Inflammatory Response in Rats
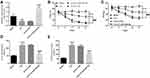
To investigate the effect of abnormally expressed miR-204-5p on the development of nerve pain in rat, miR-204-5p was overexpressed in rat spinal cord tissue by intrathecal injection of LV-miR-204-5p. The results showed that injection of LV-miR-204-5p in rats significantly up-regulated the expression level of miR-204-5p in the CCI group (Figure 3A, P < 0.001). Besides, miR-204-5p overexpression increased the threshold of mechanical and thermal pain stimulation in rats (Figure 3B and C, P < 0.001). Meanwhile, overexpression of miR-204-5p down-regulated the CCI-induced elevation of IL-6 and TNF-α (Figure 3D and E, P < 0.001).
MiR-204-5p Targeted Regulation the BRD4 Expression
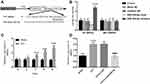
To determine the potential mechanism by which miR-204-5p regulates the development of nerve pain, the potential target genes of miR-204-5p were analyzed and predicted using the TargetScan target gene prediction database. The predicted results suggested that BRD4 might be a potential target gene of miR-204-5p. Further analysis revealed that the 3’-UTR of BRD4 had complementary sequences with miR-204-5p (Figure 4A). In order to confirm whether miR-204-5p directly binds to 3’-UTR of BRD4, the luciferase reporter gene assay was used to verify that overexpression of miR-204-3p significantly reduced luciferase activity in the WT-BRD4 group, while these effects were not observed in the MUT-BRD4 group (Figure 4B, P < 0.001). On the basis of the above studies, the expressions of BRD4 in the rat model were evaluated. Figure 4C revealed that the level of BRD4 showed a time-dependent increase after CCI (P < 0.001) in comparison to the sham group. Further examination revealed that BRD4 expression level in spinal cord tissue of rats was decreased after injection of LV-miR-204-5p (Figure 4D, P < 0.001).
Overexpression of BRD4 Reversed the Alleviative Effect of miR-204-5p on Mechanical Pain Stimulation, Thermal Pain Stimulation and Inflammatory Response in Rats
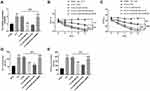
To explore the effect of BRD4 on nerve pain in rats, the expression of BRD4 was regulated by intrathecal injection of oe-BRD4. As shown in Figure 5A, the addition of oe-BRD4 relieved the inhibitory effect of miR-204-5p on BRD4 expression (P < 0.001). The results of the sensitivity of rats to mechanical and thermal pain showed that overexpression of BRD4 made rats more sensitive to mechanical and thermal pain stimuli, which showed that PWL and PWT were significantly reduced (Figure 5B and C, P < 0.001). Additionally, overexpression of BRD4 can also reverse the inhibitory effect of miR-204-5p on IL-6 and TNF-α (Figure 5D and E, P < 0.001).
Discussion
More and more attention has been paid to the function of non-coding RNAs. Numerous studies have reported that miRNA is involved in neuropathic pain. For instance, miR-194 has been shown to alleviate neuropathic pain and prevent neuroinflammation and down-regulate the cytokines, including COX-2, IL-6 and IL-10 by targeting FOXA1.15 In our study, we mainly focused on the role of miR-204-5p in neuropathic pain and tried to find out the possible regulatory mechanism through rat experiments. Based on the literature, the model of chronic compression injury of the sciatic nerve, a recognized classical animal model of neuropathic pain was selected. An analysis of cerebrospinal fluid from 215 healthy subjects showed high levels of miR-204-5p in normal brain and spinal cord tissues, and the authors speculated that diseases in brain and spinal cord tissues may directly lead to the abnormal expression of miR-204-5p.16 In the present study, it was found that miR-204-5p expression was down-regulated in the CCI rat model, and intrathecal injection of miR-204-5p lentivirus not only increased the concentration of miR-204-5p in the spinal cord but also improved the thresholds of mechanical and thermal pain stimulation in rats. This result was consistent with the down-regulation of miR-204-5p in the SCC model described by Shen et al.12
From the perspective of pathological mechanism, the release of inflammatory mediators, such as IL-6, IL-1β and TNF-α, is associated with the occurrence or maintenance of hyperalgesia.17 It has been reported that chronic constriction injury of the sciatic nerve can cause nerve injury and lead to activation of microglia, which are involved in the development and maintenance of neuropathic pain by releasing pro-inflammatory factors.18 There is sufficient evidence that miR-204-5p plays an important regulatory role in inflammatory diseases. Studies have shown that miR-204-5p is an inhibitor of inflammatory response, and miR-204-5p can inhibit inflammation and chemokine production in renal tubular epithelial cells by regulating the IL-6/IL-6 receptor axis, while inflammatory cytokine-mediated neuroinflammation is associated with the progression of neuropathic pain.19 Other studies have confirmed that miR-204 is involved in neuroinflammation induced by spinal cord compression in rats.12 In this study, CCI surgery induced an inflammatory response in rats, with elevated levels of IL-6 and TNF-α. It is worth investigating that overexpression of miR-204-5p significantly reduced the levels of IL-6 and TNF-α in CCI rats, thereby alleviating the inflammatory response.
MiRNAs usually play regulatory roles in various physiological and pathological processes as competing endogenous RNAs (ceRNAs). Salmen et al speculated that miRNAs complete the communication between genes by binding to the corresponding miRNA response element (MREs) at the 3’-UTR.20 For example, miR-101 plays a role in regulating nerve pain by targeting KPBN1.21 The binding site of miR-204-5p to BRD4 were analyzed using the database, and this result was verified via luciferase reporter gene assay. We could see that miR-204-5p was negatively correlated with BRD4 level. In the CCI rat model, miR-204-5p was under-expressed and BRD4 was overexpressed. BRD4, a member of the BET family, can bind to acetylated histones or non-histone proteins to regulate gene replication and transcription, thereby affecting cell cycle, differentiation, signal transduction and other processes.22,23 Abnormal up-regulation of BRD4 is closely associated with tumor and inflammation, and inhibition of BRD4 could effectively delay the progression and metastasis of the disease. It was reported that reduced BRD4 expression improves peri-neuropathy and neuropathic pain induced by the anticancer drug vincristine by inhibiting inflammatory response and oxidative stress.24 In addition, the relationship between miR-204-5p and BRD4 has been confirmed in some other studies. Zheng et al reported that miR-204-5p could be directly combined with BRD4 and negatively regulates BRD4 expression in squamous cell carcinoma.25
Our current studies on miR-204-5p mainly focus on its effects on pain threshold and inflammatory response in animal models, while previous reports suggest that patients with chronic pain may suffer from long-term mood disorders, such as depression. Animal models show that rats appear anxiety, tension, and other emotional abnormalities after CCI (30130302, 30284123, 22854119). We can hypothesize that modulation of miR-204-5p can improve neuropathic pain and, in turn, the emotional impact of pain. Therefore, it will be very meaningful to design experiments to further evaluate whether miR-204-5p can alleviate the emotional problems caused by neuropathic pain, and it also helps to further confirm the alleviation effect of miR-204-5p on neuropathic pain.
Conclusion
In conclusion, abnormal miR-204-5p plays a crucial role in neuropathic pain. MiR-204-5p alleviated neuropathic pain and inflammatory responses in CCI rats by targeting BRD4, which preliminarily indicated that miR-204-5p may be a breakthrough point in the treatment of neuropathic pain.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yu C, Li P, Wang YX, Zhang KG, Zheng ZC, Liang LS. Sanguinarine attenuates neuropathic pain by inhibiting P38 MAPK activated neuroinflammation in rat model. Drug Des Devel Ther. 2020;14:4725–4733. doi:10.2147/dddt.S276424
2. Tsymbalyuk S, Smith M, Gore C, et al. Brivaracetam attenuates pain behaviors in a murine model of neuropathic pain. Mol Pain. 2019;15:1744806919886503. doi:10.1177/1744806919886503
3. Lee JB, Choi SS, Ahn EH, et al. Effect of perioperative perineural injection of dexamethasone and bupivacaine on a rat spared nerve injury model. Korean J Pain. 2010;23(3):166–171. doi:10.3344/kjp.2010.23.3.166
4. Li T, Wan Y, Sun L, et al. Inhibition of microRNA-15a/16 expression alleviates neuropathic pain development through upregulation of G protein-coupled receptor kinase 2. Biomol Ther. 2019;27(4):414–422. doi:10.4062/biomolther.2018.073
5. Farhana L, Antaki F, Anees MR, et al. Role of cancer stem cells in racial disparity in colorectal cancer. Cancer Med. 2016;5(6):1268–1278. doi:10.1002/cam4.690
6. Zhang MY, Wang LQ, Chim CS. miR-1250-5p is a novel tumor suppressive intronic miRNA hypermethylated in non-Hodgkin’s lymphoma: novel targets with impact on ERK signaling and cell migration. Cell Commun Signal. 2021;19(1):62. doi:10.1186/s12964-021-00707-0
7. Henriksen M, Johnsen KB, Andersen HH, Pilgaard L, Duroux M. MicroRNA expression signatures determine prognosis and survival in glioblastoma multiforme–a systematic overview. Mol Neurobiol. 2014;50(3):896–913. doi:10.1007/s12035-014-8668-y
8. Imai S, Saeki M, Yanase M, et al. Change in microRNAs associated with neuronal adaptive responses in the nucleus accumbens under neuropathic pain. J Neurosci. 2011;31(43):15294–15299. doi:10.1523/jneurosci.0921-11.2011
9. Arai M, Genda Y, Ishikawa M, Shunsuke T, Okabe T, Sakamoto A. The miRNA and mRNA changes in rat hippocampi after chronic constriction injury. Pain Med. 2013;14(5):720–729. doi:10.1111/pme.12066
10. Sarti S, De Paolo R, Ippolito C, Pucci A, Pitto L, Poliseno L. Inducible modulation of miR-204 levels in a zebrafish melanoma model. Biol Open. 2020;9(11). doi:10.1242/bio.053785
11. Wang Y, Ye F, Huang C, et al. Bioinformatic analysis of potential biomarkers for spinal cord-injured patients with intractable neuropathic pain. Clin J Pain. 2018;34(9):825–830. doi:10.1097/ajp.0000000000000608
12. Shen WS, Li CF, Zhou ZS, Zhai NN, Pan LP. MicroRNA-204 silencing relieves pain of cervical spondylotic radiculopathy by targeting GDNF. Gene Ther. 2020;27(6):254–265. doi:10.1038/s41434-019-0114-3
13. Tian J, Song T, Wang H, et al. Intrathecal injection of SIRT1-modified human mesenchymal stem cells alleviates neuropathic pain in rat. J Mol Neurosci. 2021;71(5):972–980. doi:10.1007/s12031-020-01717-2
14. Zhang QL, Li SY, Li P. 人参皂苷Rg2对慢性坐骨神经损伤大鼠痛觉敏化和抑郁状态的影响. [Effects of ginsenoside-Rg(2) on mechanical allodynia, heat hyperalgesia, depressive state of rats with chronic sciatic nerve constriction injury]. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2019;35(3):228–231. Chinese. doi:10.12047/j.cjap.5763.2019.049
15. Zhang X, Chen Q, Shen J, Wang L, Cai Y, Zhu KR. miR-194 relieve neuropathic pain and prevent neuroinflammation via targeting FOXA1. J Cell Biochem. 2020;121(5–6):3278–3285. doi:10.1002/jcb.29598
16. Muñoz-San Martín M, Gomez I, Miguela A, et al. Description of a CSF-enriched miRNA panel for the study of neurological diseases. Life. 2021;11(7):594. doi:10.3390/life11070594
17. Gangadharan V, Kuner R. Pain hypersensitivity mechanisms at a glance. Dis Model Mech. 2013;6(4):889–895. doi:10.1242/dmm.011502
18. Homburger JR, Green EM, Caleshu C, et al. Multidimensional structure-function relationships in human β-cardiac myosin from population-scale genetic variation. Proc Natl Acad Sci U S A. 2016;113(24):6701–6706. doi:10.1073/pnas.1606950113
19. Zilberstein D, Philosoph H, Gepstein A. Maintenance of cytoplasmic pH and proton motive force in promastigotes of Leishmania donovani. Mol Biochem Parasitol. 1989;36(2):109–117. doi:10.1016/0166-6851(89)90183-7
20. Sumazin P, Yang X, Chiu HS, et al. An extensive microRNA-mediated network of RNA-RNA interactions regulates established oncogenic pathways in glioblastoma. Cell. 2011;147(2):370–381. doi:10.1016/j.cell.2011.09.041
21. Liu JC, Xue DF, Wang XQ, Ai DB, Qin PJ. MiR-101 relates to chronic peripheral neuropathic pain through targeting KPNB1 and regulating NF-κB signaling. Kaohsiung J Med Sci. 2019;35(3):139–145. doi:10.1002/kjm2.12025
22. Feng L, Wang G, Chen Y, et al. Dual-target inhibitors of bromodomain and extra-terminal proteins in cancer: a review from medicinal chemistry perspectives. Med Res Rev. 2022;42(2):710–743. doi:10.1002/med.21859
23. Rhyasen GW, Yao Y, Zhang J, et al. BRD4 amplification facilitates an oncogenic gene expression program in high-grade serous ovarian cancer and confers sensitivity to BET inhibitors. PLoS One. 2018;13(7):e0200826. doi:10.1371/journal.pone.0200826
24. Zhang K, Xu Y. Suppressing BRD4 exhibits protective effects against vincristine-induced peripheral neuropathy by alleviating inflammation and oxidative stress. Biochem Biophys Res Commun. 2020;532(2):271–279. doi:10.1016/j.bbrc.2020.06.142
25. Zheng J, Zhang YW, Li TK, Bao Y, Zhang SX. miR-204-5p靶向溴结构域蛋白4对舌鳞状细胞癌细胞SCC25增殖和迁移侵袭的影响研究. [Effect of miR-204-5p on the proliferation, migration, and invasion on tongue squamous cell carcinoma SCC25 cells by targeting bromodomain-containing protein 4]. Hua xi kou qiang yi xue za zhi. 2020;38(2):185–192. Chinese. doi:10.7518/hxkq.2020.02.013
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.