Back to Journals » OncoTargets and Therapy » Volume 12
miR-181a Upregulation Promotes Radioresistance of Nasopharyngeal Carcinoma by Targeting RKIP
Authors Huang W , Liu J, Hu S , Shi G, Yong Z, Li J, Qiu J, Cao Y, Yuan L
Received 27 August 2019
Accepted for publication 18 November 2019
Published 11 December 2019 Volume 2019:12 Pages 10873—10884
DOI https://doi.org/10.2147/OTT.S228800
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Wei Huang,1,2 Jie Liu,3 Shanbiao Hu,4 Guangqing Shi,1 Zhong Yong,1 Jian Li,1 Juan Qiu,1 Yan Cao,1 Li Yuan1
1Department of Nuclear Medicine, The Third Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China; 2Research Center of Carcinogenesis and Targeted Therapy, Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China; 3Department of Pathology, Changsha Central Hospital, Changsha, Hunan, People’s Republic of China; 4Department of Urological Organ Transplantation, The Second Xiangya Hospital of Central South University, Changsha, Hunan, People’s Republic of China
Correspondence: Li Yuan
Department of Nuclear Medicine, The Third Xiangya Hospital, Central South University, 138 Tongzipo Road, Changsha, Hunan 410013, People’s Republic of China
Email [email protected]
Background: Radioresistance is the leading cause of treatment failure for nasopharyngeal carcinoma (NPC). Therefore, screening the critical regulators in radioresistance and revealing the underlying mechanisms is imperative for improvement of therapeutical efficacy in NPC.
Materials and methods: Our previous study has proved that miR-181a may serve as a pro-radioresistant miRNA. In this study, we explored the expression of miR-181a in NPC, especially in radioresistant NPC samples, by qPCR. Moreover, the clinical significance of miR-181a level was also analyzed. Furthermore, the functions of miR-181a, both in vitro and in vivo, were detected via a serial of assays such as CCK-8, plate clone survival, apoptosis, and xenograft tumor model. The downstream target of miR-181a was also validated by dual luciferase reporter assay and the roles of miR-181a’s target in the regulation of NPC radioresistance were investigated.
Results: The results revealed that miR-181a was significantly upregulated in NPC, especially in radioresistant NPC. MiR-181a level is positively correlated to lymph node metastasis and advanced TNM stages and negatively associated with overall survival rate in NPC. Ectopic expression of miR-181a in radiosensitive NPC cells, or overexpression of miR-181a inhibitor in radioresistant NPC cells, could enhance or impair the radioresistance of NPC cells supported by the results from both in vitro and in vivo, respectively. Mechanistically, dual luciferase report assay indicated that miR-181a could directly target RKIP. Moreover, both in vitro and in vivo experimental outcomes indicated that RKIP restoration and knockdown could antagonize the effects of miR-181a and miR-181a inhibitor in the regulation of NPC radioresistance.
Conclusion: Collectively, the findings of this study proved that miR-181a is upregulated and promotes radioresistance by targeting RKIP in NPC. Targeting miR-181a/RKIP axis may be a valid path for reinforcing radiosensitivity and eventually improving the outcomes of clinical treatment in NPC.
Keywords: nasopharyngeal carcinoma, radioresistance, miR-181a, RKIP
Introduction
Nasopharyngeal carcinoma (NPC), originating from the nasopharyngeal mucosal lining, is one of malignant head and neck tumors and is prevalent in South China. Radiotherapy is the primary treatment for NPC, and most patients at an early stage can benefit a lot from radiotherapy alone. However, some NPC patients, especially for patients at advanced stages, tend to exhibit resistance to radiotherapy. Although combined radiotherapy and chemotherapy indeed improve the treatment outcome, radioresistance still accounts for the leading causes of relapse and eventual treatment failure.1–3 Therefore, it is vital and urgent to explore the molecular mechanisms of radioresistance and identify new targets for clinical treatments.
miRNAs, a class of no coding RNAs consisting of 18–22 nt, play vital roles in almost every physiological and pathological processes via regulating genes expression. Aberrant expression of miRNAs has been implicated in every malignant characteristic of cancer, absolutely including radioresistance.4,5 A host of radioresistance related miRNAs in different cancers have been revealed by studies.5,6 Recently, we performed miRNA microarrays analysis to explore the distinct expression profiles between CNE2-IR, a radioresistant NPC cell line, and CNE2, a radiosensitive NPC cell line. A series of miRNAs, maybe involving radioresistance regulation, have been presented.7 Subsequently, we confirmed that miR-203, −23a and −125b could regulate radiosensitivity of NPC by modulating Stat3, AKT and NF-κB signaling activities via targeting IL-8 and A20, respectively.8–10 However, more efforts are needed to identify the expressions of the rest miRNAs and to explore its roles in radioresistance of NPC.
The aberrant expression of RKIP (Raf kinase inhibitory protein) and its suppressive roles, in a Raf dependent or independent manner, has been exclusively revealed in a plethora of cancers. Downregulation of RKIP releases the suppression on downstream oncogenic signaling regulators like ERK, PI3K/AKT, NF-κB, STAT3, and subsequently promotes malignant progression involving proliferation, metastasis, and radiotherapy and chemotherapy resistance.11–13 Compared with its downstream effect mechanisms, the upstream mechanisms, accounting for the inactivation of RKIP, have been explored much less. Both transcriptional, like promoter hypermethylation14 and Snail mediated transcription inhibition,15 and post-transcriptional mechanisms, like miRNAs mediated mRNA degradation,16 have been involved in the regulation of RKIP among multiple cancers. Recently, we also revealed that loss of RKIP could enhance the radioresistance of NPC cells by regulating AKT and ERK signaling.17 However, the underlying reason for RKIP inactivation has still been obscure in NPC.
Interestingly, online bioinformatic analysis implied that miR-181, one of the potentially upregulated miRNAs in CNE2-IR cells, may be a negative regulator of RKIP, suggesting that miR-181a may exert pro-radioresistant roles in NPC by targeting RKIP. Therefore, in this study, we verified the expression of miR-181a in NPC cells and tissues. Subsequently, we explored the functions of miR-181a and the role of RKIP in miR-181a associated functions in NPC. Our outcomes showed that miR-181a is upregulated in NPC, especially in radioresistant NPC tissues. Moreover, miR-181a could promote radioresistance of NPC by targeting RKIP, indicating miR-181a/RKIP axis could be an effective target for radiosensitization in NPC treatment.
Materials and Methods
Cell Lines
CNE2-IR, a radioresistant NPC cell line, and CNE2, a radiosensitive NPC cell line, were applied in the study. The establishment procedure of CNE2-IR cell were indicated in our previous study.18 The usage of CNE2 and CNE2-IR cells were approved by the ethics committee of The Third Xiangya Hospital, Central South University, Changsha, China. CNE2-IR and CNE2 cells were maintained with RPMI-1640 (BI, Jerusalem, Israel) medium plus 10% fetal bovine serum (BI, Jerusalem, Israel).
Tissue Samples
One hundred one cases of paraffin-embedded NPC specimens from newly diagnosed NPC patients without distal metastasis, who received radiation therapy alone, and 25 cases of paraffin-embedded normal nasopharyngeal mucosa (NNM) specimens from non-cancerous patients, from the First Hospital of Chengzhou City between Jan 2007 and Dec 2009, were enrolled in the study. The criteria for differentiating radioresistance and radiosensitivity and the procedure of follow-up were detailly described in our previous study.17 This study was approved by the ethics committee of the First Hospital of Chengzhou City, China. Written informed consent was obtained from all enrolled participants in the study.
RNA Isolation and qPCR Detection
Total RNA was isolated from paraffin-embedded tissues and NPC cells using RecoverAll™ Total Nucleic Acid Isolation Kit (Thermo Fisher, CA, USA) and TrizolTM reagent (Thermo Fisher, CA, USA), respectively, according to the instructions. Then, miDETECT A Track miRNA qPCR Kit (RiBoBio, Guangzhou, China) was applied to reversely transcribe mRNAs into cDNA and detect the relative expression of miR-181a using specific primers. The level of snRNA U6 was simultaneously detected for normalization of miR-181a expression employing the comparative Ct method. The primers for miR-181a (catalog number, MQPS0000317-1) and U6 (catalog number, miRA0000256-1) were purchased from RiBoBio Inc (Guangzhou, China) and the sequence information was not offered for commercial confidentiality.
Stable Cell Line Establishment
Lentivirus particles for expression of miR-181a, miR-181a inhibitor, RKIP, and shRKIP were purchased from Genechem Inc (Shanghai, China). CNE2 and CNE2-IR cells were infected with the miR-181a and miR-181a inhibitor lentivirus particles, respectively, which simultaneously express the anti-hygromycin gene. Then, CNE2-miR-181a and CNE2-IR-miR-181a-inhibitor cell lines were obtained by culturing cells with medium containing hygromycin for two weeks. Accordingly, CNE2-miR-181a-RKIP and CNE2-IR-miR-181a-inhibitor-shRKIP were acquired by infecting CNE2-miR-181a and CNE2-IR-miR-181a-inhibitor cells with RKIP and shRNA lentivirus particles, which also express the anti-puromycin gene, and subsequently cultured with medium containing puromycin. The control cell lines were also constructed similarly.
Cell Viability Assay
The CCK-8 assay was adopted to analyze cell viability according to our previous description.17 Briefly, cells were seeded into a 96-well plate at a density of 1×104 cells per well. One day later, the cells were irradiated at a dose of 4Gy. Then, the CCK-8 reagent(Beyotime, Shanghai, China) was added into the cell medium every day for four days. One hour after CCK-8 addition, the absorbance of every well at 450nm was detected with a spectrophotometer (BioTek, WI, USA). Three paralleled wells were set for every group, and the experiments were independently performed for three times.
Plate Clone Survival Assay
Plate clone survival assay was similarly applied as previously described by us.17 Simply, 1×103 cells/well were seeded into 6-well plate and received 4Gy irradiation 24 hrs later. Then, the cells were successively cultured for eight days and subsequently stained with 0.5% crystal violet. The clones containing ≥ 50 cells were counted under an inverse microscope (Olympus, Tokyo, Japan). The mean clones of three paralleled wells were calculated, and the experiments were independently repeated in triplicate.
Flow Cytometric Apoptosis Assay
The apoptotic rates of cells exposed to 4Gy irradiation were detected by BD FACSCelesta™ Flow cytometer (BD, LA, USA) using Annexin V-FITC Apoptosis Detection Kit (Bioss, Beijing, China) according to the manufacturer’s instruction. Briefly, 48 hrs after irradiation, the cells were collected and washed by pre-cold PBS for twice. Then the cells were resuspended by 1×bind buffer and simultaneously stained with Annexin V-FITC and propidium iodide for 15 mins at room temperature. The data were analyzed by Flowjo software version 7.6 (OR, USA). At last, the apoptosis of cells was detected by flow cytometer analysis.
Western Blot
The procedures of total proteins extraction and Western blot were performed according to our previous description.17 Simply, the cells were disrupted by RIPA lysis and the total proteins were collected by centrifugation. The denatured proteins (30 μg per lane) were separated by SDS-PAGE and subsequently transmitted into 0.22μm PVDF membrane. After blocked with 5% nonfat milk at room temperature for 1 hr, the membrane was incubated with rabbit anti-RKIP antibody (BBI, Shanghai, China) and mouse anti-GAPDH antibody (Abclonal, Wuhan, China), respectively, overnight at 4°C. Next, after incubated with anti-rabbit or anti-mouse IgG HRP-conjugated secondary antibodies, the protein amounts were visualized by chemiluminescent HRP substrate (EpiZyme, Shanghai, China). The equipment and the software of visualization are FluorChem FC3 (Protein Simple, CA, USA) and AlphaView SA (Protein Simple, CA, USA), respectively.
Dual Luciferase Reporter Assay
Luciferase reporter plasmids, the psiCHECK2-Wt-RKIP plasmid containing wild 3ʹ-UTR and the psiCHECK2-Mut-RKIP containing mutant 3ʹ-UTR, were constructed from the psiCHECK2 vector following the manufacturer’s instruction (Promega Corporation, WI, USA). Then, miR-181a mimics were co-transfected with psiCHECK2-Wt-RKIP and psiCHECK2-Mut-RKIP into CNE2 cells, respectively, using HighGene Transfection reagent (Abclonal, Wuhan, China). Forty-eight hours later, the luciferase activity was detected using a Dual-Luciferase Reporter detection System (Promega Corporation, WI, USA). The relative luciferase activity was demonstrated by the ratio of firefly luciferase to Renilla luciferase activity.
In vivo Tumor Radioresponse Assay
In vivo tumor radioresponse assay was performed according to our previous study with slight modification.17 Briefly, Nude male mice, four weeks old, were raised under specific pathogen-free circumstances. NPC cells were subcutaneously injected into the flanks of mice at 2 × 106 cells/mouse (n = 5 each group). Upon the volumes of xenograft tumors reached approximately 50 mm3, the mice were subjected to irradiation at 6 Gy for one time. Three weeks later, the mice were killed by cervical dislocation, and the xenograft tumors were excised, weighted, and cut in half, with one half fixed and embedded in paraffin for immunohistochemical staining, and the remaining half flash-frozen in liquid nitrogen for reserve. The animal experiment was performed following the Guide for the Care and Use of Laboratory Animals of Central South University, with the approval of the Scientific Investigation Board of Central South University, Changsha, China.
Immunohistochemistry
The level of RKIP (BBI, Shanghai, China) and γH2AX (phospho-S139, Abclonal, Wuhan, China), a marker for DNA double-strand breaks,19 in xenografts were detected by immunohistochemical staining as our previous description. The score of IHC staining was calculated according to the rules based on staining intensity and relative staining area. Absent, weak, moderate, and strong staining was assigned as 0, 1, 2, and 3, respectively. 0%, 0–30%, 30~60%, and > 60% of staining cells were assigned as 0, 1, 2, and 3, respectively. The eventual staining score was the sum of the intensity score and area score. The rate of DNA damaged cells, indicated by γH2AX staining, was obtained by counting the percentage of staining cells in ten random selected microscopic fields under an inverse microscope (Leica, Solms, Germany).
Statistical Analysis
Statistical analysis and charts were carried out using Graphpad Prism 6.0 software. Student’s t-test or chi-square test was applied in comparisons between the two groups. The survival curve was achieved via the Kaplan–Meier method, and the statistical comparison was performed by using the Log rank test. P < 0.05 were considered to be statistically significant.
Results
miR-181a Is Upregulated and Negatively Correlates to the Prognosis in NPC
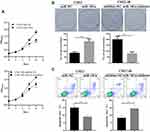
Our miRNAs microarray screening results indicated that miR-181a might be upregulated in radioresistant CNE2-IR cells.7 Therefore, qPCR was applied to verify the expression of miR-181a in CNE2 and CNE2-IR cells. As Figure 1A indicating, the miR-181a level is significantly upregulated in CNE2-IR cells. Subsequently, we further detected the expression of miR-181a in NPC and NNM tissue samples and analyzed the relationships between miR-181a expression and clinicopathological factors. Accordingly, the miR-181a level in NPC was obviously higher than that in NNM (Figure 1B). Furthermore, the level of miR-181a in radioresistant NPC tissues was significantly higher than that in radiosensitive NPC tissues (Figure 1C, Table 1). Similarly, miR-181a upregulation positively correlated to primary T stage, lymph node metastasis, and advanced TNM stage (Table 1), implying that miR-181a may correlate with NPC prognosis. Indeed, the expression of miR-181a demonstrated an inverse correlation to the overall survival of NPC patients indicating by Kaplan-Meier survival analysis (Figure 1D). Therefore, we revealed that miR-181a is upregulated in NPC, especially for radioresistant NPC, and negatively correlates to the prognosis in NPC.
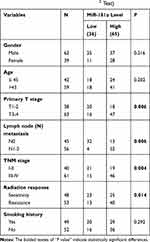 |
Table 1 Correlation Between miR-181a Level and Clinicopathological Characteristics in NPC (N=101, χ2 Test) |
miR-181a Promotes Radioresistance of NPC Cells
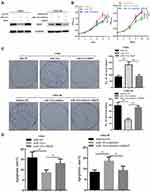
Since miR-181a is upregulated in radioresistant CNE2-IR cells, we subsequently explored the influences of miR-181a expression fluctuation on the radioresistance of NPC cells. Firstly, stable cell lines, CNE2-IR-miR181a-inhibitor, and CNE2-miR181a, along with control cells, were established by lentivirus particles transfection. Then, the radiation sensitivity of NPC cells was analyzed by CCK-8, plate clone survival, and apoptosis assays under irradiation treatment (4Gy). miR-181a inhibitors significantly sensitized CNE2-IR cells to irradiation indicating by reduced cell viability (Figure 2A, upper panel), fewer survival clones (Figure 2B, left panel), and increased apoptotic rate(Figure 2C, left panel); whereas, ectopic expression of miR-181a remarkably reinforced the tolerance of CNE2 cells to irradiation demonstrating by improved cell viability (Figure 2A, lower panel), more survival clones (Figure 2B, right panel), and decreased apoptotic rate (Figure 2C, right panel). Thus, these results manifested that miR-181a can promote radioresistance of NPC cells.
miR-181a Can Target RKIP in NPC
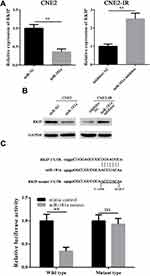
The functions of miRNAs depend on its regulated mRNAs. Therefore, we next tried to identify the target of miR-181a in NPC. The potential targets of miR-181a were subsequently analyzed by using Starbase 3.0, an integrated database for RNA interactome based on the large-scale CLIP-Seq data.20 A multitude of targets of miR-181a were obtained such as TNPO1, HMGB2, and CBX4 etc. Interestingly, according to the prediction of Starbase 3.0, RKIP,20 whose radiosensitization function in NPC has been revealed by our recent study,17 may be a target of miR-181a, suggesting miR-181a may promote radioresistance of NPC by targeting RKIP. Therefore, we analyzed the mRNA and protein level of RKIP in CNE2 and CNE2-IR cells transfected with miR-181a and miR-181a inhibitor, respectively. As the qPCR and Western blot shown (Figure 3A and B), miR-181a overexpression significantly downregulated, while miR-181a inhibitor upregulation remarkably upregulated the mRNA and protein level of RKIP in CNE2 and CNE2-IR cells, respectively. Indeed, these results indicated that miR-181a could regulate RKIP in NPC cells; however, whether miR181a could directly target RKIP in NPC cells remains unknown. Thus, dual luciferase reporter assay was performed to confirm the detailed mechanism. As shown in Figure 3C, miR-181a mimics could notably inhibit luciferase signaling in CNE2 cells transfected with wild 3ʹUTR RKIP plasmids, but not in cells transfected with mutant 3ʹUTR RKIP plasmids. Taken together, our results proved that miR-181a could directly target RKIP in NPC cells.
miR-181a Promotes Radioresistance of NPC Cells via Suppressing RKIP in vitro
Considering miR-181a can directly inhibit RKIP in NPC cells, we further explored the role of RKIP in miR-181a related NPC radioresistance. Thus, we detected the effects of ectopic RKIP expression or RKIP knockdown on the radiation sensitivity of NPC cells. Accordingly, we re-infected CNE2-miR181a and CNE2-IR-miR181a-inhibitor cells with RKIP expression and knockdown lentivirus particles, respectively. Ectopic expression of RKIP (without 3ʹ untranslated region) could rescue the level of RKIP (Figure 4A, left panel) and re-sensitize the cells to irradiation, indicated by impaired cell viability (Figure 4B, left panel), decreased survival clones (Figure 4C, upper panel), and upregulated apoptosis (Figure 4D, left panel), in CNE2-miR181a cells. Whereas, RKIP knockdown successfully re-reduced the level of RKIP (Figure 4A, right panel) and re-enhanced the tolerance of cells to irradiation, demonstrated by improved cell viability (Figure 4B, right panel), increased survival clones (Figure 4C, upper panel), and downregulated apoptosis (Figure 4D, right panel), in CNE2-IR-miR181a-inhibitor cells. Therefore, these in vitro outcomes demonstrated that miR-181a could promote radioresistance of NPC cells by inhibiting RKIP.
miR-181a Can Promote Radioresistance of NPC Cells via Suppressing RKIP in vivo
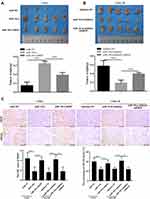
Next, we further explored whether miR-181a could enhance the tolerance of NPC cells to irradiation by targeting RKIP in vivo. NPC cells were subcutaneously injected into nude mice, and the NPC xenografts were treated by 6 Gy irradiation. By comparing the weight of xenografts, we observed that ectopic expression of miR-181a improved the sufferance of CNE2 xenografts to irradiation, and this effect could be antagonized by ectopic expression of RKIP CNE2 cells (Figure 5A); while miR-181a inhibitor overexpression significantly sensitized the CNE2-IR xenografts to irradiation, and this effect could be abolished when RKIP was further knocked down in CNE2-IR cells (Figure 5B). Consistent with the expression in NPC cells in vitro, the expression pattern of RKIP in xenografts was confirmed by IHC staining (Figure 5C). Moreover, as the γH2AX staining pattern indicated (Figure 5C), miR-181a could alleviate the degree of DNA damage in tumors originated from CNE2 cells and this effect could be counteracted by RKIP knockdown; whereas miR-181a inhibitor could aggravate the degree of DNA damage in tumors originated from CNE2-IR cells and this effect could be antagonized by ectopic expression of RKIP. Thus, these results indicated that miR-181a could promote radioresistance of NPC cells via suppressing RKIP in vivo.
Discussion
Radioresistance is the critical cause of progression and treatment failure in NPC.1–3 The prominent roles of miRNAs in the regulation of radioresistance have been indicated by dozens of studies in multiple tumors, especially for NPC, which is preferably sensitive to irradiation.5,21,22 Differential expression of various miRNAs has been observed between radioresistant and radiosensitive NPC. For example, we have explored the differential miRNA profiles between radioresistant and radiosensitive NPC cells7 and proved that miR-23a and miR-203 are downregulated in radioresistant NPC cell. Moreover, miR-23a and miR-203 downregulation positively correlate to TNM stage and prognoses like OS and disease-free survival (DFS).8,9 Aberrant expression of miRNAs such as miR-19b-3p,23 −24-3p,24,25 185-3p,26 and −324-3p,27 etc. in radioresistant NPC have also been revealed by other groups. According to the outcome of miRNA profiles,7 we further revealed that miR-181a was upregulated in NPC, especially in radioresistant NPC, and miR-181a upregulation inversely correlated with the prognosis of NPC, suggesting that miR-181a plays vital roles in the regulation of radioresistance in NPC.
Aberrant expression of miR-181a and its dual roles in carcinogenesis have been widely revealed in various cancers. miR-181a upregulation, as a result of transcriptional activation caused by activity alteration of upstream transcription regulators,28 can exert oncogenic roles in the regulation of malignant progressions in multiple cancers including ovarian cancer,29 gastric cancer,30 clear cell kidney cancer31 and thyroid cancer,32 etc. Meanwhile, miR-181a deregulation or inhibition, due to promoter methylation, lnc RNA and circ RNA related sponge, and the anti-tumor roles of miR-181a has also been observed in other cancers such as lung cancer,33 colorectal cancer,34 cutaneous35 and laryngeal squamous cell carcinoma.36 The roles of miR-181a in the regulation of irradiation sensitivity are dependent on cancer types. For example, miR-181a is upregulated in radioresistant cervical cancer tissues and accounts for the radioresistance of cervical cancer.37 In contrast, upon exposure to irradiation, miR-181a was downregulated in glioma U87MG cells, and miR-181a restoration could sensitize U87MG cells to radiation.38 In line with the study in cervical cancer, ectopic expression of miR-181a could reinforce the irradiation tolerance of CNE2 cells; meanwhile, miR-181a inhibitor could sensitize CNE2-IR cells to irradiation demonstrating miR-181a could promote radioresistance of NPC.
The function of miRNAs is dependent on its target genes. Quiet a few targets of miR-181a, which are involved in regulation of multiple biological processes of cancers, have been identified by previous studies. PRKCD, one of the pro-apoptotic genes, and BCL-2, one of the anti-apoptotic genes, have mediated the radiosensitive and radioresistant effects of miR-181a in cervical cancer37 and glioma,38 respectively, proving the tissue-specific functions of miR-181a again. In NPC, Wang et al indicated that miR-181a could also target CPEB2 to regulate the sensitivity of NPC cells to paclitaxel.39 However, the target, which accounts for the pro-radioresistant roles of miR-181a in NPC, has not been validated. RKIP, one of the candidate targets of miR-181a, whose downregulation and pro-radiosensitive roles in cancers, including NPC,17 prostate cancer40 and lung cancer,41 have been confirmed by other groups and us, was selected for validation. Indeed, we proved that miR-181a could target RKIP and RKIP restoration could antagonize the pro-radioresistant effects of miR-181a both in vitro and in vivo.
Collectively, in the present work, we demonstrate that miR-181a is upregulated in radioresistant NPC and negatively correlates with prognosis of NPC. miR-181a could promote radioresistance of NPC via directly target RKIP suggesting miR-181a/RKIP axis is critical in the regulation of irradiation sensitivity and may serve as a potential target to improve radiation sensitivity in NPC treatment.
Acknowledgments
This study was found by the New Xiangya Talent Project of the Third Xiangya Hospital of Central South University (nos. JY201722), the National Natural Science Foundation of China (nos. 81602686 and 81702924), and the Natural Science Foundation of Hunan Province of China (nos. 2018JJ3811).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Chen YP, Chan ATC, Le QT, et al. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. doi:10.1016/S0140-6736(19)30956-0
2. Leong YH, Soon YY, Lee KM, et al. Long-term outcomes after reirradiation in nasopharyngeal carcinoma with intensity-modulated radiotherapy: a meta-analysis. Head Neck. 2018;40(3):622–631. doi:10.1002/hed.v40.3
3. Lam WKJ, Chan JYK. Recent advances in the management of nasopharyngeal carcinoma. F1000Res. 2018;7. doi:10.12688/f1000research.15066.1
4. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203. doi:10.1038/nrd.2016.246
5. Ahmad P, Sana J, Slavik M, et al. MicroRNAs involvement in radioresistance of head and neck cancer. Dis Markers. 2017;2017:8245345. doi:10.1155/2017/8245345
6. Fanale D, Castiglia M, Bazan V, Russo A. Involvement of non-coding RNAs in chemo- and radioresistance of colorectal cancer. Adv Exp Med Biol. 2016;937:207–228.
7. Li XH, Qu JQ, Yi H, et al. Integrated analysis of differential miRNA and mRNA expression profiles in human radioresistant and radiosensitive nasopharyngeal carcinoma cells. PLoS One. 2014;9(1):e87767. doi:10.1371/journal.pone.0087767
8. Qu JQ, Yi HM, Ye X, et al. MiR-23a sensitizes nasopharyngeal carcinoma to irradiation by targeting IL-8/Stat3 pathway. Oncotarget. 2015;6(29):28341. doi:10.18632/oncotarget.5117
9. Qu JQ, Yi HM, Ye X, et al. MiRNA-203 reduces nasopharyngeal carcinoma radioresistance by targeting IL8/AKT signaling. Mol Cancer Ther. 2015;14(11):2653–2664. doi:10.1158/1535-7163.MCT-15-0461
10. Zheng Z, Qu JQ, Yi HM, et al. MiR-125b regulates proliferation and apoptosis of nasopharyngeal carcinoma by targeting A20/NF-kappaB signaling pathway. Cell Death Dis. 2017;8(6):e2855. doi:10.1038/cddis.2017.211
11. Zaravinos A, Bonavida B, Chatzaki E, Baritaki S. RKIP: a key regulator in tumor metastasis initiation and resistance to apoptosis: therapeutic targeting and impact. Cancers (Basel). 2018;10(9):287. doi:10.3390/cancers10090287
12. Farooqi AA, Li Y, Sarkar FH. The biological complexity of RKIP signaling in human cancers. Exp Mol Med. 2015;47:e185. doi:10.1038/emm.2015.70
13. Raquel-Cunha A, Cardoso-Carneiro D, Reis RM, Martinho O. Current status of Raf Kinase Inhibitor Protein (RKIP) in lung cancer: behind RTK signaling. Cells. 2019;8(5):442. doi:10.3390/cells8050442
14. Guo W, Dong Z, Guo Y, et al. Aberrant methylation and loss expression of RKIP is associated with tumor progression and poor prognosis in gastric cardia adenocarcinoma. Clin Exp Metastasis. 2013;30(3):265–275. doi:10.1007/s10585-012-9533-x
15. Nieh S, Jao SW, Yang CY, et al. Regulation of tumor progression via the snail-RKIP signaling pathway by nicotine exposure in head and neck squamous cell carcinoma. Head Neck. 2015;37(12):1712–1721. doi:10.1002/hed.23820
16. Du Y, Liu XH, Zhu HC, et al. MiR-543 promotes proliferation and epithelial-mesenchymal transition in prostate cancer via targeting RKIP. Cell Physiol Biochem. 2017;41(3):1135–1146. doi:10.1159/000464120
17. Yuan L, Yi HM, Yi H, et al. Reduced RKIP enhances nasopharyngeal carcinoma radioresistance by increasing ERK and AKT activity. Oncotarget. 2016;7(10):11463. doi:10.18632/oncotarget.7201
18. Feng XP, Yi H, Li MY, et al. Identification of biomarkers for predicting nasopharyngeal carcinoma response to radiotherapy by proteomics. Cancer Res. 2010;70(9):3450–3462. doi:10.1158/0008-5472.CAN-09-4099
19. Ivashkevich A, Redon CE, Nakamura AJ, Martin RF, Martin OA. Use of the gamma-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett. 2012;327(1–2):123–133. doi:10.1016/j.canlet.2011.12.025
20. Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42(Database issue):D92–D97. doi:10.1093/nar/gkt1248
21. Malla B, Zaugg K, Vassella E, Aebersold DM, Dal Pra A. Exosomes and exosomal MicroRNAs in prostate cancer radiation therapy. Int J Radiat Oncol Biol Phys. 2017;98(5):982–995. doi:10.1016/j.ijrobp.2017.03.031
22. Malik A, Sultana M, Qazi A, et al. Role of natural radiosensitizers and cancer cell radioresistance: an update. Anal Cell Pathol (Amst). 2016;2016:6146595.
23. Huang T, Yin L, Wu J, et al. MicroRNA-19b-3p regulates nasopharyngeal carcinoma radiosensitivity by targeting TNFAIP3/NF-kappaB axis. J Exp Clin Cancer Res. 2016;35(1):188. doi:10.1186/s13046-016-0465-1
24. Wang S, Zhang R, Claret FX, Yang H. Involvement of microRNA-24 and DNA methylation in resistance of nasopharyngeal carcinoma to ionizing radiation. Mol Cancer Ther. 2014;13(12):3163–3174. doi:10.1158/1535-7163.MCT-14-0317
25. Wang S, Pan Y, Zhang R, et al. Hsa-miR-24-3p increases nasopharyngeal carcinoma radiosensitivity by targeting both the 3ʹUTR and 5ʹUTR of Jab1/CSN5. Oncogene. 2016;35(47):6096. doi:10.1038/onc.2016.147
26. Li G, Wang Y, Liu Y, et al. miR-185-3p regulates nasopharyngeal carcinoma radioresistance by targeting WNT2B in vitro. Cancer Sci. 2014;105(12):1560–1568. doi:10.1111/cas.2014.105.issue-12
27. Li G, Liu Y, Su Z, et al. MicroRNA-324-3p regulates nasopharyngeal carcinoma radioresistance by directly targeting WNT2B. Eur J Cancer. 2013;49(11):2596–2607. doi:10.1016/j.ejca.2013.03.001
28. Zhang X, Li X, Tan F, Yu N, Pei H. STAT1 inhibits MiR-181a expression to suppress colorectal cancer cell proliferation through PTEN/Akt. J Cell Biochem. 2017;118(10):3435–3443. doi:10.1002/jcb.v118.10
29. Yang M, Zhai X, Ge T, Yang C, Lou G. miR-181a-5p promotes proliferation and invasion and inhibits apoptosis of cervical cancer cells via regulating Inositol Polyphosphate-5-Phosphatase A (INPP5A). Oncol Res. 2018;26(5):703–712. doi:10.3727/096504017X14982569377511
30. Mi Y, Zhang D, Jiang W, et al. miR-181a-5p promotes the progression of gastric cancer via RASSF6-mediated MAPK signalling activation. Cancer Lett. 2017;389:11–22. doi:10.1016/j.canlet.2016.12.033
31. Lei Z, Ma X, Li H, et al. Up-regulation of miR-181a in clear cell renal cell carcinoma is associated with lower KLF6 expression, enhanced cell proliferation, accelerated cell cycle transition, and diminished apoptosis. Urol Oncol. 2018;36(3):93e23–93e37. doi:10.1016/j.urolonc.2017.09.019
32. Le F, Luo P, Yang QO, Zhong XM. MiR-181a promotes growth of thyroid cancer cells by targeting tumor suppressor RB1. Eur Rev Med Pharmacol Sci. 2017;21(24):5638–5647. doi:10.26355/eurrev_201712_14007
33. Shi Q, Zhou Z, Ye N, et al. MiR-181a inhibits non-small cell lung cancer cell proliferation by targeting CDK1. Cancer Biomark. 2017;20(4):539–546. doi:10.3233/CBM-170350
34. Han P, Li JW, Zhang BM, et al. The lncRNA CRNDE promotes colorectal cancer cell proliferation and chemoresistance via miR-181a-5p-mediated regulation of Wnt/beta-catenin signaling. Mol Cancer. 2017;16(1):9. doi:10.1186/s12943-017-0583-1
35. Neu J, Dziunycz PJ, Dzung A, et al. miR-181a decelerates proliferation in cutaneous squamous cell carcinoma by targeting the proto-oncogene KRAS. PLoS One. 2017;12(9):e0185028. doi:10.1371/journal.pone.0185028
36. Zhao X, Zhang W, Ji W. miR-181a targets GATA6 to inhibit the progression of human laryngeal squamous cell carcinoma. Future Oncol. 2018;14(17):1741–1753. doi:10.2217/fon-2018-0064
37. Ke G, Liang L, Yang JM, et al. MiR-181a confers resistance of cervical cancer to radiation therapy through targeting the pro-apoptotic PRKCD gene. Oncogene. 2013;32(25):3019–3027. doi:10.1038/onc.2012.323
38. Chen G, Zhu W, Shi D, et al. MicroRNA-181a sensitizes human malignant glioma U87MG cells to radiation by targeting Bcl-2. Oncol Rep. 2010;23(4):997–1003. doi:10.3892/or_00000725
39. Wang Q, Zhang W, Hao S. LncRNA CCAT1 modulates the sensitivity of paclitaxel in nasopharynx cancers cells via miR-181a/CPEB2 axis. Cell Cycle. 2017;16(8):795–801. doi:10.1080/15384101.2017.1301334
40. Woods Ignatoski KM, Grewal NK, Markwart SM, et al. Loss of raf kinase inhibitory protein induces radioresistance in prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72(1):153–160. doi:10.1016/j.ijrobp.2008.04.072
41. Xie SY, Li G, Han C, Yu YY, Li N. RKIP reduction enhances radioresistance by activating the Shh signaling pathway in non-small-cell lung cancer. Onco Targets Ther. 2017;10:5605. doi:10.2147/OTT.S149200
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.