Back to Journals » OncoTargets and Therapy » Volume 12
miR-129-5p inhibits prostate cancer proliferation via targeting ETV1
Authors Gao G, Xiu D, Yang B, Sun D, Wei X, Ding Y, Ma Y, Wang Z
Received 10 August 2018
Accepted for publication 26 October 2018
Published 9 May 2019 Volume 2019:12 Pages 3531—3544
DOI https://doi.org/10.2147/OTT.S183435
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Leo Jen-Liang Su
Ge Gao,1,* Dianhui Xiu,2,* Bin Yang,3 Daju Sun,1 Xin Wei,4 Youpeng Ding,4 Yanan Ma,4 Zhixin Wang4
1Department of Pathology, China-Japan Union Hospital of Jilin University, Changchun, Jilin 130033, P.R. China; 2Department of Radiology, China-Japan Union Hospital of Jilin University, Changchun, Jilin 130033, P.R. China; 3Department of Breast Surgery, China-Japan Union Hospital of Jilin University, Changchun, Jilin 130033, P.R. China; 4Department of Urology, China-Japan Union Hospital of Jilin University, Changchun, Jilin 130033, P.R. China
*These authors contributed equally to this work
Background: Prostate cancer is one of the most commonly diagnosed diseases in males.
Methods: RT-qPCR was used to detect miR-129-5p expression in tumor tissues and adjacent normal tissues from patients with prostate cancer. The cell proliferation assay and colony forming assay were used to study the role of miR-129-5p in mediating prostate cancer cell growth. Bioinformatic analysis and dual luciferase assay were performed to predict and confirm ETV1 as a target gene of miR-129-5p.
Results: We found that miR-129-5p levels were decreased significantly in human prostate cancer tissues compared with matched normal tissues from patients with prostate cancer. Overexpression of miR-129-5p suppressed prostate cancer cell growth while antagonist of miR-129-5p promoted cell proliferation in immortal prostate cell line RWPE-1. In addition, elevation of miR-129-5p decreased ETV1 expression in prostate cancer cells while downregulation of miR-129-5p increased ETV1 expression in RWPE-1. Mechanistically, ETV1 is confirmed a direct target of miR-129-5p in prostate cancer cells. Through repression of ETV1 expression, miR-129-5p could inactivate YAP signaling in prostate cancer cells. In addition, overexpression of ETV1 attenuated miR-129-5p induced cell proliferation in prostate cancer cells. Correlation analysis further revealed that there was a negative correlation between miR-129-5p levels and ETV1 mRNA levels in tumor tissues from patients with prostate cancer.
Conclusion: Our results identified miR-129-5p as a tumor suppressor in prostate cancer via repression of ETV1.
Keywords: microRNA, prostate cancer, ETV1, proliferation
Introduction
Prostate cancer is the most commonly diagnosed malignant cancer types in males and ranks third among cancer-related deaths in men worldwide.1 Globally, there were estimated 903,500 new prostate cancer cases and 258,400 estimated deaths in 2012.2 Currently, prostate-specific antigen is used for early prediction of prostate cancer.3 When patients are diagnosed as early stage prostate cancer, radical prostatectomy that can greatly improve patients’ overall survival is performed.4 Although many patients with prostate cancer are curable, tumor recurrence, metastasis, and development of castration resistance frequently occur and eventually lead to patient death.5–7 Advances in genetics have deepened our understandings on the carcinogenesis of prostate cancer,8 but the precise molecular mechanism of prostate cancer progression is not fully elucidated. As a result, further investigation is still needed to provide valuable targets for prostate cancer treatment.
MicroRNAs (miRNAs) belong to a class of noncoding RNAs with the feathers of small (19–25 nucleotides), single-strand, and ubiquitously expression in human.9 Mechanistically, miRNAs bind to complementary sites on 3′-untranslated region (3′UTR) of messenger RNA (mRNA) and lead to mRNA degradation or translational inhibition, resulting in repression of target gene expression.10 MiRNAs are important regulators of normal biological processes including cell proliferation, cell differentiation, cell motility, and cell death. Accumulating evidences suggested that deregulation of miRNAs was associated with cancer initiation and progression.11,12 In prostate cancer, many miRNAs were reported as oncogenes or tumor suppressors via regulation of their numerous target genes.13–15 For example, miR-203 was reported as an upregulated miRNA in breast cancer and promoted breast cancer progression via targeting SOCS3.16 Moreover, several miRNAs were proved to be promising biomarkers for prostate cancer diagnosis.17 Profiling of miRNAs suggested that four miRNAs (miR-4289, miR-326, miR-152-3p, and miR-98-5p) were biomarkers to identify patients with prostate cancer from healthy people.17 Using microarray, many cancer-associated miRNAs have been identified in prostate cancer.18,19 In 2016, one study involving miRNA profiling of 21 normal prostate tissues and 56 prostate tumor tissues has discovered many deregulated miRNAs in prostate cancer; among them, miR-424 was experimentally validated as an oncogene.20 However, the potential function of other aberrant expressed miRNAs has not been studied yet.
ETS factors are transcription factors that can directly bind to promoters and enhancers to recruit other transcriptional machinery components.21 Overexpression of ETS family members such as ETV1, ETV4, and ETV5 are associated with prostate cancer progression.22 In prostate cancer cells, ETV1 cooperates with androgen receptor signaling and contributes to highly aggressive phenotype in mice and human.23 ETV1 also activated transcription of TAZ to promote prostate cancer development.24 Upregulated by JMJD2A, ETV1 activated transcription of YAP, a TAZ homologue, in prostate cancer.25 Therefore, the regulation of ETV1 in prostate cancer needs to be investigated.
In the current study, we showed that miR-129-5p was downregulated in prostate cancer tissues and cell lines. Overexpression of miR-129-5p inhibited cell proliferation of PC-3, while antagonizing of miR-129-5p promoted RWPE-1 cell growth. Mechanistic studies indicated that miR-129-5p negatively regulated ETV1 to inactivate YAP signaling in prostate cancer. Forced overexpression of ETV1 reversed miR-129-5p mimics inducing cell growth arrest. Reverse transcription-quantitative PCR (RT-qPCR) suggested a negative correlation between miR-129-5p and ETV1 mRNA expression in tumor tissues from patients with prostate cancer. Our findings revealed miR-129-5p as a tumor suppressor in prostate cancer via repression of ETV1.
Materials and methods
Patient samples and patient tumor data analysis
Prostate tumor tissues and matched normal prostate tissues were collected from 30 patients with prostate cancer receiving radical prostatectomy during 2015–2017 in China-Japan Union Hospital of Jilin University. Written informed consents were provided by all patients before experiments. All procedures were carried out under the supervision of medical ethics committee of Jilin University. The study was conducted in accordance with the Declaration of Helsinki. For bioinformatic analysis of miRNA profiling in prostate cancer, GSE60117 containing data of 21 normal tissues and 56 prostate tumor tissues were analyzed on Gene Expression Omnibus (GEO) using GEO2R to obtain most significantly deregulated miRNAs.
Cell lines
Human normal epithelial cell line RWPE-1 and human prostate cancer cell lines PC-3, DU145, and LNCaP were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). All cell lines were used within 6 months after receipt. PC-3, DU145, and LNCaP cells were cultured in DMEM (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (HyClone, Thermo Fisher Scientific) and 1% Penicillin–Streptomycin solution (Invitrogen, Thermo Fisher Scientific). RWPE-1 cells were maintained in Keratinocyte Serum Free Medium (Invitrogen, Thermo Fisher Scientific) supplemented with bovine pituitary extract (Invitrogen, Thermo Fisher Scientific) and human recombinant EGF (Invitrogen, Thermo Fisher Scientific). All cell lines were maintained in a 37°C incubator with 5% CO2.
MiR-129-5p overexpression and antagonizing
MiR-NC mimics, miR-129-5p mimics, miR-NC antagonist, and miR-129-5p antagonist were bought from RiboBio (Guangzhou, P.R. China). For miR-129-5p overexpression or downregulation, miR-129-5p mimics or miR-129-5p antagonist was mixed with Lipofectamine 2000 (Invitrogen, Thermo Fisher Scientific), maintained for 15 minutes, and then added into culture medium of cells. Seventy-two hours after transfection, the cells were harvested and the RNA and/or protein was extracted for the following experiments.
Western blot
Protein lysates were prepared using RIPA lysis buffer (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) according to the manufacturer’s protocol. Antibodies for ETV1, YAP, CTGF, and CYR61 were bought from Cell Signaling Technology (CST, Danvers, MA, USA). β-actin antibody was purchased from Sigma-Aldrich. Secondary antibodies against rabbit and mouse were products of Proteintech (Chicago, IL, USA). Western blot was performed in a standard procedure. Briefly, lysates were loaded on SDS gel; proteins were separated and then transferred on a PVDF membrane. The membrane was blocked by 5% nonfat milk and incubated in indicated primary antibodies overnight. On the next day, membrane was incubated in secondary antibodies for 1 hour. The membrane was developed by ECL Western blot substrate (Pierce, Thermo Fisher Scientific).
RNA extraction and real-time RT-PCR
Total RNA from tissues and cells were extracted using TRIzol Reagent (Invitrogen, Thermo Fisher Scientific) according to the manufacturer’s protocol. For miR-129-5p expression detection, RNA was reverse transcribed using a stem-loop primer with RevertAid First Strand cDNA kit (Thermo Fisher Scientific). For gene expression, RNA was reverse transcribed into first-stranded cDNA using PrimeScript RT Master Mix (Takara, Kusatsu, Japan). Real-time RT-PCR was carried out using SYBR Premix Ex Taq kit (Takara). U6 and β-actin were served as internal controls for miRNA and mRNA, respectively. The primer sequences were listed as follows: ETV1-forward: 5′-CTGAACCCTGTAACTCCTTTCC-3′; ETV1-reverse: 5′-AGACATCTGGCGTTGGTACATA-3′; CTGF-forward: 5′-CAGCATGGACGTTCGTCTG-3′; CTGF-reverse: 5′-AACCACGGTTTGGTCCTTGG-3′; CYR61-forward: 5′-CTCGCCTTAGTCGTCACCC-3′; CYR61-reverse: 5′-CGCCGAAGTTGCATTCCAG-3′; β-actin-forward: 5′-CATGTACGTTGCTATCCAGGC-3′; β-actin-reverse: 5′-CTCCTTAATGTCACGCACGAT-3′.
ETV1 overexpression
Full length of ETV1 open reading frame was amplified from RWPE-1 cDNA and ligated into pcDNA3.1 plasmid (OriGene, Rockville, MD, USA). For ETV1 overexpression, pcDNA3.1-ETV1 plasmid was mixed with Lipofectamine 2000 (Invitrogen, Thermo Fisher Scientific) in serum-free DMEM for 15 minutes and added into culture medium.
Cell proliferation assay
For analysis of cell growth ability, cells were seeded in 96-well plate. Every 24 hours from day 0 to day 3, 10 μL CCK-8 solution (Cell Counting Kit-8, Dojindo, Kumamoto, Japan) was added into culture medium in each well for 2 hours. After that, medium containing CCK-8 solution was transferred into a new 96-well plate and the absorbance at 450 nm was detected to reflect cell proliferation ability.
Colony forming assay
For colony forming assay, 500 treated cells were seeded in each well in 6-well plates. After growing for 16 days, the culture medium was discarded, and each well was slightly washed with PBS. Then, cell colonies were fixed with 4% paraformaldehyde (Sigma-Aldrich) followed by staining with 0.5% crystal violet (Sigma-Aldrich). Images of colony were captured, and colony numbers were counted using ImageJ software.
Dual luciferase reporter assay
ETV1 3′UTR was cloned from cDNA of RWPE-1 and annealed into pGL3-basic (Promega, Fitchburg, WI, USA). The primer sequences were as follows: ETV1 3′UTR-forward: 5′-GCTCTAGATTCTGTAAATGTGAT-3′; ETV1 3′UTR-reverse: 5′-GCTCTAGATGAAATTGGAGTACTT-3′. Two site mutations were introduced into pGL3-ETV1 3′UTR-WT (wild type) to construct pGL3-ETV1 3′UTR-Mut (mutant) using GeneArt™ Site-Directed Mutagenesis PLUS System (Thermo Fisher Scientific). For dual luciferase reporter assay, cells were cotransfected with pGL3-ETV1 3′UTR-WT or pGL3-ETV1 3′UTR-Mut in combination with miR-NC mimics or miR-129-5p mimics and pRL plasmid. After 48 hours, relative luciferase activity was detected using Dual Luciferase Reporter Assay System (Promega) in accordance with the manufacturer’s protocol.
Statistical analysis
All data were analyzed using GraphPad Prism 7 and expressed as mean ± SD. Student’s t-test was used to compare differences between two groups. One-way ANOVA was carried out to compare differences among three groups, followed by Newman–Keuls test. Correlation analysis between miR-129-5p and ETV1 expression was conducted using Pearson correlation analysis. P-values <0.05 were considered to be statistically significant.
Results
Expression of miR-129-5p was downregulated in prostate cancer tissues and cell lines
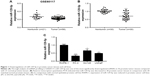
To explore deregulated miRNAs in prostate cancer, we analyzed miRNA profiling of 21 normal prostate tissues and 56 prostate cancer tissues from data set GSE60117 in GEO database. As shown in Figure 1A, miR-129-5p was significantly downregulated in prostate cancer tissues compared with normal prostate tissues (P<0.001). To confirm this result, RT-qPCR was performed to detect miR-129-5p expression in 30 pairs of prostate cancer tissues and matched normal tissues collected. Similarly, a decrease of miR-129-5p expression was observed in prostate cancer tissues compared with normal tissues (Figure 1B, P<0.001). Next, association between miR-129-5p expression and clinicopathological factors in 30 patients with prostate cancer was analyzed. No significant association was observed between miR-129-5p expression with age, Gleason score, or distant metastasis, but high expression of miR-129-5p was associated with early pathologic stage (P<0.01) (Table 1). Additionally, compared with normal epithelial prostate cell RWPE-1, expression of miR-129-5p was decreased in prostate cancer cell lines (PC-3, DU145, and LNCaP) (Figure 1C, P<0.001, P<0.05, and P<0.01, respectively).
  | Table 1 Association of miR-129-5p with clinicopathological features of 30 prostate cancer patients |
MiR-129-5p regulated prostate cancer cell proliferation
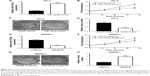
To investigate the potential role of miR-129-5p in prostate cancer, miR-129-5p mimics was transfected into PC-3 cells. Overexpression of miR-129-5p by transfection of miR-129-5p mimics significantly inhibited cell growth of PC-3 cells (Figure 2A and B, P<0.001). Additionally, in the colony forming assay, miR-129-5p mimics reduced colony number formed by PC-3 cells (Figure 2C and D, P<0.01). We next downregulated miR-129-5p expression in RWPE-1 cells by transfection of miR-129-5p antagonist (Figure 2E). In contrast, miR-129-5p downregulation promoted cell proliferation of RWPE-1 cells (Figure 2F, P<0.05). The colony forming assay showed that miR-129-5p antagonist increased colony number formed by RWPE-1 cells (Figure 2G and H, P<0.001).
Thus, these data suggested that miR-129-5p could suppress prostate cancer cell proliferation.
MiR-129-5p repressed ETV1 expression by directly binding to its 3′UTR
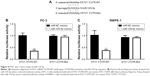
MiRNA exerts its function via binding to 3′UTR on its target gene mRNA.10 ETV1 functions as an oncogene and is overexpressed in prostate cancer.23 Using RT-qPCR, we found that ETV1 was overexpressed in all prostate cancer cell lines examined including PC-3 (P<0.01), DU145 (P<0.05), and LNCaP (P<0.01) compared with normal epithelial prostate cell RWPE-1 (Figure 3A). In PC-3, overexpression of miR-129-5p decreased ETV1 mRNA levels (Figure 3B, P<0.001). Additionally, Western blot showed that miR-129-5p mimics induced reduction of ETV1 protein expression (Figure 3C) and quantification of protein levels suggested that ETV1 protein expression was significantly downregulated toward miR-129-5p overexpression (Figure 3D, P<0.01). Moreover, in RWPE-1 cells, downregulation of miR-129-5p elevated ETV1 mRNA levels (Figure 3E). Western blot showed that miR-129-5p antagonist induced elevation of ETV1 protein expression (Figure 3F) and quantification of protein levels suggested that ETV1 was significantly upregulated toward miR-129-5p overexpression (Figure 3G, P<0.01). Using miRanda, our bioinformatic analysis showed that there was a complementary site between miR-129-5p sequence and ETV1 mRNA 3′UTR sequence (Figure 4A). To validate ETV1 as a target gene of miR-129-5p, we carried out the Dual Luciferase Reporter Assay in PC-3 cells. As expected, miR-129-5p mimics reduced relative luciferase activity in PC-3 cells transfected with ETV1 3′UTR-WT not ETV1 3′UTR-Mut (Figure 4B). Similarly, in RWPE-1 cells, overexpression of miR-129-5p repressed luciferase activity of ETV1 3′UTR-WT (Figure 4C, P<0.01). These data suggested that miR-129-5p might inhibit prostate cancer progression via regulation of ETV1.
MiR-129-5p repressed YAP and its target gene expression
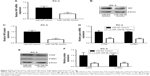
ETV1 is a transcription factor and accelerates prostate cancer cell growth via transcriptional activation of YAP.25 Consistent with ETV1 downregulation, miR-129-5p mimics also triggered the decrease of YAP mRNA expression (Figure 5A). Also, the protein levels of YAP were decreased upon miR-129-5p overexpression (Figure 5B and C, P<0.001). As a transcription coactivator, YAP stimulated transcription of CTGF and CYR61.26 RT-qPCR results indicated that CTGF and CYR61 expression was decreased (Figure 5D) and Western blot showed that CTGF and CYR61 protein expression was decreased (Figure 5E and F, P<0.01 and P<0.001, respectively) toward miR-129-5p overexpression, suggesting that miR-129-5p suppressed YAP target gene expression. In contrast, downregulation of miR-129-5p significantly elevated YAP mRNA and protein (P<0.01) expression in RWPE-1 cells (Figure 6A–C). Additionally, miR-129-5p antagonist also stimulated transcription of CTGF and CYR61 (Figure 6D, P<0.001), leading to enhanced protein expression of CTGF and CYR61 in RWPE-1 cells (Figure 6E and F, P<0.05 and P<0.01, respectively). The results demonstrated that miR-129-5p could repress YAP signaling via targeting ETV1 in prostate cancer.
ETV1 overexpression reversed miR-129-5p mimics-induced cell proliferation inhibition in PC-3 cells
To examine whether ETV1 was pivotal for miR-129-5p function in prostate cancer cells, we constructed plasmid containing recombinant ETV1. Western blot showed that miR-129-5p mimics reduced ETV1 protein (P<0.001) expression, which could be reversed (P<0.01) by pcDNA3.1-ETV1 transfection in PC-3 cells (Figure 7A and B, P<0.001 and P<0.01, respectively). In the cell proliferation assay, forced expression of ETV1 reversed cell growth arrest induced by miR-129-5p mimics in PC-3 (Figure 7C, P<0.001 and P<0.05, respectively). Moreover, the colony forming assay showed that reduced colony number toward miR-129-5p mimics was recovered after ETV1 overexpression (Figure 7D and E, P<0.001 and P<0.01, respectively). Collectively, these results manifested that miR-129-5p relied on regulation of ETV1 to inhibit prostate cancer cell proliferation.
Expression of miR-129-5p was negatively associated with ETV1 mRNA levels in prostate cancer tissues
Next, we sought to investigate the relationship between miR-129-5p expression and ETV1 expression levels in prostate cancer tissues. Consistent with previous study, ETV1 protein (P<0.001) and mRNA levels were elevated in prostate cancer tissues compared with matched normal prostate tissues (Figure 8A–C, P<0.001 and P<0.05, respectively). Furthermore, Pearson correlation assay showed that miR-129-5p expression was inversely correlated with ETV1 mRNA levels in prostate cancer tissues (Figure 8D, P=0.0018). Our data suggested an miR-129-5p/ETV1/Hippo-YAP axis in regulating proliferation of prostate cancer cells (Figure 8E).
Discussion
It is well characterized that aberrant expression of miRNAs is one of hallmarks of carcinogenesis.27 Recent years, many researches carried out miRNA profiling of prostate cancer tissues and discovered several miRNAs with oncogene or tumor suppressor potential.20,28,29 Although studies have identified numerous differentially expressed miRNAs in prostate cancer tissues compared with normal tissues, little was known about the function and underlying molecular mechanism of specific miRNA in prostate cancer. In the current study, we found that miR-129-5p was downregulated in prostate cancer tissues and functioned as a tumor suppressor via regulation of ETV1.
MiR-129-5p has been reported as a tumor suppressor in many cancer types including glioblastoma, papillary thyroid cancer, colorectal cancer, and breast cancer.30–33 Several studies provided evidences that miR-129-5p inhibited cancer progression via repression of HMGB1.34,35 In prostate cancer, miR-129-5p was found as one of the most significantly downregulated miRNA in prostate cancer cell and was involved in the regulation of metabolism and cell proliferation via repression of key proteins in carnitine cycle.36 Most recently, miR-129 was discovered to inhibit cell proliferation, migration, and invasiveness in prostate cancer cells.37 Here, through screen of differentially expressed miRNAs in GSE60117, we also observed the decreased expression of miR-129-5p in prostate tumor tissues and further validated its downregulation in 30 pairs of prostate tumor and normal tissues we collected. Our cell functional assays validated tumor suppressor role of miR-129-5p in PC-3 and RWPE-1 cells; these results were consistent with previous reports.
Several oncogenes, such as PAK5 and RET, have been validated as target genes of miR-129-5p in cancer cells.38,39 Study in mice and human showed that ETV1 cooperated with androgen receptor signaling to promote highly aggressive nature of advanced prostate cancer.23 ETV1 was proved to be regulated by several miRNAs such as miR-17-5p and miR-34b.40,41 However, whether and how ETV1 was regulated by miR-129-5p was not known. In PC-3, overexpression of miR-129-5p decreased ETV1 at both mRNA and protein levels. In contrast, downregulation of miR-129-5p increased ETV1 mRNA and protein expression in RWPE-1 cells. Bioinformatic analysis of miR-129-5p and ETV1 3′UTR sequences indicated a complementary site between them. The Dual Luciferase Reporter Assay confirmed ETV1 as a target gene of miR-129-5p in PC-3 and RWPE-1 cells. Similar to ETV1, YAP was overexpressed in prostate cancer and mediated prostate cancer metastasis, stemness, cell growth, and development of castration resistance.42,43 A recent study declared that ETV1 stimulated prostate cancer development through activation of YAP and its target gene expression.25 In PC-3 and RWPE-1 cells, miR-129-5p suppressed YAP and its target gene CTGF and CYR61 expression at both mRNA and protein levels, suggesting that miR-129-5p might suppress YAP expression via repression of ETV1. RT-qPCR further showed a strong negative correlation between miR-129-5p expression and ETV1 mRNA levels in prostate cancer tissues, implying a regulatory relationship between ETV1 and miR-129-5p in prostate cancer. In a recent study, overexpression of miR-129 was shown to inactivate PI3K/AKT signaling in the PC-3.37 Since the cross talk between PI3K/AKT and YAP signaling existed in cancer cells,44 miR-129-5p might play a central role in prostate cancer via regulating a complicated signaling network.
Conclusion
Our study provided evidences confirming that miR-129-5p was a tumor suppressor in prostate cancer and was downregulated in tissues of prostate cancer. Mechanistic study discovered an miR-129-5p/ETV1/YAP axis in prostate cancer cells. Thus, miR-129-5p might be a promising target for the treatment of patients with prostate cancer.
Data sharing statement
Data are available on request.
Disclosure
The authors report no conflicts of interest in this work.
References
Litwin MS, Tan HJ. The diagnosis and treatment of prostate cancer. JAMA. 2017;317(24):2532–2542. | ||
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. | ||
Antenor JA, Roehl KA, Eggener SE, Kundu SD, Han M, Catalona WJ. Preoperative PSA and progression-free survival after radical prostatectomy for stage T1c disease. Urology. 2005;66(1):156–160. | ||
Cozzi G, Musi G, Bianchi R, et al. Meta-analysis of studies comparing oncologic outcomes of radical prostatectomy and brachytherapy for localized prostate cancer. Ther Adv Urol. 2017;9(11):241–250. | ||
Huang EY, Chang YJ, Huang SP, et al. A common regulatory variant in SLC35B4 influences the recurrence and survival of prostate cancer. J Cell Mol Med. 2018;22(7):3661–3670. | ||
Zheng QS, Chen SH, Wu YP, et al. Increased paxillin expression in prostate cancer is associated with advanced pathological features, lymph node metastases and biochemical recurrence. J Cancer. 2018;9(6):959–967. | ||
Miller ET, Chamie K, Kwan L, Lewis MS, Knudsen BS, Garraway IP. Impact of treatment on progression to castration-resistance, metastases, and death in men with localized high-grade prostate cancer. Cancer Med. 2017;6(1):163–172. | ||
Boström PJ, Bjartell AS, Catto JW, et al. Genomic predictors of outcome in prostate cancer. Eur Urol. 2015;68(6):1033–1044. | ||
Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9(2):102–114. | ||
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. | ||
Andersen GB, Knudsen A, Hager H, Hansen LL, Tost J. miRNA profiling identifies deregulated miRNAs associated with osteosarcoma development and time to metastasis in two large cohorts. Mol Oncol. 2018;12(1):114–131. | ||
Hu X, Wang Y, Liang H, et al. miR-23a/b promote tumor growth and suppress apoptosis by targeting PDCD4 in gastric cancer. Cell Death Dis. 2017;8(10):e3059. | ||
Okato A, Arai T, Yamada Y, et al. Regulation of NCAPG by miR-99a-3p (passenger strand) inhibits cancer cell aggressiveness and is involved in CRPC. Cancer Med. 2018;7(5):1988–2002. | ||
Hao P, Kang B, Yao G, Hao W, Ma F. MicroRNA-211 suppresses prostate cancer proliferation by targeting SPARC. Oncol Lett. 2018;15(4):4323–4329. | ||
Shao N, Ma G, Zhang J, Zhu W. miR-221-5p enhances cell proliferation and metastasis through post-transcriptional regulation of SOCS1 in human prostate cancer. BMC Urol. 2018;18(1):14. | ||
Muhammad N, Bhattacharya S, Steele R, Ray RB. Anti-miR-203 suppresses ER-positive breast cancer growth and stemness by targeting SOCS3. Oncotarget. 2016;7(36):58595–58605. | ||
Matin F, Jeet V, Moya L, et al. A plasma biomarker panel of four microRNAs for the diagnosis of prostate cancer. Sci Rep. 2018;8(1):6653. | ||
Josson S, Gururajan M, Hu P, et al. miR-409-3p/-5p promotes tumorigenesis, epithelial-to-mesenchymal transition, and bone metastasis of human prostate cancer. Clin Cancer Res. 2014;20(17):4636–4646. | ||
Casanova-Salas I, Rubio-Briones J, Calatrava A, et al. Identification of miR-187 and miR-182 as biomarkers of early diagnosis and prognosis in patients with prostate cancer treated with radical prostatectomy. J Urol. 2014;192(1):252–259. | ||
Dallavalle C, Albino D, Civenni G, et al. MicroRNA-424 impairs ubiquitination to activate STAT3 and promote prostate tumor progression. J Clin Invest. 2016;126(12):4585–4602. | ||
Hollenhorst PC, McIntosh LP, Graves BJ. Genomic and biochemical insights into the specificity of Ets transcription factors. Annu Rev Biochem. 2011;80(1):437–471. | ||
Currie SL, Lau DKW, Doane JJ, et al. Structured and disordered regions cooperatively mediate DNA-binding autoinhibition of ETS factors ETV1, ETV4 and ETV5. Nucleic Acids Res. 2017;45(5):2223–2241. | ||
Baena E, Shao Z, Linn DE, et al. ETV1 directs androgen metabolism and confers aggressive prostate cancer in targeted mice and patients. Genes Dev. 2013;27(6):683–698. | ||
Liu CY, Yu T, Huang Y, Cui L, Hong W. ETS (E26 transformation-specific) up-regulation of the transcriptional co-activator TAZ promotes cell migration and metastasis in prostate cancer. J Biol Chem. 2017;292(22):9420–9430. | ||
Kim TD, Jin F, Shin S, et al. Histone demethylase JMJD2A drives prostate tumorigenesis through transcription factor ETV1. J Clin Invest. 2016;126(2):706–720. | ||
Zhou Y, Huang T, Cheng AS, Yu J, Kang W, To KF. The TEAD family and its oncogenic role in promoting tumorigenesis. Int J Mol Sci. 2016;17(1):pii:E138. | ||
Ashraf NM, Imran K, Kastner DW, et al. Potential involvement of mi-RNA 574-3p in progression of prostate cancer: a bioinformatic study. Mol Cell Probes. 2017;36:21–28. | ||
Doldi V, Pennati M, Forte B, Gandellini P, Zaffaroni N. Dissecting the role of microRNAs in prostate cancer metastasis: implications for the design of novel therapeutic approaches. Cell Mol Life Sci. 2016;73(13):2531–2542. | ||
Zhang Y, Jiang F, He H, et al. Identification of a novel microRNA-mRNA regulatory biomodule in human prostate cancer. Cell Death Dis. 2018;9(3):301. | ||
Zeng A, Yin J, Li Y, et al. miR-129-5p targets Wnt5a to block PKC/ERK/NF-κB and JNK pathways in glioblastoma. Cell Death Dis. 2018;9(3):394. | ||
Zhang H, Cai Y, Zheng L, Zhang Z, Lin X, Jiang N. Long noncoding RNA NEAT1 regulate papillary thyroid cancer progression by modulating miR-129-5p/KLK7 expression. J Cell Physiol. 2018;233(10):6638–6648. | ||
Lin J, Shi Z, Yu Z, He Z. LncRNA HIF1A-AS2 positively affects the progression and EMT formation of colorectal cancer through regulating miR-129-5p and Dnmt3a. Biomed Pharmacother. 2018;98:433–439. | ||
Lu X, Ma J, Chu J, et al. MiR-129-5p sensitizes the response of HER-2 positive breast cancer to trastuzumab by reducing rpS6. Cell Physiol Biochem. 2017;44(6):2346–2356. | ||
Liu K, Huang J, Ni J, et al. MALAT1 promotes osteosarcoma development by regulation of HMGB1 via miR-142-3p and miR-129-5p. Cell Cycle. 2017;16(6):578–587. | ||
Wu Q, Wy M, Jie Y, Zhao H. LncRNA MALAT1 induces colon cancer development by regulating miR-129-5p/HMGB1 axis. J Cell Physiol. 2018;233(9):6750–6757. | ||
Valentino A, Calarco A, Di Salle A, et al. Deregulation of microRNAs mediated control of carnitine cycle in prostate cancer: molecular basis and pathophysiological consequences. Oncogene. 2017;36(43):6030–6040. | ||
Xu S, Ge J, Zhang Z, Zhou W. MiR-129 inhibits cell proliferation and metastasis by targeting Ets1 via PI3K/Akt/mTOR pathway in prostate cancer. Biomed Pharmacother. 2017;96:634–641. | ||
Zhai J, Qu S, Li X, et al. miR-129 suppresses tumor cell growth and invasion by targeting PAK5 in hepatocellular carcinoma. Biochem Biophys Res Commun. 2015;464(1):161–167. | ||
Duan L, Hao X, Liu Z, Zhang Y, Zhang G. MiR-129-5p is down-regulated and involved in the growth, apoptosis and migration of medullary thyroid carcinoma cells through targeting RET. FEBS Lett. 2014;588(9):1644–1651. | ||
Li J, Lai Y, Ma J, et al. miR-17-5p suppresses cell proliferation and invasion by targeting ETV1 in triple-negative breast cancer. BMC Cancer. 2017;17(1):745. | ||
Shiina M, Hashimoto Y, Kato T, et al. Differential expression of miR-34b and androgen receptor pathway regulate prostate cancer aggressiveness between African-Americans and Caucasians. Oncotarget. 2017;8(5):8356–8368. | ||
Guo Y, Cui J, Ji Z, et al. miR-302/367/LATS2/YAP pathway is essential for prostate tumor-propagating cells and promotes the development of castration resistance. Oncogene. 2017;36(45):6336–6347. | ||
Liu H, Dai X, Cao X, et al. PRDM4 mediates YAP-induced cell invasion by activating leukocyte-specific integrin beta2 expression. EMBO Rep. 2018;19(6):pii:e45180. | ||
Yu S, Cai X, Wu C, et al. Adhesion glycoprotein CD44 functions as an upstream regulator of a network connecting ERK, Akt and Hippo-YAP pathways in cancer progression. Oncotarget. 2015;6(5):2951–2965. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.