Back to Journals » OncoTargets and Therapy » Volume 13
miR-1297 Suppresses Osteosarcoma Proliferation and Aerobic Glycolysis by Regulating PFKFB2
Authors Pan X, Li H, Tan J, Weng X, Zhou L, Weng Y, Cao X
Received 10 August 2020
Accepted for publication 1 October 2020
Published 3 November 2020 Volume 2020:13 Pages 11265—11275
DOI https://doi.org/10.2147/OTT.S274744
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev K. Srivastava
Xiaohui Pan,1,* Haibo Li,1,* Jingxue Tan,1,* Xiaokun Weng,2 Li Zhou,1 Yiping Weng,1 Xiaojian Cao3
1Department of Orthopedics, The Affiliated Hospital of Nanjing Medical University, Changzhou No.2 People’s Hospital, Changzhou, Jiangsu 213003, People’s Republic of China; 2Department of Radiotherapy, The Affiliated Hospital of Nanjing Medical University, Changzhou No.2 People’s Hospital, Changzhou, Jiangsu 213003, People’s Republic of China; 3Department of Orthopedics, The First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yiping Weng; Xiaojian Cao Email [email protected]; [email protected]
Background: MiR-1297 is reported to function as a tumor suppressor of various cancers. However, the role of miR-1297 in the development of osteosarcoma (OS) has not been elaborated. The purpose of this study was to investigate the functional effects of miR-1297 on OS progression and the underlying mechanism.
Methods: The expression of protein and mRNA in OS cells was evaluated by Western blotting and quantitative real-time polymerase chain reaction. Cellular proliferation was investigated by cell counting kit-8, colony formation and apoptosis assays. Bioinformatics methods were used to predict target genes. The relationship between PFKFB2 and miR-1297 was demonstrated by dual-luciferase reporter assay. Metabolic changes in OS cells were monitored using an XF96 metabolic flux analyzer.
Results: We found that miR-1297 was downregulated in OS and that lower expression of miR-1297 promoted proliferation and contributed to the Warburg effect in OS cells. Furthermore, we showed that silencing PFKFB2 inhibited proliferation and reduced aerobic glycolysis while overexpression of PFKFB2 reduced the anti-tumor function of miR-1297 in OS cells. Mechanistically, miR-1297 acted as a tumor suppressor in OS and reduced the expression of PFKFB2 by directly targeting its 3ʹUTR.
Conclusion: The miR-1297/PFKFB2 axis regulated OS proliferation by controlling the Warburg effect. Our results revealed a previously undiscovered function of miR-1297 in OS, which strongly linked metabolic alterations with cancer progression. Targeting miR-1297 may become a promising therapeutic approach for OS.
Keywords: osteosarcoma, miR-1297, PFKFB2, Warburg effect
Introduction
Osteosarcoma (OS), one of the most common primary bone tumors, arises most often in adolescents and children, with a second peak in incidence among the elderly.1 Up to now, the main therapies for OS are surgery, immunotherapy, and chemotherapy. Nonetheless, the survival rate of patients has not markedly improved in the last few decades and many mysteries regarding better management still remain.2 Thus, it is vital to explore the mechanism underlying OS progression as well as to investigate potential effective therapeutic targets.
MicroRNAs (miRNAs) are short single-stranded, non-coding RNAs consisting of about 18–25 nucleotides, which function as regulators of target genes by binding to the target mRNAs.3,4 The development of OS is a complex process and miRNAs play extensive roles in these processes. As previously described, some miRNAs have the potential to become therapeutic targets and biomarkers for OS patients.5–7 Recently, miR-1297 has been reported to play a role as a tumor suppressor in many cancers. Gao et al demonstrated that downregulation of miR-1297 enhanced cell proliferation via regulation of CREB1 in gastric cancer.8 Wang et al reported that miR-1297 inhibited the progression of colorectal cancer by suppressing cyclin D2 expression.9 However, the biological functions and specific roles of miR-1297 in OS are as yet unknown.
In this work, we confirmed that the expression level of miR-1297 in OS cell lines and tissues is downregulated. MiR-1297 inhibited aerobic glycolysis in OS cells, resulting in the suppression of cell proliferation. Further studies indicated that miR-1297 inhibited aerobic glycolysis by targeting PFKFB2 directly. Therefore, these results indicated that miR-1297 has the potential to be a new anti-cancer target in OS.
Materials and Methods
Cell Culture and Reagents
The Cell Bank of the Chinese Academy of Sciences (Shanghai, China) provided the human OS cell lines (MNNG-HOS, U-2OS Saos-2 and MG63 cells) and human osteoblast hFOB1.19 cells. All cell lines were cultured according to ATCC instructions. The human OS cells were grown at 37°C in 5% CO2, while the hFOB1.19 cells were grown at 34.5°C in 5% CO2. Antibodies against PFKFB2 (ab234865; Abcam, Cambridge, UK) and GAPDH (bsm-33033M; Bioss, Beijing, China) were used.
Transfection Assay
For the experiments, MNNG-HOS and U-2OS cells were transfected with small interfering RNAs (siRNAs); the siRNA oligos were purchased from Gene Pharma (Shanghai, China). Transfection procedures were carried out in accordance with the manufacturer’s operating procedures. Sequences of PFKFB2 siRNAs were as follows: si-1, 5ʹ-AACUAACACGCUACCUCAA-3ʹ (sense) and 5ʹ-CCAUUUACCUUUGCC GGCA-3ʹ (antisense); si-2, 5ʹ-AAGAUGCAGCCUACCUGAA-3ʹ (sense) and 5ʹ-CCUAACUCAUACGGCGACA-3ʹ (antisense).
The plasmids containing PFKFB2 or miR-1297-FLAG and the negative control plasmid were purchased from OBiO Technology (Shanghai, China). These plasmids were packaged into virus particles using HEK 293T cells to determine the virus titer. In order to establish stable cells that overexpressed miR-1297 or PFKFB2, target cells were infected with 1 × 108 lentiviral transduction units containing 6 mg/mL of Polybrene (Sigma-Aldrich, St. Louis, MO, USA). After 72 h, infected cells were screened with 2.5 mg/mL puromycin. Western blotting or quantitative real-time polymerase chain reaction (qRT-PCR) verified the efficiency of overexpression.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
The extraction of total RNA and reverse transcription of total RNA were performed as previously described.10 A SYBR® Premix Ex Taq™ kit (Takara Bio, Otsu, Japan) was used to quantify the PFKFB2 transcripts. The setup of the thermal cycler program was as previously described.11 For miRNA qRT-PCR, 1 mg of total RNA was reverse-transcribed using stem-loop primers from a Bio-TNT miRNA qRT-PCR Detection Primer Set (BioTNT Biotechnologies, Shanghai, China). The qRT-PCR was performed using SYBR-green Master mix (BioTNT Biotechnologies). The reaction conditions were: 95°C for 5 min, followed by 95°C for 5 s, 60°C for 30 s, for a total of 40 cycles, and fluorescence signals were detected. The endogenous controls were 18S and U48 small-nuclear RNA.
Western Blot Assay
Western blotting was conducted as described previously.11 In simple terms, whole-cell proteins were extracted and the equivalent protein was subjected to electrophoresis on a 10% sodium dodecyl sulfate polyacrylamide gel. After the protein was electrically transferred to the membrane and blocked, membranes were incubated overnight with the primary antibodies at 4°C and were washed three times with TBST. After incubation with the secondary antibody for 1 h at room temperature, it was detected and combined with a western electrochemical luminescent substrate (Share-Bio, Shanghai, China).
CCK-8 and Colony Formation Assays
The CCK-8 assay was performed according to the vendor’s instructions (Dojindo Molecular Technologies, Japan). Briefly, 3 × 103 cells were seeded into 96-well plates. The absorbance at a wavelength of 450 nm was measured at 0, 24, 48, 72, 96, and 120 h using a tablet reader (Thermo Fisher Scientific, Waltham, MA, USA).
The infected OS cells were cultured in a 6-well plate at an initial cell density of 1 × 103 cells/well. After two weeks, ice-cold PBS was used to wash the colonies. Then, the cell pellet was fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Finally, photographic images were captured and cell numbers were counted.
Fluorescence in situ Hybridization (FISH)
A microarray containing tissue from 40 OS patients was obtained from Alena Biotechnology Co., Ltd. (Xi’an, China). OS tissue sections were hybridized with the miR-1297 probes (Servicebio, Wuhan, China). Fluorescence in situ hybridization was performed as described previously.12
Measurement of Oxidative Phosphorylation and Glycolysis
The extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) of cultured cells were tested using an XF96 metabolic flux analyzer (Seahorse Biosciences, Billerica, MA, USA) in accordance with the manufacturer’s instructions. In brief, 80 µL of a suspension of the target cells (3 × 104) were plated into each well of an XF96 96-well plate (Seahorse Biosciences) and incubated overnight at 37°C. XF calibration solution (Seahorse Biosciences) was added to the XF sensor box (seahorse bioscience company) and incubated at 37°C without adding CO2 overnight. On the second day, DMEM (1 g/mL glucose, pH 7.4; Seahorse Biosciences) modified by XF assay was used to replace the complete medium, and the cells were incubated for 1 h at 37°C without CO2, while 10 mM glucose, 1 mM oligomycin (Sigma-Aldrich) and 80 mM 2-deoxyglucose (D8375; Sigma-Aldrich) were injected continuously to assess the ECAP and the OCR. XFe Wave software (Seahorse Biosciences) was used to analyze the results.
Cell Apoptosis
In order to evaluate cell apoptosis, the adherent cells were separated after culturing for 24 h in serum-free medium. Cells were stained using an Annexin V/FITC Kit (BD Biosciences, San Jose, CA, USA) following the manufacturer’s instructions. Finally, flow cytometry was used to analyze the results.
Measurement of Cellular ATP Level and Lactate Production
An ATP assay kit (Promega, Madison, WI, USA) was used to detect cellular ATP level according to the manufacturer’s instructions. A fluorescence luminometer (Perkin Elmer, Waltham, MA, USA) was used to determine bioluminescence. The ATP level was calculated from a standard curve. A lactate assay kit (BioVision, Zurich, Switzerland) was used to detect extracellular lactate levels according to the manufacturer’s protocols. All values were normalized to the cellular protein level. Three independent experiments were performed.
Dual-Luciferase Reporter Assay
Luciferase reporter plasmids were cloned into the pMIR vector, which contained both mutant 3ʹ-UTR and wild-type PFKFB2. The cells were transfected with luciferase reporters, either wild-type or mutant PFKFB2 3ʹ-UTR, combined with miR-1297 mimics or negative control (NC) for miRNA mimics using Roche X-tremeGENE HP DNA Transfection Reagent (Roche, Basel, Switzerland). Luciferase activity was analyzed using the Dual-Glo Luciferase Assay System (Promega) after 48 h.
Database Analysis
TargetScan (http://www.targetscan.org) and miRDB (http://www.mirdb.org) were used to predict potential target genes of miR-1297 and potential target sites of miR-1297 on PFKFB2.
Statistical Analyses
Graphpad software was used for statistical analyses. All data are represented as mean ± SD. The chi-square test was used for proportion comparison. The comparison between different groups was performed by Student’s t-test. A P value < 0.05 was considered to show a statistically-significant difference.
Results
MiR-1297 is Downregulated in OS Cell Lines and Inhibits the Proliferation and Promotes the Apoptosis of OS Cells
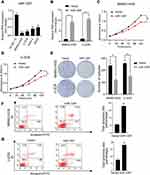
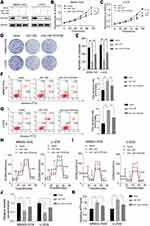
To investigate the expression of miR-1297 in OS, we initially made an assessment of the expression level of miR-1297 in hFOB1.19 cells and OS cell lines (Saos-2, MG63, MNNG-HOS, and U-2OS) by qRT-PCR. The results suggested that, compared to the expression level in normal human osteoblast cell lines, miR-1297 was significantly downregulated in OS cell lines, especially in MNNG-HOS and U-2OS cell lines (Figure 1A). Furthermore, we detected differences in the expression of miR-1297 in OS (n = 40) tissue samples through fluorescence in situ hybridization (FISH). We found that the lower expression of miR-1297 was related to the advanced pathological staging in OS (Supplementary Figure 1A and B). In order to explore the exact function of miR-1297 in the progression of OS, we developed miR-1297-overexpressing cell lines and confirmed the efficiency of overexpression by qRT-PCR (Figure 1B). According to the CCK-8 proliferation assay (Figure 1C and D) and colony formation assay (Figure 1E), the results showed that overexpression of miR-1297 inhibited the proliferation of MNNG-HOS and U-2OS cells. Next, we explored the effect of miR-1297 overexpression on the apoptosis of OS cells. As shown in Figure 1F and G, compared with the control cells, overexpression of miR-1297 significantly increased the apoptosis of OS cells. These results suggested that miR-1297 inhibited the proliferation of OS cells and promoted their apoptosis.
MiR-1297 is Involved in the Regulation of the Warburg Effect in OS Cells
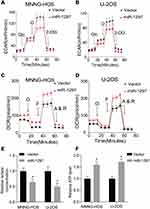
It is well known that the Warburg effect plays a vital role in tumor development, contributing to the growth of cancer cells.13 We used a metabolic flux analyzer to explore whether miR-1297 has any relationship with the Warburg effect in the development of OS by measuring the glycolytic rate (ECAR) and mitochondrial respiration (OCR). Overexpression of miR-1297 significantly suppressed the ECAR in MNNG-HOS and U-2OS cells (Figure 2A and B) but enhanced OCR in these cells (Figure 2C and D). Furthermore, the overexpression of miR-1297 in OS cells also augmented the level of ATP produced by oxidative phosphorylation and decreased the production of lactate by aerobic glycolysis (Figure 2E and F). Collectively, these data suggested that miR-1297 inhibited aerobic glycolysis in OS cells.
PFKFB2 is Identified as a Direct Target of miR-1297
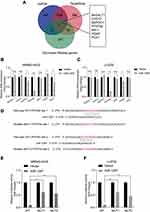
In order to predict the latent target genes of miR-1297, we utilized two widely-applied prediction algorithms, TargetScan (http://www.targetscan.org) and miRDB (http://www.mirdb.org). We found that PFKFB2, CHST2 (carbohydrate sulfotransferase 2), DEPDC1 (DEP domain containing 1), B4GALT1 (beta-1,4-galactosyltransferase 1), MXI1 (MAX interactor 1, dimerization protein), PGM2 phosphoglucomutase 2), and PCK1 (phosphoenolpyruvate carboxykinase 1) were related to miR-1297 and the Warburg effect (Figure 3A). We analyzed the expression level of these genes when miR-1297 was overexpressed in OS cell lines. We found that the expression level of PFKFB2 was notably downregulated in the miR-1297-overexpressing cell lines (MNNG-HOS and U-2OS Figure 3B–C). As shown in Figure 3D, there are two possible target sites of miR-1297 in PFKFB2. We found that miR-1297 significantly suppressed the luciferase activity of the wild-type PFKFB2 3ʹ-UTR (WT), but not the Mut 3ʹ-UTR of PFKFB2 in MNNG-HOS and U-2OS cells (Figure 3E and F). In summary, all these data suggested that PFKFB2 was a target of miR-1297 in OS cells.
PFKFB2 Promotes the Proliferation of OS Cells and Contributes to the Warburg Effect in OS Cells
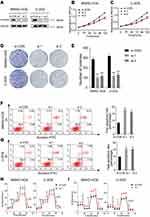
PFKFB2 belongs to the PFKFB family, and has been found to be overexpressed in many tumors such as glioma, prostate cancer and acute lymphoblastic leukemia.14–16 However, further study is necessary to explore the mechanism underlying its actions. We knocked down PFKFB2 using siRNA and used Western blotting to confirm the efficiency of the knockdown (Figure 4A). CCK-8 proliferation assay (Figure 4B and C) and colony formation assay (Figure 4D and E) indicated that knocking down of PFKFB2 significantly suppressed the multiplication of the OS cells, while an apoptosis assay indicated that silencing of PFKFB2 induced OS cell apoptosis (Figure 4F and G). In OS cells, knockdown of PFKFB2 also increased the OCR and reduced the ECAR (Figure 4H and I). In conclusion, all these experimental results indicated that PFKFB2 promoted the Warburg effect and contributed to the multiplication of OS cells.
MiR-1297 Suppresses Proliferation and Aerobic Glycolysis by Inhibiting PFKFB2 Expression in OS Cells
In our research, once we had identified that miR-1297 inhibited the multiplication of OS cells (Figure 1), we next further probed whether or not miR-1297 suppressed proliferation and aerobic glycolysis by targeting PFKFB2 in OS cells. We first overexpressed miR-1297 in wild-type and stable PFKFB2-overexpressing cell lines. Western blotting was used to confirm the overexpression efficiency (Figure 5A). As shown in Figure 5B–G, colony formation assays, CCK8 assays and apoptosis assays indicated that overexpression of miR-1297 inhibited cell growth while overexpression of PFKFB2 reduced the anti-tumor function of miR-1297. Furthermore, the aerobic glycolysis which was previously suppressed by miR-1297, was partly restored by overexpression of PFKFB2 (Figure 5H–K). All these results indicated the miR-1297 suppressed proliferation and aerobic glycolysis by inhibiting PFKFB2 expression in OS cells.
Discussion
A large number of studies have indicated that miR-1297 is closely related to the growth, apoptosis and metastasis of cancer cells. An in vitro study revealed that miR-1297 suppresses growth of pancreatic cancer by targeting MTDH.17 Wang et al found that miR-1297 inhibited metastasis by targeting AEG-1 in cervical cancer.18 Wang et al reported that serum miR-1297 is a promising diagnostic biomarker in esophageal squamous cell carcinoma.19 Therefore, miR-1297 is an effective target for the treatment of tumors. In our present study, we ascertained that miR-1297 was downregulated in OS cell lines and tissues and inhibited the proliferation of OS cells (Figure 1). More importantly, we found that miR-1297 was involved in metabolic reprogramming and that overexpressing miR-1297 suppressed aerobic glycolysis in OS cells (Figure 2).
In tumors or other cells under development, the rate of glucose uptake markedly increases and large amounts of lactate are produced, even in the presence of oxygen and fully functioning mitochondria. This process is known as the Warburg effect.20,21 Previous studies have reported that the Warburg effect plays a vital role in supporting proliferation, aggressiveness and drug resistance of OS cells. Yuan et al reported that PKM2, which is a rate-limiting enzyme of the glycolytic process, promotes tumor survival in OS cells both in vitro and in vivo.22 In addition, lactate dehydrogenase A (LDHA) is a glycolytic enzyme in the final step of anaerobic glycolysis and was reported to play a crucial role in promoting OS tumor metastasis.23 Furthermore, aldolase A (ALDOA) which catalyzes the conversion of fructose-1,6-bisphosphate was demonstrated to be involved in the development of OS chemoresistance.24 Moreover, glycolysis inhibition by 2-deoxy-D-glucose has been shown to significantly delay metastasis and prolong survival in an orthotopic postsurgical OS model.25 Therefore, inhibition of aerobic glycolysis could be an effective approach for the treatment of OS. Recently, some miRNAs, such as miR-644a, miR-30a-5p and miR-214 have been reported to regulate cell growth and the Warburg effect.26–28 MiR-1297 has also been reported to be connected with metabolic reprogramming in glioblastoma.29 In our study, we found that miR-1297 directly targeted PFKFB2, resulting in the impairment of aerobic glycolysis in OS.
The enzyme fructose-2, 6-bisphosphatase 2(PFKFB2) is a member of the PFKFB family, which is a family of bifunctional enzymes that control the levels of fructose 2,6-bisphosphate (Fru-2,6-P2).30 Previous research has found that the expression level of PFKFB2 is evidently increased in a variety of tumors.31–33 A recent study revealed that RSK regulates PFKFB2 activity in order to promote metabolic rewiring in melanoma.34 It is also reported that PFKFB2 regulates glycolysis and proliferation in pancreatic cancer cells.31 Through database analysis, qRT-PCR, Western blotting and luciferase assays we found that PFKFB2 was directly regulated by miR-1297 in OS. Our study provides insight into the biology of OS progression and shows that miR-1297 is an inhibitor of PFKFB2 that suppresses the growth of OS cells by inhibiting PFKFB2-mediated aerobic glycolysis (Figures 3–5).
In conclusion, our study indicated that the expression level of miR-1297 was evidently downregulated in OS cells. MiR-1297 inhibited the proliferation of OS cells by inhibiting aerobic glycolysis via downregulation of PFKFB2 expression. Our findings provide evidence of the underlying mechanisms of OS development and suggest that miR-1297 could be a potential target for the treatment of OS.
Funding
This work was supported by grants from the Changzhou Sci & Tech Program (CJ20190083) and Young Talent Development Plan of Changzhou Health Commission (CZQM2020069).
Disclosure
The authors declare no conflicts of interest.
References
1. Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33(27):3029–3035. doi:10.1200/JCO.2014.59.4895
2. Cancer statistics. JAMA. 2013;310(9):982. doi:10.1001/jama.2013.5289
3. Karius T, Schnekenburger M, Dicato M, Diederich M. MicroRNAs in cancer management and their modulation by dietary agents. Biochem Pharmacol. 2012;83(12):1591–1601. doi:10.1016/j.bcp.2012.02.004
4. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297.
5. Weng Y, Shen Y, He Y, et al. The miR-15b-5p/PDK4 axis regulates osteosarcoma proliferation through modulation of the Warburg effect. Biochem Biophys Res Commun. 2018;503(4):2749–2757. doi:10.1016/j.bbrc.2018.08.035
6. Wang SN, Luo S, Liu C, et al. miR-491 inhibits osteosarcoma lung metastasis and chemoresistance by targeting αb-crystallin. Mol Ther. 2017;25(9):2140–2149. doi:10.1016/j.ymthe.2017.05.018
7. Shekhar R, Priyanka P, Kumar P, et al. The microRNAs miR-449a and miR-424 suppress osteosarcoma by targeting cyclin A2 expression. J Biol Chem. 2019;294(12):4381–4400. doi:10.1074/jbc.RA118.005778
8. Gao W, Cao Y, Guo P, et al. Downregulation of MiR-1297 predicts poor prognosis and enhances gastric cancer cell growth by targeting CREB1. Biomed Pharmacothe/Biomedecine & Pharmacotherapie. 2018;105:413–419.
9. Wang Y, Xue J, Kuang H, Zhou X, Liao L, Yin F. microRNA-1297 inhibits the growth and metastasis of colorectal cancer by suppressing cyclin D2 expression. DNA Cell Biol. 2017;36(11):991–999. doi:10.1089/dna.2017.3829
10. Zhao SJ, Jiang YQ, Xu NW, et al. SPARCL1 suppresses osteosarcoma metastasis and recruits macrophages by activation of canonical WNT/beta-catenin signaling through stabilization of the WNT-receptor complex. Oncogene. 2018;37(8):1049–1061. doi:10.1038/onc.2017.403
11. Zhang C, Chi YL, Wang PY, et al. miR-511 and miR-1297 inhibit human lung adenocarcinoma cell proliferation by targeting oncogene TRIB2. PLoS One. 2012;7(10):e46090. doi:10.1371/journal.pone.0046090
12. Shen Y, Xu J, Pan X, et al. LncRNA KCNQ1OT1 sponges miR-34c-5p to promote osteosarcoma growth via ALDOA enhanced aerobic glycolysis. Cell Death Dis. 2020;11(4):278.
13. Bartrons R, Caro J. Hypoxia, glucose metabolism and the Warburg’s effect. J Bioenerg Biomembr. 2007;39(3):223–229. doi:10.1007/s10863-007-9080-3
14. Ji D, Lu ZT, Li YQ, et al. MACC1 expression correlates with PFKFB2 and survival in hepatocellular carcinoma. Asian Pac J Cancer Prev. 2014;15(2):999–1003. doi:10.7314/APJCP.2014.15.2.999
15. Moon JS, Jin WJ, Kwak JH, et al. Androgen stimulates glycolysis for de novo lipid synthesis by increasing the activities of hexokinase 2 and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 2 in prostate cancer cells. Biochem J. 2011;433(1):225–233. doi:10.1042/BJ20101104
16. Minchenko O, Opentanova I, Minchenko D, Ogura T, Esumi H. Hypoxia induces transcription of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase-4 gene via hypoxia-inducible factor-1alpha activation. FEBS Lett. 2004;576(1–2):14–20. doi:10.1016/j.febslet.2004.08.053
17. Chen Z, Ma Y, Pan Y, Zhu H, Yu C, Sun C. MiR-1297 suppresses pancreatic cancer cell proliferation and metastasis by targeting MTDH. Mol Cell Probes. 2018;40:19–26. doi:10.1016/j.mcp.2018.06.003
18. Wang Z, He S, Guo P, Guo X, Zheng J. MicroRNA-1297 inhibits metastasis and epithelial-mesenchymal transition by targeting AEG-1 in cervical cancer. Oncol Rep. 2017;38(5):3121–3129. doi:10.3892/or.2017.5979
19. Wang C, Li Q, Liu F, et al. Serum miR-1297: a promising diagnostic biomarker in esophageal squamous cell carcinoma. Biomarkers. 2016;21(6):517–522.
20. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
21. Hsu PP, Sabatini DM. Cancer cell metabolism: warburg and beyond. Cell. 2008;134(5):703–707. doi:10.1016/j.cell.2008.08.021
22. Yuan Q, Yu H, Chen J, Song X, Sun L. Knockdown of pyruvate kinase type M2 suppresses tumor survival and invasion in osteosarcoma cells both in vitro and in vivo. Exp Cell Res. 2018;362(1):209–216. doi:10.1016/j.yexcr.2017.11.020
23. Gao S, Tu DN, Li H, et al. Pharmacological or genetic inhibition of LDHA reverses tumor progression of pediatric osteosarcoma. Biomed Pharmacother. 2016;81:388–393. doi:10.1016/j.biopha.2016.04.029
24. Long F, Cai X, Luo W, Chen L, Li K. Role of aldolase A in osteosarcoma progression and metastasis: in vitro and in vivo evidence. Oncol Rep. 2014;32(5):2031–2037. doi:10.3892/or.2014.3473
25. Sottnik JL, Lori JC, Rose BJ, Thamm DH. Glycolysis inhibition by 2-deoxy-D-glucose reverts the metastatic phenotype in vitro and in vivo. Clin Exp Metastasis. 2011;28(8):865–875. doi:10.1007/s10585-011-9417-5
26. Ebron JS, Shankar E, Singh J, et al. MiR-644a disrupts oncogenic transformation and warburg effect by direct modulation of multiple genes of tumor-promoting pathways. Cancer Res. 2019;79(8):1844–1856. doi:10.1158/0008-5472.CAN-18-2993
27. Li L, Kang L, Zhao W, et al. miR-30a-5p suppresses breast tumor growth and metastasis through inhibition of LDHA-mediated Warburg effect. Cancer Lett. 2017;400:89–98. doi:10.1016/j.canlet.2017.04.034
28. Yang L, Zhang W, Wang Y, et al. Hypoxia-induced miR-214 expression promotes tumour cell proliferation and migration by enhancing the Warburg effect in gastric carcinoma cells. Cancer Lett. 2018;414:44–56. doi:10.1016/j.canlet.2017.11.007
29. Li H, Yuan H. MiR-1297 negatively regulates metabolic reprogramming in glioblastoma via repressing KPNA2. Hum Cell. 2020;33(3):619–629. doi:10.1007/s13577-019-00316-7
30. Pilkis SJ, Claus TH, Kurland IJ, Lange AJ. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase: a metabolic signaling enzyme. Annu Rev Biochem. 1995;64:799–835. doi:10.1146/annurev.bi.64.070195.004055
31. Ozcan SC, Sarioglu A, Altunok TH, et al. PFKFB2 regulates glycolysis and proliferation in pancreatic cancer cells. Mol Cell Biochem. 2020;470(1–2):115–129. doi:10.1007/s11010-020-03751-5
32. Yang H, Shu Z, Jiang Y, et al. 6-Phosphofructo-2-Kinase/Fructose-2,6-Biphosphatase-2 Regulates TP53-dependent paclitaxel sensitivity in ovarian and breast cancers. Clin Cancer Res. 2019;25(18):5702–5716. doi:10.1158/1078-0432.CCR-18-3448
33. Zhao L, Ji G, Le X, et al. Long noncoding RNA LINC00092 acts in cancer-associated fibroblasts to drive glycolysis and progression of ovarian cancer. Cancer Res. 2017;77(6):1369–1382. doi:10.1158/0008-5472.CAN-16-1615
34. Houles T, Gravel SP, Lavoie G, et al. RSK regulates PFK-2 activity to promote metabolic rewiring in melanoma. Cancer Res. 2018;78(9):2191–2204. doi:10.1158/0008-5472.CAN-17-2215
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.