Back to Journals » OncoTargets and Therapy » Volume 10
miR-199a modulates cisplatin resistance in ovarian cancer by targeting Hif1α
Authors Feng X, Liu N, Deng S, Zhang DD, Wang KX, Lu MS
Received 8 July 2017
Accepted for publication 14 September 2017
Published 12 December 2017 Volume 2017:10 Pages 5899—5906
DOI https://doi.org/10.2147/OTT.S145833
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Samir Farghaly
Xue Feng,1 Ning Liu,2 Suo Deng,1 Dandan Zhang,1 Kexin Wang,1 Meisong Lu1
1Department of Obstetrics and Gynecology, 2Department of Orthopedic Surgery, The First Affiliated Hospital of Harbin Medical University, Heilongjiang, Harbin, People’s Republic of China
Abstract: Resistance to chemotherapy is a primary problem for the effective treatment of ovarian cancer. Recently, increasing evidence has demonstrated that miRNAs modulate many important molecular pathways involved in chemotherapy. Previous studies demonstrated that miR-199a affected ovarian cancer cell resistance to cisplatin (DDP). However, the role of miR-199a and its target genes in determination of ovarian cancer sensitivity to DDP remains unclear. Quantitative reverse transcription polymerase chain reaction was used to detect the expression levels of miR-199a in ovarian cancer tissues and C13* and OV2008 cell lines. After transfection of miR-199a mimic or inhibitor, flow cytometry was used to detect cell apoptosis exposed to DDP. Enzyme-linked immunosorbent assay and Western blot assay were applied to detect tumor necrosis factor-α levels and protein expression levels of Bax, Fas, Fas-associated death domain, and caspase-8. The results indicated that the expression of miR-199a was downregulated and hypoxia-inducible factor 1α (Hif1α) upregulated in the ovarian tumors compared with those in the corresponding normal tissues. Besides, the expression levels of miR-199a were significantly higher in OV2008 cells compared with those in C13* cells. Moreover, suppression of Hif1α reversed the inhibiting function of miR-199a inhibitor on DDP-induced apoptosis in the OV2008 cells. However, overexpression of both miR-199a and Hif1α reduced DDP-induced apoptosis in C13* cells. In conclusion, miR-199a may change DDP resistance in ovarian cancer by regulating Hif1α.
Keywords: miR-199a, ovarian cancer, cisplatin resistance, Hif1α
Introduction
Ovarian cancer is one of the most common gynecologic malignancies and the primary cause of gynecologic cancer deaths for women.1 Nowadays, ovarian cancer has become a serious threat to women’s health in China because of its high incidence and mortality.2 One of the current standard treatments for women with ovarian cancer is platinum-based chemotherapy.3 Cisplatin (DDP) is a platinum compound that is used as a first line of therapy in ovarian cancer.4 Moreover, DDP directly covalently binds to nuclear DNA, leading to transcription inhibition processes.5 More than 70% of patients initially respond to DDP, but the 5-year survival rate is only about 30%.6 DDP resistance is the major cause of chemotherapy failure.7 As the apoptotic response of cancer cells to drugs is used to evaluate the sensitivity to chemotherapy,8 the decreased cellular uptake of chemotherapeutic drugs accompanied by increased antiapoptotic capacity may be important in DDP resistance in ovarian cancer.9 Therefore, exploring the molecular mechanisms of DDP resistance in ovarian cancer cells has the potential to improve the therapy of the disease.
miRNAs are endogenous, noncoding RNAs that bind to complementary sequences on target mRNA transcripts to cause translational repression or target degradation.10 miR-199a is located on human chromosome 19q13.2, and a low expression of miR-199a has been detected in human ovarian carcinoma.11 Although recent reports demonstrate that miR-199a may affect ovarian cancer cell resistance to DDP,12 the role of miR-199a and its target mRNAs in determination of ovarian cancer sensitivity to DDP still needs to be investigated.
Hypoxia-inducible factor 1 (Hif1) is overexpressed in human cancers under intratumoral hypoxia.13 In addition, its subunit Hif1α has been widely studied as an endogenous hypoxia marker.14 The Hif1α protein overexpression is more frequently observed in the ovarian cancer specimens than in noncancerous ovarian tissues.15 Besides, a previous study demonstrated that Hif1α contained a potential binding site in its 3′ untranslated region for miR-199a in the ovarian cancer cells.16
The purpose of this study was to investigate the regulatory mechanism of miR-199a in DDP drug resistance in the human OV2008 and C13* ovarian cancer cell lines. Also, miR-199a may be a new strategy for DDP resistance chemotherapy in patients with ovarian cancer.
Patients and methods
Patients
This study was approved by the Research Ethics Committee of The First Affiliated Hospital of Harbin Medical University. Written informed consent was obtained from all of the patients. The ovarian cancer tissue samples and corresponding normal tissues were collected from 23 patients diagnosed with primary ovarian cancer after operation at The First Affiliated Hospital of Harbin Medical University from January 2008 to December 2012. The normal tissues were >1.5 cm away from the edge of the ovarian cancer tissues in 23 patients. None of the patients received any chemotherapy or radiotherapy before operation. The samples were immediately snap-frozen in liquid nitrogen and kept at −80°C.
Cell lines and cell culture
The cisplatin-resistant ovarian cancer cell line (C13*) and its sensitive variant (OV2008) were gifts from Dr Rakesh Goel at Ottawa Regional Cancer Center (Ottawa, Canada). Cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 media supplemented with 2 mM L-glutamine, 10% fetal bovine serum, 100 U/mL penicillin, and 100 mg/mL streptomycin at 37°C in a humidified atmosphere containing 5% CO2.
Transfection
The miR-199a mimics and inhibitors and the miR-mimic negative control (NC) and inhibitor NC were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Lentiviral Hif1α shRNA and the nontargeting shRNA negative control (sh-NC) were obtained from The RNAi Consortium (Broad Institute, Cambridge, MA, USA). For gene overexpression, Hif1α full-length cDNAs were cloned into pcDNA3.1-vector (Thermo Fisher Scientific). For transfection, OV2008 and C13* cells were plated in six-well plates using Lipofectamine 2000™ (Thermo Fisher Scientific) according to the manufacturer’s instructions. After transfection for 48 h, cells were harvested and stored as cell pellets at −80°C until use.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
To measure the relative level of transcript, qRT-PCR was performed. Briefly, total RNA was extracted by TRIzol reagent (Thermo Fisher Scientific). cDNA was obtained by reverse transcription using M-MLV reverse transcriptase (Promega). Also, qRT-PCR was performed using the Maxima SYBR Green qPCR mastermix (Fermentas) in a 7900 HT qPCR machine (Thermo Fisher Scientific). A specific forward primer of miR-199a (5′-CCCAGTGTTCAGACTACCTGTTC-3′) and a common universal reverse primer were used. Also, U6 or GAPDH was used for normalization using the comparative CT-method.17 Each sample was performed in triplicate.
Measurement of tumor necrosis factor alpha (TNF-α) by enzyme-linked immunosorbent assay
To measure the amount of TNF-α in cell lysates, cells were lysed in PBS. After centrifugation, supernatant was taken for determination of intracellular TNF-α level by enzyme-linked immunosorbent assay (ELISA). The amount of TNF-α was measured using a Duoset ELISA (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Western blot analysis
Protein levels were measured by Western blot. Briefly, cells were lysed in ice-cold radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich, St Louis, MO, USA) and protein was quantified by Bradford assay. Then, an equal amount of protein was separated on sodium dodecyl sulfate polyacrylamide electrophoresis gel and transferred to a nitrocellulose membrane (Merck Millipore, Burlington, MA, USA). After being blocked with 10% skim milk, the membranes were incubated with primary antibodies overnight at 4°C. Then, the HRP-conjugated secondary antibodies were used. The bound antibodies were detected with enhanced chemiluminescence system (Bio-Rad Laboratories, Hercules, CA, USA). GAPDH was used as a loading control.
Apoptosis assay
After transfection as described above, the OV2008 and C13* cells were treated with DDP at a concentration of 40 μM for 48 h. After incubation, the cells were harvested and stained with 5 μL annexin V-fluorescein isothiocyanate (FITC) and 10 μL propidium iodide (PI; 20 μg/mL; Thermo Fisher Scientific) in the dark for 15 min at room temperature. Then, the apoptotic cells were analyzed by a FACScan (BD Biosciences, Franklin Lakes, NJ, USA). The signal of annexin V-FITC was detected using the FITC signal detector (FL1), and PI was measured with the phycoerythrin signal detector (FL2).
Statistical analysis
All experiments were repeated at least three times. Data are presented as the mean ± SD. Statistical significance was determined with Student’s t-test by using the SPSS 16.0 software (IBM Corp., Armonk, NY, USA). P<0.05 was considered as statistically significant.
Results
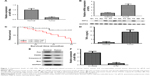
miR-199a was downregulated and Hif1α upregulated in ovarian cancer specimens
To detect the detailed relationship between miR-199a and ovarian cancer, the expression of miR-199a was determined in ovarian tumors and their corresponding normal tissues. We found that miR-199a levels were significantly lower in ovarian tumors compared with those in corresponding normal tissues (Figure 1A). Conversely, the relative mRNA and protein expression of Hif1α increased in ovarian tumors compared with the normal tissues (Figure 1B).
Downregulation of miR-199a associated with poor prognosis of patients with ovarian tumors
Ovarian cancer patients with low miR-199a expression showed shorter survival time than those with high miR-199a expression (Figure 1C).
Apoptosis was involved in the development of ovarian cancer
Next, we further investigated the expression levels of apoptosis-related proteins in ovarian tumors and their corresponding normal tissues. Figure 1D illustrates that the levels of TNF-α were markedly increased in ovarian tumors compared to those in corresponding normal tissues. In addition, our data showed the protein expression levels of Fas, Fas-associated death domain (FADD), caspase-8, and Bax were significantly decreased in ovarian tumors compared to those in corresponding normal tissues (Figure 1E). These results revealed that the apoptosis-related proteins were involved in the development and progression of ovarian cancer.
Expression of miR-199a in DDP-resistant and -sensitive cell lines
Subsequently, we assessed the expression of miR-199a in cisplatin-sensitive and -resistant ovarian cancer cell lines OV2008 and C13*. The expression levels of miR-199a were significantly higher in OV2008 cells compared with to in C13* cells (Figure 1F).
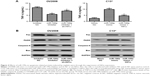
Effect of miR-199a on DDP-induced apoptosis in the resistant and sensitive cell lines
To investigate whether miR-199a could modulate the resistance of OV2008 and C13* cells to DDP, the OV2008 and C13* cells were transfected with miR-199a inhibitor and miR-199a mimic, respectively. Then, the cells were treated with 40 μM DDP for 48 h. Figure 2A reveals that the apoptosis assays show a significant decline of apoptosis ratios in the OV2008 cells transfected with miR-199a inhibitor compared to those in the blank and inhibitor NC groups. However, apoptosis assays using annexin V staining showed that miR-199a mimic in the C13* cells enhanced DDP-induced apoptosis compared with that in the blank and mimic NC groups (Figure 2B). These results revealed that miR-199a could change the resistance to DDP in the ovarian cancer cells through promoting DDP-induced apoptosis in vitro.
miR-199a promoted DDP-induced apoptosis of ovarian cancer cells through targeting Hif1α
We further examined whether miR-199a was involved in the regulation of the expression of Hif1α. In Western blot, Hif1α expression exhibited a dramatic increase in the OV2008 cells transfected with miR-199a inhibitor compared to that in the cells transfected with inhibitor NC (Figure 3A). Next, we investigated whether the effect of miR-199a was involved in DDP-induced apoptosis through targeting Hif1α. As shown in Figure 3B, OV2008 cells transfected with the miR-199a inhibitor displayed lower levels of apoptosis in response to DDP. However, suppression of Hif1α reversed the inhibiting effects of miR-199a inhibitor on DDP-induced apoptosis in OV2008 cells. In contrast, the upregulation of miR-199a by the miR-199a mimic led to a significant downregulation of Hif1α protein expression in the C13* cells (Figure 3C). An inverse correlation between miR-199a and Hif1α protein expression levels existed in the ovarian cancer cells.
Moreover, overexpression of miR-199a promoted DDP-induced apoptosis in C13* cells. However, the effects were abolished by Hif1α (Figure 3D). Taken together, the results indicate that miR-199a may promote DDP-induced apoptosis through suppression of Hif1α in the ovarian cancer cells.
Finally, we investigated the expression levels of apoptosis-related proteins in the ovarian cancer cells treated with DDP. Inhibition of Hif1α abolished the suppression effect of miR-199a inhibitor on the levels of TNF-α and Fas, FADD, caspase-8, and Bax protein expression levels induced by DDP in the OV2008 cells (Figure 4A). However, overexpression of Hif1α blocked the acceleration effect of miR-199a mimic on the levels of TNF-α and Fas, FADD, caspase-8, and Bax protein expression levels induced by DDP in the C13* cells (Figure 4B). These results confirmed that miR-199a may increase the sensitivity of ovarian cancer cells to DDP through suppression of Hif1α.
Discussion
Cisplatin results in the formation of DNA adducts to trigger apoptosis in cancer cells.18 However, development of drug resistance is the main obstacle for DDP treatment.19 A critical mechanism of drug resistance in the ovarian cancer cells is the defective apoptosis pathway.20 Increasing evidence indicates that aberrant expression of miRNAs is related to the drug resistance of ovarian cancer cells through this mechanism. Kong et al found miR-125b conferred DDP resistance to ovarian cancer cells by blocking DDP-induced apoptosis.21 Rao et al demonstrated that knockdown of miR-106a dramatically decreased DDP-induced apoptosis in DDP-sensitive cells A2780, while overexpression of miR-106a significantly increased DDP-induced apoptosis in DDP-resistant cells A2780/DDP.22 However, the molecular mechanisms of miR-199a in DDP-resistant ovarian cancer remain unclear.
Previous studies indicated that miR-199a was deregulated in ovarian cancers through targeting IKKβ.23 Besides, miR-199a expression is found to be significantly downregulated in patients with ovarian cancer in comparison with matched normal controls.24 Moreover, miR-199a can reverse DDP resistance in human ovarian cancer cells through the inhibition of mTOR.25 In this study, we found the miR-199a levels were significantly lower in ovarian tumors compared with those in corresponding normal tissues. In addition, miR-199a was downregulated in DDP-resistant C13* cells as compared with that in DDP-sensitive OV2008 cells. Furthermore, results from annexin V-FITC/PI double staining showed that inhibition of miR-199a in the OV2008 cells decreased the sensitivity and apoptosis induced by DDP, while the upregulation of miR-199a in C13* cells caused a notable increase of apoptotic index induced by DDP. These results suggested that miR-199a could change DDP resistance in ovarian cancer cells.
Apoptosis plays a key role in preventing tumorigenesis. Two main apoptotic pathways are reported, the intrinsic pathway and the extrinsic pathway.26 The intrinsic apoptosis pathway contains the Bcl-2 family. Bcl-2 can limit the proapoptotic effects of Bax.27 Furthermore, DDP activates Bax, which induces permeabilization of the outer mitochondrial membrane, leading to cytochrome c release and activation of caspases.28 In addition, DDP upregulates the expression of TNF-α.29 Also, activation of the extrinsic pathway triggers the activation of death receptors such as Fas and TNF-α–related apoptosis-inducing ligand receptors.30 DDP also upregulates Fas ligand/receptor system. Besides, Fas can interact with FADD, which results in caspase-8 activation and cell death.31 In this study, inhibition of miR-199a in the OV2008 cells significantly decreased the levels of TNF-α and the protein expression levels of Fas, FADD, caspase-8, and Bax induced by DDP. However, upregulation of miR-199a in the C13* cells dramatically increased the levels of TNF-α and the protein expression levels of Fas, FADD, caspase-8, and Bax induced by DDP. These results confirmed that miR-199a could change cell apoptosis and sensitivity to DDP in the ovarian cancer cells.
To investigate the possible mechanism by which miR-199a modulated DDP resistance in ovarian cancer, we predicted Hif1α might be a downstream target of miR-199a. It has been demonstrated that miR-199a directly targets and inhibits translation of Hif1α mRNA in cardiac myocytes.32 Moreover, increased expression of miR-199a-5p was associated with decreased expression of Hif1α in the lungs from patients with COPD.33 Furthermore, miR-199a inhibits the hypoxia-induced proliferation of non-small cell lung cancer cells by suppressing the expression of Hif1α.34 Here, we proved that downregulation of miR-199a was associated with increased expression of Hif1α. In addition, we found that the inhibition or overexpression of both miR-199a and Hif1α changed the apoptosis ratios and the expression levels of apoptosis-related proteins induced by DDP. Thus, miR-199a may play a key role in modulating the DDP resistance of OV2008 and C13* cells through the regulation of Hif1α expression.
In conclusion, our data demonstrated that miR-199a might lead to change of DDP resistance by inhibiting the expression of Hif1α in the ovarian cancer cells. Additionally, the novel finding may provide a drug target for the drug resistance of ovarian cancer cells and be adopted to treat chemotherapy resistance in patients with ovarian cancer.
Acknowledgment
This work was supported by the Post Doctoral Foundation of Heilongjiang Province, People’s Republic of China (Grant No LBH-Z14141).
Disclosure
The authors report no conflicts of interest in this work.
References
Han Z, Feng J, Hong Z, et al. Silencing of the STAT3 signaling pathway reverses the inherent and induced chemoresistance of human ovarian cancer cells. Biochem Biophys Res Commun. 2013;435(2):188–194. | ||
Chen W, Zheng R, Zhang S, et al. Report of incidence and mortality in China cancer registries, 2009. Chin J Cancer Res. 2012;25(3):171–180. | ||
Yen MS, Juang CM, Lai CR, Chao GC, Ng HT, Yuan CC. Intraperitoneal DDP-based chemotherapy vs intravenous DDP-based chemotherapy for stage III optimally cytoreduced epithelial ovarian cancer. Int J Gynaecol Obstet. 2001;72(1):55–60. | ||
Boulikas T, Vougiouka M. Cisplatin and platinum drugs at the molecular level. (Review). Oncol Rep. 2003;10(6):1663–1682. | ||
Chu G. Cellular responses to DDP. The roles of DNA-binding proteins and DNA repair. J Biol Chem. 1994;269(2):787–790. | ||
Thigpen T, duBois A, Mcalpine J, et al; Gynecologic Cancer InterGroup. First-line therapy in ovarian cancer trials. Int J Gynecol Cancer. 2011;21(4):756–762. | ||
Kigawa J. New Strategy for overcoming resistance to chemotherapy of ovarian cancer. Yonago Acta Med. 2013;56(2):43–50. | ||
Azmi AS, Wang Z, Burikhanov R, et al. Critical role of prostate apoptosis response-4 in determining the sensitivity of pancreatic cancer cells to small-molecule inhibitor-induced apoptosis. Mol Cancer Ther. 2008;7(9):2884–2893. | ||
Rosen DG, Yang G, Liu G, et al. Ovarian cancer: pathology, biology, and disease models. Front Biosci (Landmark Ed). 2009;14:2089–2102. | ||
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. | ||
Nam EJ, Yoon H, Kim SW, et al. MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res. 2008;14(9):2690–2695. | ||
Wang Z, Ting Z, Li Y, Chen G, Lu Y, Hao X. microRNA-199a is able to reverse DDP resistance in human ovarian cancer cells through the inhibition of mammalian target of rapamycin. Oncol Lett. 2013;6(3):789–794. | ||
Semenza GL. Involvement of hypoxia-inducible factor 1 in human cancer. Intern Med. 2002;41(2):79–83. | ||
Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–732. | ||
Zhong H, De Marzo AM, Laughner E, et al. Overexpression of hypoxia-inducible factor 1α in common human cancers and their metastases. Cancer Res. 1999;59(22):5830–5835. | ||
Joshi H, Subramanian I, Ghosh G, Zeng Y, Zhao M, Ramakrishnan S. Abstract 2070: Mir-199a is downregulated in hypoxia and targets HIF1α in ovarian cancer cells. Cancer Res. 2010;70(8 Suppl):2070. | ||
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3(6):1101–1108. | ||
Materna V, Liedert B, Thomale J, Lage H. Protection of platinum-DNA adduct formation and reversal of DDP resistance by anti-MRP2 hammerhead ribozymes in human cancer cells. Int J Cancer. 2005; 115(3):393–402. | ||
Galluzzi L, Senovilla L, Vitale I, et al. Molecular mechanisms of DDP resistance. Oncogene. 2012;31(15):1869–1883. | ||
Tan L, Kwok RP, Shukla A, et al. Trichostatin A restores Apaf-1 function in chemoresistant ovarian cancer cells. Cancer. 2011;117(4):784–794. | ||
Kong F, Sun C, Wang Z, et al. miR-125b confers resistance of ovarian cancer cells to DDP by targeting pro-apoptotic Bcl-2 antagonist killer 1. J Huazhong Univ Sci Technolog Med Sci. 2011;31(4):543–549. | ||
Rao YM, Shi HR, Ji M, Chen CH. MiR-106a targets Mcl-1 to suppress DDP resistance of ovarian cancer A2780 cells. J Huazhong Univ Sci Technol Med Sci. 2013;33(4):567–572. | ||
Chen R, Alvero AB, Silasi DA, et al. Regulation of IKKbeta by miR-199a affects NF-kappaB activity in ovarian cancer cells. Oncogene. 2008;27(34):4712–4723. | ||
Zuberi M, Khan I, Gandhi G, Ray PC, Saxena A. The conglomeration of diagnostic, prognostic and therapeutic potential of serum miR-199a and its association with clinicopathological features in epithelial ovarian cancer. Tumor Biol. 2016;37(8):11259–11266. | ||
Wang Z, Zhou T, Ya LI, Chen G, Yunping LU, Hao X. microRNA-199a is able to reverse DDP resistance in human ovarian cancer cells through the inhibition of mammalian target of rapamycin. Oncol Lett. 2013;6(3):789–794. | ||
Ramesh G, Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in DDP nephrotoxicity. J Clin Invest. 2002;110(6):835–842. | ||
Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26(9):1324–1337. | ||
Cullen KJ, Yang Z, Schumaker L, Guo Z. Mitochondria as a critical target of the chemotheraputic agent DDP in head and neck cancer. J Bioenerg Biomembr. 2007;39(1):43–50. | ||
Sánchez-González PD, López-Hernández FJ, López-Novoa JM, Morales AI. An integrative view of the pathophysiological events leading to DDP nephrotoxicity. Crit Rev Toxicol. 2011;41(10):803–821. | ||
Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16(2):139–144. | ||
Tsuruya K, Ninomiya T, Tokumoto M, et al. Direct involvement of the receptor-mediated apoptotic pathways in DDP-induced renal tubular cell death. Kidney Int. 2003;63(1):72–82. | ||
Rane S, He M, Sayed D, et al. Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. 2009;104(7):879–886. | ||
Mizuno S, Bogaard HJ, Gomez Arroyo J, et al. MicroRNA-199a-5p is associated with hypoxia-inducible factor-1α expression in lungs from patients with COPD. Chest. 2012;142(3):663–672. | ||
Ding G, Huang G, Liu HD, et al. MiR-199a suppresses the hypoxia-induced proliferation of non-small cell lung cancer cells through targeting HIF1α. Mol Cell Biochem. 2013;384(1–2):173–180. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.