Back to Journals » OncoTargets and Therapy » Volume 9
miR-203 facilitates tumor growth and metastasis by targeting fibroblast growth factor 2 in breast cancer
Authors He S, Zhang G, Dong H, Ma M, Sun Q
Received 17 March 2016
Accepted for publication 8 August 2016
Published 11 October 2016 Volume 2016:9 Pages 6203—6210
DOI https://doi.org/10.2147/OTT.S108712
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Li
Shuqian He, Guihui Zhang, He Dong, Maoqiang Ma, Qing Sun
Department of Pathology, Qianfoshan Hospital Affiliated to Shandong University, Jinan, Shandong, People’s Republic of China
Abstract: Breast cancer is the second leading cause of cancer mortality in women worldwide. Molecular therapy is needed to improve the outcome in patients with breast cancer. miR-203 participates in cancer cell proliferation, transformation, and apoptosis. This study showed that miR-203 was upregulated in breast cancer tissues and the MCF-7 cell line. miR-203 knockdown suppressed colony formation and transformation and also limited migration in MCF-7 cells. Fibroblast growth factor 2 (FGF2) was confirmed as a novel target of miR-203, as miR-203 knockdown induced an enhanced expression of FGF2 in MCF-7 cells. Moreover, FGF2 can reverse transforming growth factor-β signal pathway to suppress breast cancer. These findings provide new insights with potential therapeutic applications for the treatment of breast cancer.
Keywords: breast cancer, miR-203, FGF2
Introduction
As the second leading cause of cancer mortality in women, breast cancer is a serious public health problem worldwide, and the age of onset tends to be younger in recent years.1 According to the International Agency for Research on Cancer, ~1.7 million women were diagnosed with breast cancer in 2012, and breast cancer incidence has increased by >20% since 2008.2 Although early diagnosis and more effective treatment can decline the incidence of breast cancer, morbidity and mortality remain high and the prognosis is poor.3 Therefore, there is a growing need to understand the molecular pathogenesis of breast cancer. Fortunately, molecular cancer biology has led to an increased understanding of factors that contribute to breast cancer pathogenesis and progression in recent years.4
MicroRNAs (miRNAs) are a kind of small noncoding single-stranded RNAs from the endogenous chromosome, which are highly conservative in evolution. miRNAs mediate posttranslational regulation via base pairing with target messenger RNAs,5 thereby playing an important role in the control of cancer cell growth.6–8 A number of studies have demonstrated that the expression levels of miRNAs are significantly changed in at least one tumor type, and the regulation of miRNAs is closely related to the proliferation and transformation of cancer cells.9 For instance, miR-15a and miR-16-1 were shown to be downregulated in patients with B-cell chronic lymphocytic leukemia, while miR-21 was found to be upregulated in human non-small cell lung cancer.10,11 As the first skin-specific miRNA reported recently, miR-203 not only involved in regulating embryonic epidermal differentiation, building the protective layer of skin as well as skin diseases such as psoriasis but also participated in cancer cell proliferation, transformation, and apoptosis by cooperating with target gene as suppressor or carcinogen factor.12 To explore the action mechanism of miR-203, ~588 target sites of miR-203 were predicted by TargetScan and miRanda bioinformatics software.13 As a well-known tumor suppressor, the downregulated expression of miR-203 has been described in several types of cancer, such as human esophageal squamous cell carcinoma,14 lung cancer,15 pancreatic cancer,16 colon cancer,17 bladder cancer,18 and hepatocellular carcinomas.19 On the contrary, further research has shown that miR-203 is highly expressed in breast cancer tissue compared with the adjacent noncancer breast tissue.20,21 However, the functional role and mechanistic action of miR-203 in breast cancer are still unclear.
In this study, we demonstrate the biological function, molecular mechanisms, and target gene of miR-203 in breast cancer.
Patients and methods
Patients
A total of ten patients with breast cancer were enrolled in our study, who were recruited from Qianfoshan Hospital Affiliated to Shandong University. A total of ten patient-matched noncancerous tissues were obtained from patients with breast cancer undergoing surgery. The diagnosis of breast cancer was established using the World Health Organization’s morphological criteria.22 A written form of informed consent was obtained from all patients participating in the study. The study was approved by the Medical Ethics Committee of Qianfoshan Hospital Affiliated to Shandong University.
Quantitative real-time polymerase chain reaction
Total RNA was extracted from breast cancer tissues or cultured MCF-7 cells using the Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA). cDNA was synthesized from 1 μg of total RNA using One Step RT-PCR Kit (TaKaRa). The expression level of miR-203 was measured using a TaqMan miRNA assay (Thermo Fisher Scientific) according to the provided protocol and using U6 small nuclear RNA as an internal control. The expression of miR-203 was detected using Power SYBR Green Kit (Thermo Fisher Scientific). All experiments were performed in triplicate.
miRNAs transfection
MCF-7 cells were seeded in six-well plates at a concentration of 1×105 and cultured in medium without antibiotics for ~24 hours before transfection. Cells were transiently transfected with miRNA inhibitor negative control and anti-miR-203 (Thermo Fisher Scientific) at a final concentration of 200 nM.
Cell proliferation, transformation, and migration assay
Cloning formation assay
MCF-anti-miR-203 and MCF-control (cont) cells were seeded into a 10 cm cell culture plate and incubated for 10 days at 37°C after treatment. Then, cells were washed twice with phosphate-buffered saline, fixed with cold methanol, and then counted and analyzed by Wright’s stain.
Wound healing assay
According to the provided protocol, 70 μL of MCF-anti-miR-203 and MCF-cont suspended cells were seeded into Culture-Inserts (Ibidi) each well and incubated for 24 hours at 37°C and 5% CO2. After appropriate cell attachment, the culture inserts were gently removed using sterile tweezers. The situation of wound healing was observed by measuring the wound width over time.
Transwell assay
MCF-anti-miR-203 and MCF-cont cells were serum starved overnight and resuspended in serum-free medium. According to the provided protocol, 400 μL cell suspension was placed in the upper transwell of Matrigel (BD Biosciences, San Jose, CA, USA). The chambers were placed in 24-well plates and cultured in 600 μL RPMI medium with 20% fetal bovine serum for 24 hours at 37°C to allow the cells to migrate through the porous membrane. The migration capacity was evaluated as the total number of cells on the lower surface of the membrane counted by microscopy.
Luciferase assay
The miR-203 putative conserved binding sites from 3′ untranslated region (UTR) fibroblast growth factor 2 (FGF2) was cloned into the reporter luciferase vector (Ambion, Austin, TX, USA). Mutant FGF2 3′UTRs bearing a substitution of three nucleotides in the miR-203 target sequence were generated using a Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA, USA). Then, miR-203 inhibitor or control miRNA was cotransfected with wild-type or mutant luciferase reporter plasmid DNAs into MCF-7 cells in 24-well plates using Lipofectamine 2000. Luciferase activity was measured after 48 hours.
Western blot
MCF-7 cells were transduced with replication-deficient lentivirus expressing anti-miR-203 or control miRNA. Cells were selected with puromycin to establish a stable cell line and then lysed with radioimmunoprecipitation assay buffer supplied with the mixture of protease inhibitors (Thermo Fisher Scientific). Equal amounts of protein of cells were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After blotting on polyvinylidene difluoride membranes, the membrane was blocked with 3% fat-free milk for 2 hours, which were probed with primary antibody for FGF2, p-extracellular signal-regulated kinase (ERK), transforming growth factor (TGF)-β, p-Smad, E-cadherin, and glyceraldehyde 3-phosphate dehydrogenase (Santa Cruz Biotechnology Inc., Dallas, TX, USA) at 4°C overnight. Then, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 2 hours at room temperature. Signals were detected using enhanced chemiluminescence reagents (Thermo Fisher Scientific).
Statistical analysis
Student’s t-test was performed to compare the differences between two groups. The analysis of more than two groups was performed with a two-way ANOVA. P≤0.05 was considered statistically significant.
Results
miR-203 is upregulated in breast cancer
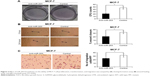
To determine the role of miR-203 in breast cancer, quantitative real-time polymerase chain reaction was performed on RNA extracted from ten pairs of breast cancer tissues and patient-matched noncancerous tissues. Figure 1A shows that miR-203 expression level is higher in breast tumor tissues (seven of ten) than in patient-matched noncancerous tissues, and Figure 1B shows that the average value of ten samples reveals remarkable miR-203 upregulated expression in tumor tissues compared with normal breast tissues, which is consistent with recent reports.20,21 Next, we measured the miR-203 expression level in breast cell line, MCF-7, and primary human mammary epithelial cells. Figure 1C shows an enhanced expression level of miR-203 in breast cancer cells, MCF-7, compared with human mammary epithelial cells. Based on the findings from miRNA array, we considered that miR-203 was overexpressed in breast cancer, and focused on miR-203 for further studies to evaluate its role in breast cancer pathogenesis.
Anti-miR-203 inhibits the proliferation, transformation, and migration capacities of MCF-7 cells
The significant upregulation of miR-203 expression in breast cancer tissues suggested possible biological significance in tumorigenesis. To explore the potential biological effect of unusually high expression of miR-203 on breast cancer, we transfected miRNA-cont and anti-miR-203 into the MCF-7 breast cancer cell line. Cloning formation assay was utilized to evaluate cell proliferation capacity, and the results showed that miR-203 knockdown could repress the cell growth of MCF-7 cells (Figure 2A). Then, wound healing assay was performed to assess cell transformation activity, and the results revealed that the healing degree was significantly inferior in anti-miR-203 MCF-7 cells compared with control (Figure 2B). Finally, transwell assay was used to estimate cell migration capacity, and the result demonstrated that few cells moved on the lower membrane surface by counting with microcopy (Figure 2C). Moreover, cell proliferation, transformation, and migration capacities were obviously lower in MCF-7 cells when miR-203 was knocked down.
miR-203 targets FGF2 via binding to its 3′UTR
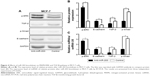
To ascertain the underlying mechanisms of how knockdown of miR-203 inhibits the growth of MCF-7 cells, potential miR-203 target genes were searched by TargetScan and miRanda computer prediction methods. We found a putative miR-203 target site in FGF2 3′UTR (Figure 3A). The putative binding site retains perfect conservation in FGF2 3′UTR. FGF2 plays an important role in regulating cell proliferation, differentiation, and apoptosis. FGF2 induces cell proliferation in various types of cancer.23 Luciferase assay was investigated to find the interaction between miR-203 and 3′UTR of FGF2. The result demonstrated that miR-203 repressed wild-type FGF2 3′UTR directly, but mutant 3′UTR of FGF2 luciferase activity was unaffected by miR-203 (Figure 3B). To examine whether miR-203 knockdown enhances the expression of FGF2, we implemented Western blot analysis in MCF-7 cells with anti-miR-203 or miR-cont. Along with the decrease in miR-203 in MCF-7 cells (Figure 3C), the expression level of FGF2 significantly increased compared with glyceraldehyde 3-phosphate dehydrogenase (Figure 3D), which was consistent with the earlier luciferase assay result. Overall, all the experiments suggested that FGF2 was a novel direct target of miR-203.
Mitogen-activated protein kinase/ERK and TGF-β pathway are involved in FGF2 effect
FGF2 participates in valve interstitial cell growth through TGF-β signal pathway24 and increases the liquidity of lung epithelial cancer cells by reversing epithelial–mesenchymal transition (EMT) via mitogen-activated protein kinase (MAPK)/ERK pathway.25 To investigate the effect of FGF2 on MAPK/ERK and TGF-β pathways in breast cancer, we generated an MCF-7 cell line overexpressing FGF2 by knocking down miR-203. Then, Western blot was utilized to examine the expression level of p-ERK, TGF-β, p-Smad, and E-cadherin, which are the key proteins in EMT. Finally, the increased expression of p-ERK and E-cadherin and the decreased expression of TGF-β and p-Smad were observed (Figure 4A and B), which was consistent with homologous miRNA expression (Figure 4C). The results suggested that FGF2 can reverse TGF-β signal pathway via activated MAPK/ERK pathway.
Discussion
In this study, we identified that miR-203 was markedly upregulated in breast cancer cell line and clinical specimens. Then, we demonstrated that the abnormal increased expression of miR-203 played an important role in carcinogenesis and progression of breast cancer, since miR-203 knockdown inhibited the proliferation, transformation, and migration of the MCF-7 breast cancer cell line. Interestingly, we proved that FGF2 was a novel direct target of miR-203 by luciferase assay. By Western blot, we discovered that FGF2 could activate MAPK/ERK pathway to inhibit TGF-β pathway. Our study demonstrated that miR-203 acts as a carcinogen factor by targeting FGF2 in breast cancer.
miR-203 not only regulates cell differentiation, proliferation, and apoptosis but also participates in tumor invasion, angiogenesis, and transformation. The relationship between miRNA and cancers has been explored using various techniques from different views, such as polymorphism, epigenetic phenomenon, and target gene.26 miR-203 was proved to act as a tumor-suppressive miRNA, and its expression was downregulated in laryngeal squamous cell carcinoma.27 A related report showed that miR-203 leads to G1 phase cell cycle arrest in laryngeal carcinoma cells by directly targeting survival.28 Boll et al29 reported that miR-203 was downregulated in prostate carcinoma and had an effect on tumor growth. Moreover, miR-203 was considered a tumor suppressor in ER (−), PgR (−), and HER2 (−) breast cancer with the low expression.30 As an antitumor, miR-203 was downregulated in prostatic cancer and mediated the LIM and SH3 domain protein 1 overexpression in progression and metastasis of tumor cell.31 Nevertheless, miR-203 was upregulated in oxaliplatin-insensitive colorectal cancer cells, which induced oxaliplatin resistance in colorectal cancer cells.32 Unlike the earlier study, we found that the expression of miR-203 enhanced abnormally in breast cancer tissues compared with patient-matched noncancerous tissues by quantitative real-time polymerase chain reaction (Figure 1). Proliferation, transformation, and migration activities were inhibited when miR-203 was knocked down in the MCF-7 breast cancer cell line; Figure 2). Based on the different findings, it is possible that miR-203 is not only a complete suppressor in cancer, but it may also play a different role to suppress or promote cell growth in different tumor tissues. In addition, miR-203 was expressed several 100-fold higher in less-invasive breast cancer cell lines as compared with the highly invasive breast cancer cell lines. Restoring miR-203 expression in highly invasive breast cancer cell lines inhibits tumor cell invasion.33,34 Due to the several mechanisms involved in tumor initiation and progression, we consider that miR-203’s two-sided influence on cancer cells is existent, and the overexpression of miR-203 in breast cancer is taken for an oncogene.
However, the molecular mechanism of tumor is still not clear enough. Only a small portion of the biological function of miR-203 target genes is identified, and little is known about the corresponding specific regulating mechanism of target genes. In the current study, luciferase assay revealed an inverse correlation between FGF2 and miR-203 expression. As shown in Figure 3, FGF2 was confirmed to be a novel target of miR-203. FGF2 can induce the proliferation, differentiation, and motility of various types of cancer cells. An earlier study had proposed that MAPK-induced phosphorylation of the Smad2/3 linker region activated TGF-β-induced Smad2/3, which is suppressed by oncogenic Ras.35 FGF2-induced MAPK signal pathway suppressed TGF-β signaling through phosphorylation of Smad2 in lymphatic endothelial cells.36 In addition, with the reduction of E-cadherin expression, epithelial cells change into migrational mesenchymal cells in EMT, which is the first step of tumor transformation and migration.37 Thus, we demonstrate that FGF2 inhibits the expression of TGF-β by activating MAPK/ERK signal pathway, while TGF-β facilitates tumor cell growth, migration, and transformation via Smad phosphorylation. The identification of FGF2 as a miR-203 target gene may explain, at least in part, the molecular mechanism of carcinogen factor by miR-203.
Conclusion
miR-203 may present a potential therapeutic target for breast cancer, and validating its clinical treatment value in large cohorts of breast cancer samples for improving treatment efficiencies is needed.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (no 81272420), Scientific and Technological Development Projects in Shandong Province of China (no 2011GSF11838), Shandong Province Natural Science Foundation (no ZR2012HM085), and Scientific and Technological Development Projects of Jinan City (no 201202039).
Disclosure
The authors report no conflicts of interest in this work.
References
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2007;57(1):43–66. | ||
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. | ||
Clarke M. Meta-analyses of adjuvant therapies for women with early breast cancer: the Early Breast Cancer Trialists’ Collaborative Group overview. Ann Oncol. 2006;17(suppl 10):x59–x62. | ||
Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that over-expresses HER2. N Engl J Med. 2001;344(11):783–792. | ||
O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. C-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435(7043):839–843. | ||
Trang P, Weidhaas JB, Slack FJ. MicroRNAs as potential cancer therapeutics. Oncogene. 2008;27(suppl 2):S52–S57. | ||
Iorio MV, Croce CM. MicroRNAs in cancer: small molecules with a huge impact. J Clin Oncol. 2009;27(34):5848–5856. | ||
Hurst DR, Edmonds MD, Welch DR. Metastamir: the field of metastasis-regulatory microRNA is spreading. Cancer Res. 2009;69(19):7495–7498. | ||
Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103(7):2257–2261. | ||
Calin GA, Sevignani C, Dumitru CD, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101(9):2999–3004. | ||
Shen YZ, Pan XF, Zhao H. The histone demethylase PHF8 is an oncogenic protein in human non-small cell lung cancer. Biochem Biophys Res Commun. 2014;451(1):119–125. | ||
Chim CS, Wong KY, Leung CY, et al. Epigenetic inactivation of the hsa-miR-203 in haematological malignancies. J Cell Mol Med. 2011;15(12):2760–2767. | ||
Cui XB, Chen YS, Pan QF, Li F. Research progress of miR-203 and relation of its target sites and cancer. J Clin Exp Pathol. 2012;28:189–191. | ||
Yuan Y, Zeng ZY, Liu XH, et al. MicroRNA-203 inhibits cell proliferation by repressing ΔNp63 expression in human esophageal squamous cell carcinoma. BMC Cancer. 2011;11:57. | ||
Wang C, Wang XL, Liang HW, et al. miR-203 inhibits cell proliferation and migration of lung cancer cells by targeting PKCα. PLoS One. 2013;8(9):e73985. | ||
Ikenaga N, Ohuchida K, Mizumoto K, et al. MicroRNA-203 expression as a new prognostic marker of pancreatic adenocarcinoma. Ann Surg Oncol. 2010;17(12):3120–3128. | ||
Li J, Chen Y, Zhao J, Kong F, Zhang Y. miR-203 reverses chemoresistance in p53-mutated colon cancer cells through downregulation of Akt2 expression. Cancer Lett. 2011;304(1):52–59. | ||
Bo J, Yang G, Huo K, et al. microRNA-203 suppresses bladder cancer development by repressing bcl-w expression. FEBS J. 2011;278(5):786–792. | ||
Wei W, Wanjun L, Hui S, Dongyue C, Xinjun Y, Jisheng Z. miR-203 inhibits proliferation of HCC cells by targeting survivin. Cell Biochem Funct. 2013;31(1):82–85. | ||
Ru P, Steele R, Hsueh EC, Ray RB. Anti-miR-203 upregulates SOCS3 expression in breast cancer cells and enhances cisplatin chemosensitivity. Genes Cancer. 2011;2(7):720–727. | ||
Zhang Z, Zhang B, Li W, et al. Epigenetic silencing of miR-203 upregulates SNAI2 and contributes to the invasiveness of malignant breast cancer cells. Genes Cancer. 2011;2(8):782–791. | ||
Hamilton SR, Aaltonen LA. Pathology and Genetics of Tumors of the Digestive System. Lyon: IARC Press; 2000. | ||
Chandler LA, Sosnowski BA, Greenlees L, Aukerman SL, Baird A, Pierce GF. Prevalent expression of fibroblast growth factor (FGF) receptors and FGF2 in human tumor cell lines. Int J Cancer. 1999;81(3):451–458. | ||
Han L, Gotlieb AI. Fibroblast growth factor-2 promotes in vitro mitral valve interstitial cell repair through transforming growth factor-β/Smad signaling. Am J Pathol. 2011;178(1):119–127. | ||
Geng WW, Zhang B, Li DH, Liang XR, Cao XC. Molecular mechanism of fibroblast growth factor 2 reversing epithelial-mesenchymal-transition. Chin J Exp Surg. 2013;30:99–101. | ||
You WY, Li F. Single nucleotide polymorphism of microRNA and esophagus cancer. J Clin Exp Pathol. 2011;27:758–759. | ||
Tian L, Li M, Ge J, et al. miR-203 is downregulated in laryngeal squamous cell carcinoma and can suppress proliferation and induce apoptosis of tumours. Tumour Biol. 2014;35(6):5953–5963. | ||
Bian K, Fan J, Zhang X, et al. MicroRNA-203 leads to G1 phase cell cycle arrest in laryngeal carcinoma cells by directly targeting survivin. FEBS Lett. 2012;586(6):804–809. | ||
Boll K, Reiche K, Kasack K, et al. MiR-130a, miR-203 and miR-205 jointly repress key oncogenic pathways and are downregulated in prostate carcinoma. Oncogene. 2012;32(3):277–285. | ||
Wang C, Zheng X, Shen C, Shi Y. MicroRNA-203 suppresses cell proliferation and migration by targeting BIRC5 and LASP1 in human triple-negative breast cancer cells. J Exp Clin Cancer Res. 2012;31:58. | ||
Hailer A, Grunewald TG, Orth M, et al. Loss of tumor suppressor mir-203 mediates over-expression of LIM and SH3 Protein 1 (LASP1) in high-risk prostate cancer thereby increasing cell proliferation and migration. Oncotarget. 2014;5(12):4144–4153. | ||
Zhou Y, Wan G, Spizzo R, et al. miR-203 induces oxaliplatin resistance in colorectal cancer cells by negatively regulating ATM kinase. Mol Oncol. 2014;8(1):83–92. | ||
Ding X, Park SI, McCauley LK, et al. Signaling between transforming growth factor Î2 (TGF-Î2) and transcription factor SNAI2 represses expression of microRNA miR-203 to promote epithelial mesenchymal transition and tumor metastasis. J Biol Chem. 2013;288:10241–10253. | ||
Taipaleenmäki H, Browne G, Akech J, et al. Targeting of Runx2 by miR-135 and miR-203 impairs progression of breast cancer and metastatic bone disease. Cancer Res. 2015;75(7):1433–1444. | ||
Kretzschmar M, Doody J, Timokhina I, Massagué J. A mechanism of repression of TGF beta/Smad signaling by oncogenic Ras. Genes Dev. 1999;13(7):804–816. | ||
Ichise T, Yoshida N, Ichise H. FGF2-induced Ras-MAPK signalling maintains lymphatic endothelial cell identity by upregulating endothelial-cell-specific gene expression and suppressing TGF-β signalling through Smad2. J Cell Sci. 2014;127(pt 4):845–857. | ||
Jiang J, Tang YL, Liang XH. EMT: a new vision of hypoxia promoting cancer progression. Cancer Biol Ther. 2011;11(8):714–723. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.