Back to Journals » OncoTargets and Therapy » Volume 12
MiR-155-5p accelerates the metastasis of cervical cancer cell via targeting TP53INP1
Authors Li N, Cui T, Guo WL, Wang DW, Mao L
Received 1 November 2018
Accepted for publication 13 March 2019
Published 29 April 2019 Volume 2019:12 Pages 3181—3196
DOI https://doi.org/10.2147/OTT.S193097
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Arseniy Yuzhalin
Ning Li,1 Tao Cui,2 Wenling Guo,1 Dianwei Wang,1 Li Mao3
1Obstetrical Department, Binzhou Central Hospital, Binzhou, Shandong 251700, People’s Republic of China; 2Anesthesiology Department, Huimin County Maternal and Child Health Hospital, Binzhou, Shandong, 251700, People’s Republic of China; 3Gynecology Department, Binzhou Central Hospital, Binzhou, Shandong 251700, People’s Republic of China
Background: The dysregulation of microRNAs has been implicated in the progression of different malignancies. Herein, we sought to identify the precise roles of miR-155-5p in the progression of cervical cancer.
Materials and methods: The expressions of miR-155-5p in cervical carcinoma cells and clinical tissues were assessed using qRT-PCR analysis. The functions of miR-155-5p on the growth of cervical cancer cell were investigated using MTT and colony formation. The Transwell and wound closure assays were selected to explore the influence of miR-155-5p on the invasion and migration of cervical cancer cell. The effect of miR-155-5p on cervical carcinoma cell growth and metastasis in vivo was investigated using xenograft model and experimental lung metastasis model. Bioinformatics analysis and luciferase reporter assay were applied to identify that tumor protein p53-inducible nuclear protein 1 (TP53INP1) was the target of miR-155-5p.
Results: MiR-155-5p was significantly upregulated in cervical cancer tissue than that in control normal tissue. Downexpression of miR-155-5p decreased the growth, migration as well as invasiveness abilities of cervical cancer cell in vitro whereas overregulation of miR-155-5p caused the opposite outcomes. In addition, the in vivo mice xenograft model suggested that downexpression of miR-155-5p restrained the progression of cervical cancer cell whereas overexpression of miR-155-5p caused opposite outcomes. Furthermore, we revealed that TP53INP1 was the target of miR-155-5p and the level of TP53INP1 was inversely associated with miR-155-5p level in cervical carcinoma. Furthermore, TP53INP1 knockdown mimicked the influence of miR-155-5p on cervical cancer proliferation, migration and invasion phenotypes. Finally, overexpression of TP53INP1 impaired the promote effect of miR-155-5p on cervical cancer cell and downregulation of TP53INP1 counteracted the suppressive impact of miR-155-5p on the aggressiveness of cervical cancer cell.
Conclusion: Our study indicated that miR-155-5p regulated the development of cervical cancer cell by regulating the expression of TP53INP1.
Keywords: miR-155-5p, cervical cancer, TP53INP1, metastasis
Introduction
As one of the fatal malignant neoplasms, the morbidity of cervical carcinoma remains has drastically increased worldwide. After cervical carcinoma cell diffuses to secondary tissues, cervical carcinoma becomes resistant to the conventional therapeutic methods and become an incurable disease.1,2 MicroRNAs, which are small non-coding RNAs, post-transcriptionally regulate the expressions of their downstream target genes.3 Substantive studies have shown that altered expressions of miRNAs are related with the progression of cancers, including proliferation, angiogenesis, metastasis and chemotherapy resistance.4 MiRNAs commonly function as oncogenes of tumor suppressors and play important roles in the diagnosis and treatment of cancer.5 Several miRNAs, such as miR-145, miR-9 and miR-182 have been demonstrated participate into the invasion and metastasis of cancer cell.6–8 Specifically, previous studies identify that several miRNAs, including miR-155, miR-21, miR-503, miR-224 and miR-1246, are closely associated with the prognosis of patients with cervical cancer.9–13 Among them, miR-155-5p is dysregulation in different types of malignancies, such as breast cancer, colon carcinoma, hepatocellular carcinoma and gastric cancer.13–17 In hepatocellular carcinoma, miR-155-5p regulates the malignant phenotypes of tumor cell through indirectly regulating the glycogen synthase kinase-3β-involved Wnt/β-catenin signaling and collagen triple helix repeat containing 1.17 Additionally, miR-155-5p overexpressing increases the growth and metastasis of colorectal cancer cell.15 However, the precise impact of miR-155-5p on the growth and metastasis of cervical carcinoma has never been investigated.
The TP53 gene is one of the vital cancer suppressors which responses to several cell stresses, including metabolic stress, oncogene activation, DNA damage and hypoxia.18 Previous investigations have demonstrated that TP53 participates into the progression of multiple tumor types including breast carcinoma, colorectal cancer, and TP53 is commonly mutated in pancreatic carcinoma and the mutations of TP53 lead to the absence or dysfunction of p53 gene.19 In addition, TP53 interacts with plenty of proteins, including the tumor protein p53-induced nuclear protein 1 (TP53INP1), which modulates malignant phenotypes of tumor cell.20 TP53INP1 is an important stress-response gene in pancreatic carcinoma and is significantly overregulated during pancreatitis.21 The expression of TP53INP1 is also lost in other types of cancer, including breast cancer or glioma.22,23 Hence, loss of its expression may contribute to deregulation of cell proliferation, a hallmark of oncogenesis. Previous investigation demonstrates that miR-155 targets TP53INP1 to regulate hepatocellular carcinoma stem cell self-renewal and acquisition.24 In addition, TGF-β1 acts through miR-155 to downregulate the expression of TP53INP1 in maintaining cancer stem cell phenotype and facilitating the epithelial–mesenchymal transition process.25 Nevertheless, the relationship between TP53INP1 and miR-155 in cervical carcinoma has not been well explored.
MiR-155-5p and miR-155-3p, two different miRNA strands, produced from the miR-155 host gene produces. In the current research, we revealed that miR-155-5p was significantly upregulated in clinical cervical cancer tissues and cells. Downregulation of miR-155-5p markedly suppressed cervical carcinoma cell proliferation, invasion and metastasis in vitro and in vivo, whereas overregulation of miR-155-5p resulted in the opposite results. In addition, we demonstrated that TP53INP1, the target gene of miR-155-5p, played very important roles in this process.
Materials and methods
Cervical carcinoma cell lines and clinical tissues
Twenty-four cases of cervical carcinoma samples and paratumor tissues were collected from Binzhou Central Hospital. Written informed consent for participation was obtained from patients before participation in this study. The ethical approval was obtained from the ethics committee of the Binzhou Central Hospital. The research conforms to the Code of Ethics of the World Medical Association (Declaration of Helsinki) printed in the British Medical Journal (July 18, 1964). The cervical cancer cell lines, including C-33 A, C-4-I, SiHa and CaSki, were purchased from the Chinese Academy of Sciences Cell Bank of Type Culture Collection (CBTCCCAS, Shanghai, People’s Republic of China). The human cervical surface epithelial cell, HcerEpic was obtained from Nanjing Cobioer (Nanjing, Jiangsu, People’s Republic of China). The HEK-293T cell was purchased from GuangZhou Jennio (Guangzhou, Guangdong, People’s Republic of China). The SiHa and CaSki cells were cultured in 1,640 media supplemented with 10% FBS (Thermo Fisher Scientific, Carlsbad, CA, USA), 100 μg/mL streptomycin/penicillin. The C-33 A, C-4-I, HcerEpic and HEK-293T cells were cultured using DMEM media containing 10% FBS (Thermo Fisher Scientific). Cells were cultured in an incubator containing 95% air and 5% CO2 at 37°C.
Cell transfection
MiR-155-5p and its negative control (miR-NC), as well as miR-155-5p inhibitor and its negative control (miR-NC inhibitor) were obtained from GeneCopoeia (Guangzhou, Guangdong, People’s Republic of China). MiR-155-5p or miR-155-5p inhibitor transfection was carried out using Lipofectamine RNAiMAX transfection reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol. The shRNAs against the human TP53INP1 gene were designed and purchased from Genepharma Co., Ltd (Shanghai, People’s Republic of China). The pcDNA3.1-TP53INP1 plasmid encoding TP53INP1 and pcDNA3.1 empty vector were bought from GeneCopoeia (Guangzhou, Guangdong, People’s Republic of China). The pcDNA3.1-TP53INP1 or empty vector was transfected into SiHa cell using the Lipofectamine 3000 transfection reagent (Thermo Fisher Scientific). For the generation of stable cell line, SiHa cell stably overexpressing miRNA was constructed by lentiviral transfection with a vector expressing miR-155-5p or miR-155-5p inhibitor (Genepharma Co., Ltd, Shanghai, People’s Republic of China) using the Lenti-miR™ miRNA precursor clones (SBI System Biosciences, Palo Alto, CA, USA).
In situ hybridization
The miR-155-5p level in normal tissue and cervical carcinoma tissue was analyzed using the biotin-labeled miR-155-5p probe (Beyotime Biotechnology, Nanjing, Jiangsu, People’s Republic of China). Paraffinized cervical carcinoma tissue or normal tissue was deparaffinized using xylene and 100% ethanol. The tissue was then incubated with the biotin-labeled probe (Beyotime Biotechnology) for 18 hrs at 40°C. DAB substrate (Beyotime Biotechnology) was used for colorimetric detection of miR-155-5p. Finally, tissue was stained using hematoxylin followed by dehydration in graded alcohols and xylene.
Luciferase reporter gene analysis
HEK-293T cell (1 x 105) was cultured into 6-well plate for 24 hrs. The luciferase reporter vector for the wild type (WT) or mutant type (MUT) 3ʹ-UTR of TP53INP1 that containing binding sites between miR-155-5p was inserted into the pMIR-reporter luciferase system (Thermo Fisher Scientific). The MUT 3ʹ-UTR of TP53INP1 was constructed by the QuikChange XL Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA, USA). MiR-155-5p or miR-NC combination with the vector expressing firefly luciferase reporter fused with WT or MUT 3ʹ-UTR of TP53INP1 was cotransfected into HEK-293T cell. After 24 hrs, cell was collected and the luciferase activity was detected utilizing the dual-luciferase reporter gene assay (Promega, Madison, WI, USA).
MTT assay
Cells (1 x 104) were cultured into 96-well plate, and were cultured for 24, 48 or 72 hrs days, respectively. MTT (5 mg/mL) was added to plates and the cell was incubated for another 4 hrs. Then, the supernatant was gently removed and 200 μL dimethylsulfoxide was added, the OD value was assessed at 490 nm.
Migration assay
Cells (1 x 105) were cultured into 6-well plates and cultured for overnight. A wound was made in the monolayer cell using a 100 μL tip, and cell was cultured using serum-free medium for 24 hrs. The pictures of wound width at 0 and 24 hrs were recorded using a common microscope, and the percent of wound closure was analyzed using the following formula: (0 hr wound width - 24 hrs wound width)/0 hr wound width×100%.26
Transwell invasion analysis
Cells (1 x 103) were seeded into a chamber with an 8 μm pore size that was pre-coated with BD Matrigel™ Basement Membrane Matrix (BD, Shanghai, People’s Republic of China) and placed in a 24-well plate. After 24 hrs, the lower chamber cell was fixed and stained using 0.1% crystal violet. The invaded cells were counted.27
Colony formation
Cells were seeded in 25 mm3 culture dish and cultured in an incubator for 3 weeks. After that, colonies were stained with 0.1% crystal violet and the cell colonies were counted.
Annexin V-FITC/PI assay
The Annexin V-FITC/PI apoptosis detection kit was obtained from Beyotime Biotechnology (Nanjing, Jiangsu, People’s Republic of China). The harvested cell (1×106 cells/mL) was resuspended in binding buffer. Then, cell was incubated with 5 µL Annexin V-fluorescein isothiocyanate (Annexin V-FITC) followed by 10 µL propidium iodide (PI). The samples were kept in the dark at room temperature for 15 mins. Flow cytometry was used to detect fluorescence.
Xenograft model of cervical cancer and cervical cancer cells metastasis in vivo
All nude mice were bred and housed in AAALAC-accredited SPF rodent facilities at Binzhou Central Hospital. MiR-155-5p or miR-155-5p inhibitor stable transfected SiHa cell was subcutaneously inoculated into nude mice. The tumor sizes were recorded every 3 days with a caliper, and tumor volume was calculated using the following formula: length×width2/2. For experimental lung metastasis model, miR-155-5p inhibitor or miR-155-5p stable transfected SiHa cell was injected into mice via tail vein. After 2 weeks, mice were sacrificed and lungs were excised. Lungs were stained with Bouin’s solution for 24 hrs and then paraffin-embedded, sectioned and stained with H&E. Animal experiments were approved by the Institutional Animal Care and Use Committee at Binzhou Central Hospital according to the NIH Guide for the Care and Use of Laboratory Animals (NIH publication no. 85–23, revised 1985).
qRT-PCR
The total RNA was extracted from tissue or cell using Trizol (TakaraBio, Tokyo, Japan). 1 μg RNA was reverse-transcripted to cDNA using a PrimeScript RT reagent kit (TakaraBio, Tokyo, Japan). qRT-PCR was conducted to detect the level of miR-155-5p or other genes using IQTM SYBR Green supermix and the iQ5 real-time detection system (Bio-Rad Laboratories, Hercules, CA, USA). The comparative cycle threshold (Ct) method was applied to quantify the expression levels through calculating the 2(-∆∆Ct) method. U6 and GAPAH were endogenous controls. The primers used for PCR were as follows: GAPDH (forward primer): 5ʹ-CTGGGCTACACTGAGCACC-3ʹ and (reverse primer): 5ʹ-AAGTGGTCGTTGAGGGCAATG-3ʹ; TP53INP1 (forward primer): 5ʹ-TTCCTCCAACCAAGAACCAGA-3ʹ and (reverse primer): 5ʹ-GCTCAGTAGGTGACTCTTCACT-3ʹ miR-155-5p (forward primer): 5ʹ-GAGGGTTAATGCTAATCGTGATAGG-3ʹ and (reverse primer): 5ʹ-GCACAGAATCAACACGACTCACTAT-3ʹ; U6 (forward primer): (forward primer): 5ʹ-GAGGGTTAATGCTAATCGTGATAGG-3ʹ and (reverse primer): 5ʹ-GCACAGAATCAACACGACTCACTAT-3ʹ and (reverse primer): 5ʹ-GCACAGAATCAACACGACTCACTAT-3ʹ.
Statistical analysis
Data were presented as mean±SD for the three experiments in each group. P<0.05 was considered as significant. The difference among groups was determined using the one-way ANOVA followed by a Bonferroni post hoc test for multiple groups or the unpaired, two-tailed Student’s t test for two groups.
Results
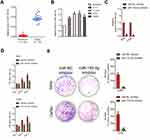
MiR-155-5p is overregulated in cervical cancer
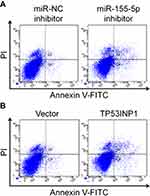
Firstly, 24 cases of cervical carcinoma and paratumor tissues were collected and the levels of miR-155-3p and miR-155-5p were detected using qRT-PCR assay. We found that miR-155-5p was overregulated in cervical cancer tissue compared to that in the control tissue (Figure 1A). Nevertheless, there was no difference of miR-155-3p level between cervical carcinoma and paratumor tissue (data not shown). The level of miR-155-5p in cervical cancer cell was also detected by qRT-PCR. Consistently, miR-155-5p was overexpressed in cervical carcinoma cells (Figure 1B). Then, the in situ hybridization assay was used to assess the level of miR-155-5p in normal tissue and cervical cancer tissue. As shown in Figure S1, miR-155-5p was obviously overregulated in tumor tissue when compared to that in peritumor tissue. To study the impact of miR-155-5p on the growth of cervical cancer cell, we constructed the miR-155-5p downexpression system using miR-155-5p inhibitor. MiR-155-5p was markedly downexpressed in miR-155-5p inhibitor transfected cervical carcinoma SiHa and CaSki cell (Figure 1C). The MTT assay results showed that miR-155-5p inhibitor transfection obviously inhibited the proliferation of cervical cancer cell (Figure 1D). Meanwhile, the colony formation analysis future indicated that downexpression of miR-155-5p inhibited the colony growth of SiHa and CaSki cell (Figure 1E). Next, the influence of miR-155-5p downregulation on SiHa cell apoptosis was investigated using the Annexin V-FITC/PI assay. As shown in Figure S2A, downregulation of miR-155-5p significantly induced the apoptosis of SiHa cell. Finally, Kaplan–Meier analysis indicated that patient who had a higher level of miR-155-5p exhibited a poor survival (Figure S3 and Table S1). These data suggested that miR-155-5p was a cancer promoter in cervical carcinoma in vitro.
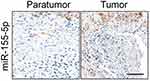 | Figure S1 The expression of miR-155-5p in cervical cancer and control normal tissues was analyzed by in situ hybridization. Scale bar represents 200 μm. |
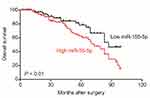 | Figure S3 Overall survival analysis of cervical cancer patients with high or low level of miR-155-5p. |
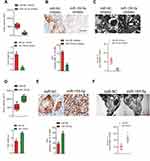
Downregulation of miR-155-5p suppresses migration and invasion of cervical carcinoma cell
Subsequently, we explored the precise role of miR-155-5p on SiHa and CaSki cell migration in vitro using the wound closure analysis. We observed that miR-155-5p inhibitor transfection significantly reduced the migration abilities of SiHa and CaSki cell (Figure 2A). Additionally, we observed the shorter distance in wound edge of miR-155-5p overexpressing SiHa and CaSki cell when compared to parental cell (Figure 2C). To future investigate the effect of miR-155-5p on cervical cancer cell invasion in vitro, we conducted the Transwell invasion assay, in which we observed that miR-155-5p inhibitor markedly impaired the invasion abilities of CaSki and SiHa cell (Figure 2B). We also proved that overregulation of miR-155-5p accelerated SiHa and CaSki cell invasion in vitro (Figure 2D).
The metastasis of cervical cancer cell is inhibited by down-regulation of mir-155-5p in vivo
In vitro, downregulation of miR-155-5p significantly impaired cervical cancer cell proliferation and aggressiveness. Whether miR-155-5p could regulate the progression of cervical carcinoma cell in vivo needs to be further investigated. MiR-155-5p inhibitor stable transfected SiHa cell was subcutaneously inoculated into nude mice. Downregulation of miR-155-5p obviously restrained cervical cancer cell growth in vivo (Figure 3A). After 28 days, mice were sacrificed and tumor tissue was subjected to immunohistochemical (IHC) staining assay. The level of Ki67 is associated with the growth of cancer cell, and is widely used in routine pathological investigation as a proliferation marker. Meanwhile, Ki67 is a meaningful prognostic indicator for the assessment of cancer biopsies.28 We found that the Ki67 positive staining was significantly inhibited in tumor tissue formed by miR-155-5p inhibitor transfected cell than that in the tumor which was formed by miR-NC inhibitor transfected cell (Figure 3B), which confirmed that downregulation of miR-155-5p inhibited the growth of SiHa cell in vivo. Moreover, the incidence of lung metastasis in mice that was injected with miR-155-5p inhibitor stable transfected cervical cancer SiHa cell was remarkably suppressed, as compared with miR-NC inhibitor group (Figure 3C). To further determine the effects of miR-155-5p on the progression of SiHa cell in vivo, miR-155-5p stable overexpressing SiHa cell was inoculated into nude mice. Overexpression of miR-155-5p significantly promoted the growth of SiHa cell in vivo (Figure 3D). Then, we analyzed the level of Ki67 in tumor tissue by IHC staining. The IHC staining assay suggested that the level of Ki67 in tumor derived from miR-155-5p transfected SiHa cell was significantly higher compared to that in tumor tissue derived from miR-NC transfected SiHa cell (Figure 3E). Finally, miR-155-5p stable transfected SiHa cell was injected into nude mice via tail vein. As expected, the lung metastasis nodules in mice that were injected with miR-155-5p transfected cell were significantly increased (Figure 3F). All results proved that miR-155-5p promoted the development of cervical carcinoma in vivo.
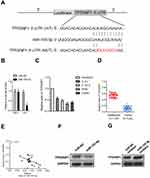
MiR-155-5p binds to the 3ʹ-UTR of TP53INP1
Generally, miRNAs regulate their target genes by binding to their 3ʹ-untranslated region (3ʹ-UTR). Bioinformatics analysis tools (miRDB, miRTarBase and TargetScan) were utilized to predict the targets of miR-155-5p. The 25 common potential target genes are summarized in Figure S4A. Then, we detected the minimum free energy of target genes bind to miR-155-5p using RNAhybrid (
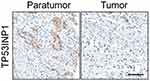 | Figure S5 The expression of TP53INP1 in cervical cancer and control normal tissues was analyzed using immunohistochemical (IHC) assay. Scale bar represents 200 μm. |
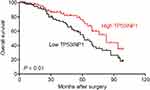 | Figure S6 Overall survival analysis of cervical cancer patients with high or low level of TP53INP1. |
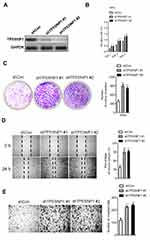
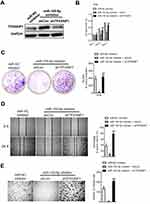
The growth and metastasis of cervical carcinoma cell suppressed by miR-155-5p is rescued TP53INP1
To explore the impact of TP53INP1 in the progression of cervical carcinoma cell, two different short hairpin RNAs (shRNAs) targeting TP53INP1 (shTP53INP1 #1 and shTP53INP1 #2) were utilized to reduce the level of TP53INP1 in SiHa cell (Figure 5A). To reveal the role of TP53INP1 on cervical carcinoma cell growth, invasion and migration, the effects of TP53INP1 knocked-down on SiHa cell proliferation, clone formation, invasion and migration were examined (Figure 5B-E). Downregulation of TP53INP1 significantly promoted the aggressiveness of SiHa cell in vitro, which suggested that TP53INP1 served as a tumor suppressor in cervical carcinoma. To investigate the function of TP53INP1 on the apoptosis of SiHa, the Annexin V-FITC/PI assay was conducted. As shown in Figure S2B, overexpression of TP53INP1 induced the apoptosis of SiHa cell. To investigate whether downregulation of miR-155-5p inhibited the proliferation, migration and invasion abilities of cervical cancer cell through inhibiting TP53INP1, miR-155-5p inhibitor and shTP53INP1 was cotransfected into SiHa cell. The western blot analysis showed that the level of TP53INP1 in SiHa cell that was promoted by miR-155-5p inhibitor was decreased by shTP53INP1 (Figure 6A). Then, MTT assay, colony formation, migration and Transwell invasion assays indicated that downregulation of TP53INP1 neutralized the suppressive effect of miR-155-5p inhibitor on cervical cancer cell proliferation and metastasis (Figure 6B-6E).
MiR-155-5p impairs the progression of cervical carcinoma cell via regulating TP53INP1
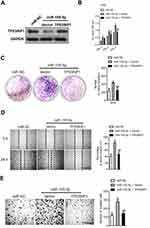
Since TP53INP1 is the functional target of miR-155-5p, we further verified whether the function of miR-155-5p on the development of cervical cancer depended on TP53INP1. Hence, miR-155-5p and pcDNA3.1-TP53INP1 plasmid were cotransfected into SiHa cell (Figure 7A). Then, the proliferation analysis indicated that the beneficial function of miR-155-5p on the proliferation of SiHa cell was inhibited by overregulation of TP53INP1 (Figure 7B). Consistently, the clone formation test showed that the promote impact of miR-155-5p on SiHa growth was suppressed by overexpression of TP53INP1 (Figure 7C). In addition, the wound closure and Transwell assay revealed that the facilitating function of miR-155-5p on SiHa migration and invasion was counteracted by overexpression of TP53INP1 (Figure 7D-E). In conclusion, these data demonstrated that miR-155-5p suppressed the proliferation and aggressive phenotypes of cervical cancer cell by regulatingTP53INP1.
Discussion
Cervical carcinoma remains one of the most aggressive gynecological oncologies, and its metastasis results in an unsatisfactory overall survival of a patient. Hence, the investigation of metastatic-associated biomarkers will contribute to improve the outcomes of cervical carcinoma patient. Nearly, miRNAs are involved into tumor cell invasion and metastasis, and might be the potential non-invasive biomarkers in various cancers.29 Previous investigations have suggested that miR-155 was markedly overexpressed in cervical cancer tissues when compared to normal tissues and miR-155 increases the proliferation of cervical carcinoma cell via suppressing its target gene liver kinase B1.30,31 Consistently, we reported that miR-155-5p was a metastasis-related miRNA that played vital functions in regulating the metastasis and growth of cervical cancer cell. We demonstrated that miR-155-5p was overexpressed in cervical cancer cell line and tissue. Moreover, overexpression of miR-155-5p accelerated the aggressiveness of cervical cancer cell by direct targeting TP53INP1. Finally, overregulation of miR-155-5p promoted the progression of cervical carcinoma in vivo. Totally, our findings proved that miR-155-5p was a potential biomarker and a therapeutic target for the treatment of cervical cancer.
Cancer cell metastasis is a complicated event including adhesion reduction, cytoskeletal rearrangement, cell detachment, extracellular matrix degradation, migration, colonization and growth. In colorectal carcinoma, overexpression of miR-155-5p increases cell proliferation, mobility and metastasis.15 Additional, miRNA-155 facilitates the growth and invasion of gastric cancer cell through negatively regulating the expression of TGF-β receptor 2.32 Nevertheless, the impact of miR-155-5p in cervical carcinoma metastasis has not been well studied, and its mechanism is not clear. In our research, we proved that miR-155-5p was upregulated in cervical carcinoma tissue and cell line. More importantly, miR-155-5p promoted cervical carcinoma cell growth and aggressiveness in vitro, indicating the great potential function of miR-155-5p in cervical cancer progression. Our study also proved the role of miR-155-5p in promoting cervical carcinoma cell growth and metastasis in vivo. In summary, these results verified the crucial functions of miR-155-5p in the progression of cervical cancer cell. It has been demonstrated that downregulation of miR-155 induces cell cycle arrest and apoptosis through regulating many anti-apoptotic genes.33 Consistently, our study revealed that downexpression of miR-155 raised SiHa cervical cancer cell apoptosis.
It is well known that miRNAs regulate gene expression by binding to the 3ʹ-UTR of the target gene.34 It has been reported that miR-155-5p directly targets certain oncogenes, and overregulation of miR-155-5p in tumor decreases the expression of target genes.14 In clear cell renal cell carcinoma, miR-155 regulates the proliferation and invasion of cancer cell by targeting E2F transcription factor 2 (E2F2).35 Additionally, downexpression of miR-155 markedly reduces the expressions of matrix metalloproteinase-2 (MMP-2), MMP-9 and vascular endothelial growth factor , thereby inhibiting the metastasis of gastric cancer cell.36 In this work, the target gene of miR-155-5p was identified using the bioinformatics analysis tools, and tumor protein 53-induced nuclear protein 1 (TP53INP1) was selected as the candidate target. The luciferase reporter assay confirmed that miR-155-5p bound to the 3ʹ-UTR of TP53INP1. The western blot assay also proved that the level of TP53INP1 was negatively regulated by miR-155-5p in SiHa cell.
TP53INP1 is a tumor suppressor gene located on chromosome 8q22.14 and participates in cancer progression in p53-independent and p53-dependent processes.37 TP53INP1 has been identified as a major tumor suppressor in diverse cancers, such as gastric cancer, pancreatic cancer and breast cancer.22,37,38 Consistent with the previous findings, we found that TP53INP1 was significantly downexpressed in cervical cancer tissues. In addition, knocked-down of TP53INP1 promoted the aggressiveness of SiHa cell, suggesting that TP53INP1 also played a crucial role in the proliferation and metastasis of cervical carcinoma cell. Importantly, the proliferation, mobility and invasion abilities of cervical carcinoma cell that was inhibited by miR-155-5p inhibitor transfection were rescued by downexpression of TP53INP1. In order to confirm it, pcDNA3.1-TP53INP1 plasmid combination with miR-155-5p was cotransfected into SiHa cell. Consistently, the proliferation, migration and invasion assay indicated that overexpression of TP53INP1 reduced the malignant phenotypes of cervical carcinoma cell that was promoted by miR-155-5p transfection. All these data indicated that TP53INP1 was the direct functional target of miR-7-5p in cervical carcinoma and miR-155-5p accelerated the progression of cervical cancer cell through targeting TP53INP1.
Conclusion
In conclusion, we demonstrated that miR-155-5p was overexpressed in cervical carcinoma. Downregulation of miR-155-5p impaired the growth, invasion and metastasis of cervical carcinoma cell, whereas overexpression of miR-155-5p resulted in the opposite results. TP53INP1, the target gene of miR-155-5p, played a crucial role in this process. These results might be helpful to more deeply understand the role of miR-155-5p in the development of cervical cancer, and might provide a potential therapeutic target combating the metastasis of cervical carcinoma.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hou C, Zhuang Z, Deng X, Xu Y, Zhang P, Zhu L. Knockdown of trio by CRISPR/Cas9 suppresses migration and invasion of cervical cancer cells. Oncol Rep. 2018;39(2):795–801. doi:10.3892/or.2017.6117
2. Zhu X, Zhou L, Li R, et al. AGER promotes proliferation and migration in cervical cancer. Biosci Rep. 2018;38(1):BSR20171329. doi:10.1042/BSR20171329
3. Qu R, Hao S, Jin X, et al. MicroRNA-374b reduces the proliferation and invasion of colon cancer cells by regulation of LRH-1/Wnt signaling. Gene. 2018;642:354–361. doi:10.1016/j.gene.2017.11.019
4. Feng ZY, Xu XH, Cen DZ, Luo CY, Wu SB. miR-590-3p promotes colon cancer cell proliferation via Wnt/beta-catenin signaling pathway by inhibiting WIF1 and DKK1. Eur Rev Med Pharmacol Sci. 2017;21(21):4844–4852.
5. Guo J, Feng Z, Huang Z, Wang H, Lu W. MicroRNA-217 functions as a tumour suppressor gene and correlates with cell resistance to cisplatin in lung cancer. Mol Cells. 2014;37(9):664–671. doi:10.14348/molcells.2014.0121
6. Sheng N, Tan G, You W, et al. MiR-145 inhibits human colorectal cancer cell migration and invasion via PAK4-dependent pathway. Cancer Med. 2017;6(6):1331–1340. doi:10.1002/cam4.1029
7. Chiang CH, Chu PY, Hou MF, Hung WC. MiR-182 promotes proliferation and invasion and elevates the HIF-1alpha-VEGF-A axis in breast cancer cells by targeting FBXW7. Am J Cancer Res. 2016;6(8):1785–1798.
8. Zhu M, Xu Y, Ge M, Gui Z, Yan F. Regulation of UHRF1 by microRNA-9 modulates colorectal cancer cell proliferation and apoptosis. Cancer Sci. 2015;106(7):833–839. doi:10.1111/cas.12689
9. Han Y, Xu GX, Lu H, et al. Dysregulation of miRNA-21 and their potential as biomarkers for the diagnosis of cervical cancer. Int J Clin Exp Pathol. 2015;8(6):7131–7139.
10. Yin ZL, Wang YL, Ge SF, et al. Reduced expression of miR-503 is associated with poor prognosis in cervical cancer. Eur Rev Med Pharmacol Sci. 2015;19(21):4081–4085.
11. Shen SN, Wang LF, Jia YF, Hao Y-Q, Zhang L, Wang H. Upregulation of microRNA-224 is associated with aggressive progression and poor prognosis in human cervical cancer. Diagn Pathol. 2013;8:69. doi:10.1186/1746-1596-8-69
12. Yang Y, Xie YJ, Xu Q, Chen JX, Shan NC, Zhang Y. Down-regulation of miR-1246 in cervical cancer tissues and its clinical significance. Gynecol Oncol. 2015;138(3):683–688. doi:10.1016/j.ygyno.2015.06.015
13. Fang H, Shuang D, Yi Z, Sheng H, Liu Y. Up-regulated microRNA-155 expression is associated with poor prognosis in cervical cancer patients. Biomed Pharmacother. 2016;83:64–69. doi:10.1016/j.biopha.2016.06.006
14. Wang M, Yang F, Qiu R, et al. The role of mmu-miR-155-5p-NF-kappaB signaling in the education of bone marrow-derived mesenchymal stem cells by gastric cancer cells. Cancer Med. 2018;7(3):856–868. doi:10.1002/cam4.1355
15. Qu YL, Wang HF, Sun ZQ, et al. Up-regulated miR-155-5p promotes cell proliferation, invasion and metastasis in colorectal carcinoma. Int J Clin Exp Pathol. 2015;8(6):6988–6994.
16. Luan T, Zhang X, Wang S, et al. Long non-coding RNA MIAT promotes breast cancer progression and functions as ceRNA to regulate DUSP7 expression by sponging miR-155-5p. Oncotarget. 2017;8(44):76153–76164. doi:10.18632/oncotarget.19190
17. Chen G, Wang D, Zhao X, et al. miR-155-5p modulates malignant behaviors of hepatocellular carcinoma by directly targeting CTHRC1 and indirectly regulating GSK-3beta-involved Wnt/beta-catenin signaling. Cancer Cell Int. 2017;17:118. doi:10.1186/s12935-017-0469-8
18. Leroy B, Anderson M, Soussi T. TP53 mutations in human cancer: database reassessment and prospects for the next decade. Hum Mutat. 2014;35(6):672–688. doi:10.1002/humu.22552
19. Parikh N, Hilsenbeck S, Creighton CJ, et al. Effects of TP53 mutational status on gene expression patterns across 10 human cancer types. J Pathol. 2014;232(5):522–533. doi:10.1002/path.4321
20. Wang X, Wang L, Mo Q, Jia A, Dong Y, Wang G. A positive feedback loop of p53/miR-19/TP53INP1 modulates pancreatic cancer cell proliferation and apoptosis. Oncol Rep. 2016;35(1):518–523. doi:10.3892/or.2015.4361
21. Giusiano S, Garcia S, Andrieu C, et al. TP53INP1 overexpression in prostate cancer correlates with poor prognostic factors and is predictive of biological cancer relapse. Prostate. 2012;72(2):117–128. doi:10.1002/pros.21412
22. Zhang CM, Zhao J, Deng HY. MiR-155 promotes proliferation of human breast cancer MCF-7 cells through targeting tumor protein 53-induced nuclear protein 1. J Biomed Sci. 2013;20(1):79. doi:10.1186/1423-0127-20-79
23. Wang Y, Lin G. TP53INP1 3ʹ-UTR functions as a ceRNA in repressing the metastasis of glioma cells by regulating miRNA activity. Biotechnol Lett. 2016;38(10):1699–1707. doi:10.1007/s10529-016-2159-3
24. Liu F, Kong X, Lv L, Gao J. MiR-155 targets TP53INP1 to regulate liver cancer stem cell acquisition and self-renewal. FEBS Lett. 2015;589(4):500–506. doi:10.1016/j.febslet.2015.01.009
25. Liu F, Kong X, Lv L, Gao J. TGF-beta1 acts through miR-155 to down-regulate TP53INP1 in promoting epithelial-mesenchymal transition and cancer stem cell phenotypes. Cancer Lett. 2015;359(2):288–298. doi:10.1016/j.canlet.2015.01.030
26. Shi X, Ran L, Liu Y, et al. Knockdown of hnRNP A2/B1 inhibits cell proliferation, invasion and cell cycle triggering apoptosis in cervical cancer via PI3K/AKT signaling pathway. Oncol Rep. 2018;39(3):939–950. doi:10.3892/or.2018.6195
27. Zhao J, Li B, Shu C, Ma Y, Gong Y. Downregulation of miR-30a is associated with proliferation and invasion via targeting MEF2D in cervical cancer. Oncol Lett. 2017;14(6):7437–7442. doi:10.3892/ol.2017.7114
28. Yang C, Zhang J, Ding M, et al. Ki67 targeted strategies for cancer therapy. Clin Transl Oncol. 2018;20(5):570–575. doi:10.1007/s12094-017-1774-3
29. Jin YP, Hu YP, Wu XS, et al. miR-143-3p targeting of ITGA6 suppresses tumour growth and angiogenesis by downregulating PLGF expression via the PI3K/AKT pathway in gallbladder carcinoma. Cell Death Dis. 2018;9(2):182. doi:10.1038/s41419-018-1111-y
30. Lao G, Liu P, Wu Q, et al. Mir-155 promotes cervical cancer cell proliferation through suppression of its target gene LKB1. Tumour Biol. 2014;35(12):11933–11938. doi:10.1007/s13277-014-2479-7
31. Park S, Eom K, Kim J, et al. MiR-9, miR-21, and miR-155 as potential biomarkers for HPV positive and negative cervical cancer. BMC Cancer. 2017;17(1):658. doi:10.1186/s12885-017-3642-5
32. Qu Y, Zhang H, Sun W, et al. MicroRNA-155 promotes gastric cancer growth and invasion by negatively regulating transforming growth factor-beta receptor 2. Cancer Sci. 2018;109(3):618–628. doi:10.1111/cas.13472
33. Higgs G, Slack F. The multiple roles of microRNA-155 in oncogenesis. J Clin Bioinforma. 2013;3(1):17. doi:10.1186/2043-9113-3-17
34. Xie WB, Liang LH, Wu KG, et al. MiR-140 expression regulates cell proliferation and targets PD-L1 in NSCLC. Cell Physiol Biochem. 2018;46(2):654–663. doi:10.1159/000488634
35. Gao Y, Ma X, Yao Y, et al. miR-155 regulates the proliferation and invasion of clear cell renal cell carcinoma cells by targeting E2F2. Oncotarget. 2016;7(15):20324–20337. doi:10.18632/oncotarget.7951
36. Wei H, Li Y, Ning Q, Suo ZM. Regulation of miR-155 affects the invasion and migration of gastric carcinoma cells by modulating the STAT3 signaling pathway. Oncol Lett. 2018;16(4):4137–4142. doi:10.3892/ol.2018.9152
37. Gironella M, Seux M, Xie MJ, et al. Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development. Proc Natl Acad Sci U S A. 2007;104(41):16170–16175. doi:10.1073/pnas.0703942104
38. Jiang PH, Motoo Y, Garcia S, Iovanna JL, Pébusque M-J, Sawabu N. Down-expression of tumor protein p53-induced nuclear protein 1 in human gastric cancer. World J Gastroenterol. 2006;12(5):691–696.
Supplementary materials
 | Table S1 The association between miR-155-5p expression and clinic-pathological factors in cervical cancer patients |
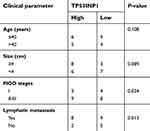 | Table S2 The association between TP53INP1 expression and clinic-pathological factors in cervical cancer patients |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.