Back to Journals » OncoTargets and Therapy » Volume 13
MiR-124-3p.1 Sensitizes Ovarian Cancer Cells to Mitochondrial Apoptosis Induced by Carboplatin
Authors Deng X, Chen Y, Liu Z, Xu J
Received 13 December 2019
Accepted for publication 9 April 2020
Published 10 June 2020 Volume 2020:13 Pages 5375—5386
DOI https://doi.org/10.2147/OTT.S242342
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Xiaohong Deng,1 Yi Chen,2 Zhao Liu,3 Jingning Xu4
1Department of Gynecology, Northwest Women and Children’s Hospital, Xi’an City, Shanxi Province 710061, People’s Republic of China; 2Department of Surgery, Affiliated Hospital of Xi’an Jiao Tong University, Chang’an District Hospital, Xi’an City, Shanxi Province 710119, People’s Republic of China; 3Department of Surgery, Xi’an Chest Hospital, Xi’an TB and Thoracic Tumor Hospital, Xi’an City, Shanxi Province 710100, People’s Republic of China; 4Department of Obstetrics and Gynecology, Northwest Women and Children’s Hospital, Xi’an City, Shanxi Province 710061, People’s Republic of China
Correspondence: Jingning Xu
Department of Obstetrics and Gynecology, Northwest Women and Children’s Hospital, No. 1616, YanXiang Road, Xi’an City, Shanxi Province 710061, People’s Republic of China
Email [email protected]
Background: Carboplatin is a platinum-based chemotherapeutic drug that is commonly used as a treatment for ovarian cancer. However, high doses and repeated use of carboplatin usually reduce the sensitivity of cancer cells to the drug. There is an urgent need to develop strategies to increase the sensitivity of ovarian cancer cells to carboplatin.
Materials and Methods: Quantitative reverse-transcriptase real-time PCR was used to detect miR-124-3p.1 levels in ovarian cancer tissues and cell lines. Transfection with miR-124-3p.1 and caveolin-1 (CAV1) was used for gain-of-function experiments. Western blot and immunoprecipitation assays were performed to evaluate the expression and function of CAV1, AKT, Bad, and Bcl-xl. Flow cytometry analysis was used to measure the apoptosis rates of SKOV3 and A2780 cells.
Results: Expression levels of miR-124-3p.1 were decreased in ovarian cancer tissues and cell lines. Furthermore, overexpression of miR-124-3p.1 enhanced carboplatin-induced apoptotic cell death of ovarian cancer cell lines. Regarding the mechanism of this effect, we showed that CAV1 was the target of miR-124-3p.1 in ovarian cancer. Overexpression of miR-124-3p.1 suppressed the expression of CAV1, thereby reducing the activation of AKT and phosphorylation of Bad. As a result, the function of Bcl-xl was inhibited and carboplatin-induced mitochondrial apoptosis was enhanced.
Conclusion: miR-124-3p.1 sensitizes carboplatin-induced mitochondrial apoptosis through suppression of CAV1 in ovarian cancer. Increasing miR-124-3p.1 expression may represent a novel strategy to improve carboplatin sensitivity in ovarian cancer.
Keywords: carboplatin, miR-124-3p.1, ovarian cancer, CAV1
Introduction
Ovarian cancer is one of the most prevalent malignancies in women. Owing to the small size and deep pelvic location of the ovary, ovarian cancer lacks typical symptoms and is difficult to diagnose in the early stages of the disease. The 5-year survival rate of ovarian cancer patients is very low.1,2 Despite the rapid development of new medical technologies, chemotherapy is still a major strategy for the treatment of ovarian cancer.3,4 However, cancer cells usually show acquired drug resistance during the course of chemotherapy.5,6
Platinum-based chemotherapy is commonly used in the treatment of ovarian cancer.7 Cisplatin is an effective treatment but causes nephrotoxicity.8–10 Carboplatin, by contrast, exhibits cytotoxicity against ovarian cancer cells without obvious nephrotoxicity.11,12 However, resistance to carboplatin is a major obstacle to achieving satisfactory effects in ovarian cancer treatment.13,14 Thus, there is an urgent need for strategies to increase the carboplatin sensitivity of ovarian cancer cells.
MicroRNAs (miRNAs) are endogenous and non-coding RNAs of 20–24 nucleotides in length. Cellular miRNAs can induce mRNA degradation through binding to the 3′-untranslated region (3′-UTR) of the targeted mRNAs. Thus, miRNAs function as gene suppressors and regulate various physiological activities, including cell growth, differentiation, apoptosis, and tumorigenesis.15–17 However, in cancer cells, the expression profile of miRNAs is usually dysregulated.18–20 Recent studies have demonstrated that aberrant expression of miRNAs is associated with development of chemoresistance in several cancers, including ovarian cancer.21,22 Furthermore, these reports indicate that some specific miRNAs are useful targets for sensitizing cancer cells to chemotherapy.23,24
It has been reported that miR-124-3p.1 is a potential tumor suppressor. miR-124-3p.1 can inhibit cell proliferation and suppress tumor growth in colorectal cancer, bladder cancer, and renal cell carcinoma.25–27 Furthermore, miR-124-3p.1 has been shown to reduce drug resistance in some cancers.28,29 However, the mechanisms underlying these effects remain unclear. Caveolin-1 (CAV1), a scaffolding protein, is the major component of caveolae within plasma membranes in most cell types.30 In cancer cells, CAV1 is usually overexpressed in multi-drug-resistant tumor cells.31,32 In the present study, we focus on the role of miR-124-3p.1 and CAV1 in carboplatin-induced cytotoxicity against ovarian cancer.
Materials and Methods
Cell Lines and Patients’ Specimens
Normal human ovarian surface epithelial cell line HOSEpiC and human ovarian cancer cell lines SKOV3 and A2780 were obtained from the China Centre for Type Culture Collection (Wuhan, China). Cells were cultured in Roswell Park Memorial Institute-1640 medium (RPMI-1640, Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal calf serum. Cells were grown at 37 °C in an incubator under 5% CO2. To detect expression of miR-124-3p.1 in ovarian cancer in vivo, tumor tissues and corresponding paracancerous tissues were derived from 25 primary ovarian cancer patients undergoing surgery at Northwest Women and Children’s Hospital between December 2017 and January 2019. We obtained written informed consent from all the patients. The experimental protocols were approved by the ethics committee of Northwest Women and Children’s Hospital.
Detection of miR-124-3p.1 Expression
Relative expression of miR-124-3p.1 was detected using quantitative real-time polymerase chain reaction (qRT-PCR). Briefly, total RNAs were extracted from ovarian cancer cell lines and tissues using TRIzol® reagent (Invitrogen). Subsequently, the extracted RNAs were reverse transcribed using a One Step PrimeScript miRNA cDNA Synthesis Kit (Takara Bio, Inc., Otsu, Japan) to obtain cDNAs. Expression of miR-124-3p.1 in ovarian cancer cell lines and tissues was measured using SYBR Premix Ex Taq (TaKaRa) on an ABI PRISM 7900 Sequence Detection System (Applied Biosystems Prism, USA). Relative expression of miR-124-3p.1 was normalized to U6 small nuclear RNA according to the 2−ΔΔCq method.
Transfection
Mature human miR-124-3p.1 mimics (5′-UAAGGCACGCGGUGAAUGCC-3′) and negative control oligonucleotides (NCO, 5′-GAUGCACGGACGUGCGAAU-3′) were purchased from GenePharma Co. Ltd. (Shanghai, China). CAV1 small interfering RNA (siRNA) was purchased from Santa Cruz Biotechnology (sc-29,241; Santa Cruz, CA, USA). The full-length sequence of the open reading frame of the CAV1 gene was inserted into pcDNA3.1 (Invitrogen) to construct a recombinant eukaryotic expression plasmid for CAV1. For transfection, cells were incubated in a six-well plate overnight. RNA (50 pmol/mL) or plasmid (2 μg/mL) was coated with Lipofectamine 2000 (Invitrogen), then mixed with serum-free medium and added to the cells. After incubation for 6 h, the serum-free medium was removed and fresh RPMI-1640 medium with 10% fetal bovine serum was added for 24 h.
Cell Viability Assay
Transfected cells were seeded into 96-well plates at a density of 5×103 cells/well. After cellular adhesion, cells were treated with different concentrations of carboplatin (0–12 μM) for 48 h. Subsequently, 10 μL CCK8 reagent (Beyotime Biotechnology, Shanghai, China) was added to the cell culture medium for 1 h. Optical density (OD) values were then measured at 450 nm wavelength on a spectrophotometer. Relative cell viability was calculated using the following formula: relative cell viability = OD (drug treatment group)/OD (NCO group).
Immunoprecipitation
Cells were counted, and equal numbers of cells were taken from each group and lysed in NP-40 buffer (Beyotime Biotechnology) on ice. After 30 min of lysis, the cell lysates were centrifuged and the supernatants were collected. The supernatants were then divided into two equal parts and used for internal reference (input) and co-immunoprecipitation (Co-IP), respectively. Primary antibodies were added to the Co-IP system and incubated overnight at 4 °C. Next, protein A/G agarose beads (Santa Cruz Biotechnology) were added into the Co-IP system for a further 2 h incubation. After centrifugation, the supernatant was removed and the beads were mixed with sodium dodecyl sulfate (SDS) loading buffer and heated at 95 °C for 5 min in preparation for Western blot analysis.
Western Blot Analysis
Equal amounts of proteins were separated by 10% SDS polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). After blocking with 5% skimmed milk, membranes were incubated with primary antibodies (Cell Signaling Technology, Danvers, MA, USA) overnight at 4°C. Next, proteins on the membranes were probed with horseradish peroxidase-conjugated secondary antibodies. An enhanced chemiluminescence detection kit (Pierce, Rockford, IL, USA) was used for signal detection.
Luciferase Reporter Assay
The CAV1 3′-UTR containing the predicted miR-124-3p.1 binding site was cloned into the pMIR-REPORT™ miRNA Expression Reporter Vector (Thermo Fisher Scientific, Inc, Waltham, MA, USA), and the resulting plasmid was named pMIR-CAV1-wt. A mutant CAV1 reporter was created by mutating the seed region of the miR-124-3p.1 binding site (GUGCCUU) using a site-directed mutagenesis kit (Takara, Dalian, China) and named pMIR-CAV1-mt. For transfection, cells were seeded into 24-well plates, and the pMIR-REPORT and pRL-TK renilla plasmids (Promega, Fitchburg, WI, USA) and miR-124-3p.1 were co-transfected into cells using Lipofectamine 2000. After 48 h, luciferase activities were measured with the Dual Luciferase Assay System (Promega) according to the manufacturer’s instructions.
Detection of Apoptosis and Mitochondrial Membrane Potential
Cell apoptosis and mitochondrial membrane potential were detected by flow cytometry (Becton Dickinson, USA). For detection of apoptosis, propidium iodide and Annexin-V were added to the cells and incubated for 20 min according to the apoptosis detection kit instructions (Beyotime Biotechnology). Flow cytometry was used to detect cell apoptosis. Annexin-V-positive cells were considered to be apoptotic cells. For measurement of mitochondrial membrane potential, JC-1 was added to the cells and incubated for 20 min according to the mitochondrial membrane potential detection kit instructions (Beyotime Biotechnology). Flow cytometry was used to detect the mitochondrial membrane potential. Cells with high mitochondrial membrane potential emitted red fluorescence.
In vivo Experiments
MiR-124-3p.1-overexpressing SKOV3 cells were constructed using a lentiviral-based system (Genechem Co., Ltd., Shanghai, China) to stably express miR-124-3p.1, and 5×106 lentivirus-transfected SKOV3 cells were inoculated subcutaneously into BALB/c nude mice (Shanghai Super-B&K Laboratory Animal Corp., Ltd., Shanghai, China). Carboplatin (1 mg/kg) was administrated by intraperitoneal injection twice a week after xenografts reached 0.5 cm in diameter. All mice were sacrificed 28 days post-injection. The animal experiments were conducted in strict accordance with the Guide for the Care and Use of Laboratory Animals, 8th edition, issued by the National Institutes of Health. Experimental protocols were approved by the ethics committee of Northwest Women and Children’s Hospital.
Statistical Analysis
All data are represented as mean ± standard deviation. Statistical analyses were conducted using SPSS 15.0 software, and data were visualized using GraphPad Prism. For comparison analyses, two-tailed Student’s t-tests were used to estimate statistical differences between two groups. One-way analysis of variance and Bonferroni’s post hoc test were used to determine differences among three or more groups. Differences were considered significant when P<0.05.
Results
Downregulation of miR-124-3p.1 in Ovarian Cancer
To study the role of miR-124-3p.1 in ovarian cancer, we first detected miR-124-3p.1 expression in 25 pairs of tumor samples from ovarian cancer patients and matched normal tissues by qRT-PCR. The results showed that expression of miR-124-3p.1 was downregulated in tumor tissues compared with the corresponding paracancerous tissues (Figure 1A). Expression of miR-124-3p.1 was detected in normal human ovarian surface epithelial cell line HOSEpiC and human ovarian cancer cell lines SKOV3 and A2780. As shown in Figure 1B, compared with HOSEpiC cells, ovarian cancer cells had significantly lower expression levels of miR-124-3p.1. These results suggest that miR-124-3p.1 is a potential tumor suppressor in ovarian cancer.
MiR-124-3p.1 Sensitizes Ovarian Cancer Cells to Carboplatin-Induced Cytotoxicity
We corrected the downregulation of miR-124-3p.1 by transfection with miR-124-3p.1 mimics in the SKOV3 and A2780 cell lines, which significantly increased cellular levels of miR-124-3p.1 (Figure 2A). To investigate the effects of miR-124-3p.1 on carboplatin treatment, we performed cell viability assays using CCK8. The results indicated that miR-124-3p.1-overexpressing SKOV3 and A2780 cells were more sensitive to various concentrations of carboplatin compared with NCO-transfected cells (Figure 2B). Thus, miR-124-3p.1 is a potential sensitizer to carboplatin-based chemotherapy.
MiR-124-3p.1 Sensitizes Ovarian Cancer Cells to Carboplatin-Induced Apoptosis
As carboplatin-triggered cell death is dependent on the activation of the apoptosis pathway,33 we next investigated the effects of miR-124-3p.1 on carboplatin-triggered apoptosis in ovarian cancer cells. As 0.5 μM carboplatin single treatment showed slight cytotoxicity against ovarian cancer cells (Figure 2B), we chose this concentration of carboplatin for combination treatment with miR-124-3p.1 in the subsequent experiments. The flow cytometry results showed that the apoptotic rates of SKOV3 and A2780 cells co-treated with miR-124-3p.1 and carboplatin were significantly higher than those of cells treated with carboplatin only (Figure 3A). Furthermore, after treatment with equal concentrations of carboplatin, the mitochondrial membrane potentials of miR-124-3p.1-overexpressing SKOV3 and A2780 cells were markedly lower than those of control SKOV3 and A2780 cells, respectively (Figure 3B). These results indicate that miR-124-3p.1 can enhance carboplatin-induced apoptosis in ovarian cancer.
MiR-124-3p.1 Targets CAV1 in Ovarian Cancer
To explore the mechanism by which miR-124-3p.1 enhanced the cytotoxicity of carboplatin against ovarian cancer, we searched for potential targets of miR-124-3p.1 using TargetScan, a public miRNA prediction database (www.targetscan.org). The results showed that CAV1, a promoter of the AKT pathway,34,35 contained a putative binding site paired with miR-124-3p.1 (Figure 4A). Furthermore, in contrast to the decrease in miR-124-3p.1 expression in ovarian cancer cell lines (Figure 1B), expression of CAV1 was increased in SKOV3 and A2780 cells (Figure 4B). Thus, we predicted that CAV1 could be a target of miR-124-3p.1. To confirm the miR-124-3p.1/CAV1 axis in ovarian cancer, we performed Western blot and luciferase reporter assays in SKOV3 and A2780 cells. Transfection with miR-124-3p.1 markedly inhibited the expression of CAV1 (Figure 4C). Furthermore, the results of luciferase reporter assays showed that miR-124-3p.1 significantly decreased the luciferase activity of the pMIR-REPORT containing the wild-type CAV1 3′-UTR but not that of the mutant luciferase reporter (Figure 4D). Taken together, these results demonstrate that miR-124-3p.1 targets CAV1 in ovarian cancer.
MiR-124-3p.1 Sensitizes Ovarian Cancer to Carboplatin Through Suppression of CAV1
We next investigated the effects of CAV1 on miR-124-3p.1-transfected ovarian cancer cells. For this purpose, we constitutively expressed CAV1 in SKOV3 and A2780 cells co-treated with carboplatin and miR-124-3p.1. The transfection efficiency of the CAV1 plasmid is shown in Figure 5A. Constitutive expression of CAV1 improved the cell viability of SKOV3 and A2780 cells co-treated with carboplatin and miR-124-3p.1 (Figure 5B), suggesting that downregulation of CAV1 is required for the sensitization of ovarian cancer to carboplatin by miR-124-3p.1. To explore the effects of CAV1 on carboplatin-induced cytotoxicity directly, we transfected SKOV3 and A2780 cells with CAV1 siRNA (Figure 5C). This treatment increased the sensitivity of the cells to carboplatin, similar to the effect of miR-124-3p.1 (Figure 5D). Taken together, these results demonstrate that the sensitization of ovarian cancer to carboplatin by miR-124-3p.1 is dependent on the suppression of CAV1.
MiR-124-3p.1 Decreases the Stability of the Mitochondrial Outer Membrane Through the CAV1/AKT/Bad Pathway
As CAV1 is known to be a promoter of the AKT pathway,34,35 we next investigated the AKT pathway in ovarian cancer cells co-treated with carboplatin and miR-124-3p.1. As shown in Figure 6A, treatment with miR-124-3p.1 but not carboplatin reduced the phosphorylation of AKT. As a downstream effect of the dephosphorylation of AKT, levels of phosphorylated Bad were also decreased in the miR-124-3p.1-treated ovarian cancer cells. Owing to the dephosphorylation of Bad, miR-124-3p.1 inhibited the interaction of Bad with Bcl-xl in ovarian cancer cells, according to the immunoprecipitation assay results (Figure 6B). After separation of mitochondria and cytosol, we detected Bax in both fractions of ovarian cancer cells. Interestingly, we found that combination treatment with carboplatin and miR-124-3p.1 increased the localization of Bax to mitochondria, although both carboplatin and miR-124-3p.1 single treatments had weak effects on Bax (Figure 6C). Furthermore, in the carboplatin and miR-124-3p.1 co-treated ovarian cancer cells, Bax was present in high levels and interacted with Bak in the mitochondrial fraction (Figure 6D). As activation of Bax and Bak induces opening of the mitochondrial outer membrane (MOM) pore,36 we thus detected the release of cytochrome c from mitochondria. As shown in Figure 6E, combination treatment with miR-124-3p.1 promoted the release of cytochrome c from mitochondria into cytosol in the carboplatin-treated SKOV3 and A2780 cells. Finally, combination treatment with carboplatin and miR-124-3p.1 induced significant activation of caspase-9 and caspase-3 (Figure 6F), which are markers of endogenous apoptosis pathway.37 Taken together, these results demonstrate that miR-124-3p.1 enhances carboplatin-induced mitochondrial apoptosis in ovarian cancer through the CAV1/AKT/Bad pathway.
MiR-124-3p.1 Enhanced the Anti-Tumor Effect of Carboplatin on Ovarian Cancer in vivo
To investigate the role of miR-124-3p.1 in carboplatin treatment in vivo, we established ovarian cancer models in mice using miR-124-3p.1-overexpressing or control SKOV3 cells. Under treatment with equal doses of carboplatin, miR-124-3p.1-overexpressing SKOV3 tumors had slower growth rates than control tumors (Figure 7A). After sacrifice of the mice, the SKOV3 tumors co-treated with miR-124-3p.1 and carboplatin were smaller than those treated with carboplatin alone (Figure 7B). These results indicate that the miR-124-3p.1-overexpressing tumors were more sensitive to carboplatin treatment than the control tumors. In resected tissues, the miR-124-3p.1-overexpressing tumors had lower CAV1 expression levels compared with control tumors (Figure 7C), as well as lower levels of phosphorylated AKT and Bad (Figure 7D). These results demonstrate that miR-124-3p.1 negatively regulates the CAV1/AKT/Bad pathway in ovarian cancer cells in vivo. Moreover, overexpression of miR-124-3p.1 may enhance the anti-tumor effect of carboplatin on ovarian cancer in vivo.
Discussion
Although carboplatin is a commonly used chemotherapeutic drug for the treatment of ovarian cancer, drug resistance is a major obstacle to achieving a satisfactory effect for patients. Recent studies have indicated that aberrant expression of some miRNAs is responsible for the resistance of ovarian cancer cells to platinum-based chemotherapy.8,24,38 We found that miR-124-3p.1 expression was significantly decreased in ovarian cancer tissues and cell lines. Furthermore, we showed that recovery of miR-124-3p.1 expression could increase the sensitivity of ovarian cancer cells to carboplatin treatment. Thus, miR-124-3p.1 is a potential sensitizer for use in carboplatin-based treatment of ovarian cancer.
In our study, we demonstrated that miR-124-3p.1 targets CAV1 in ovarian cancer cells. More importantly, we found that the miR-124-3p.1-induced sensitization of ovarian cancer to carboplatin-induced cytotoxicity was dependent on the inhibition of CAV1. Thus, CAV1 is a potential target for improving chemotherapy in ovarian cancer. Previous studies have reported that CAV1 acts as a promoter of the AKT pathway.34,35 Activation of AKT induces phosphorylation of Bad in cancer cells.39 Non-phosphorylated Bad can promote apoptosis by inactivating anti-apoptotic protein Bcl-xl through conjugation. However, phosphorylated Bad loses its ability to inactivate Bcl-xl.40,41 In the present study, we found that overexpression of miR-124-3p.1 decreased CAV1 expression and thus inhibited the phosphorylation of AKT and Bad. As a result, cellular free Bcl-xl levels increased.
The key step of mitochondrial apoptosis is dependent on damage to MOM continuity. However, this process requires activation and construction of Bax and Bak proteins to form protein–protein oligomers.36 In healthy cells, Bax is mainly localized in the cytosol and Bak is mainly located on the MOM.42 Bax levels in mitochondria are strictly controlled through continuous “reverse transportation” from MOM to cytosol.43,44 However, the regulation mechanism of this reverse transportation process remains unclear. Some studies indicate that it requires free Bcl-xl in the cytosol,36 although other results suggest that this is not the case.45 According to our results, the reverse transportation of Bax and construction of Bax and Bak proteins required free Bcl-xl and apoptotic signals such as carboplatin treatment.
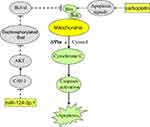
We also demonstrated that miR-124-3p.1 inhibited CAV1 expression and thus inhibited the phosphorylation of AKT and Bad. The resulting high level of dephosphorylated Bad then inhibited the function of Bcl-xl, leading to free Bax. Under the apoptosis signals caused by carboplatin treatment, the free Bax conjugated with Bak to induce MOM permeability and decrease the mitochondrial membrane potential (Δφ). This resulted in release of cytochrome c from the mitochondria into the cytosol, triggering of effector caspases, and, finally, apoptosis (Figure 8).
Conclusions
Taken together, our results indicate that miR-124-3p.1 sensitizes ovarian cancer to carboplatin-induced mitochondrial apoptosis through suppression of CAV1. Increasing miR-124-3p.1 expression may represent a novel approach to improve the carboplatin sensitivity of ovarian cancer. These findings provide an attractive strategy for efficient treatment of ovarian cancer with carboplatin.
Acknowledgments
This study is supported by the National Natural Science Foundation of China (grant no: 61805195)
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi:10.3322/caac.21254
2. Rooth C. Ovarian cancer: risk factors, treatment and management. Br J Nurs. 2013;22(Sup17):S23–30. doi:10.12968/bjon.2013.22.Sup17.S23
3. Alharbi M, Zuñiga F, Elfeky O, et al. The potential role of miRNAs and exosomes in chemotherapy in ovarian cancer. Endocr Relat Cancer. 2018;25:R663–85. doi:10.1530/ERC-18-0019
4. Elies A, Rivière S, Pouget N, et al. The role of neoadjuvant chemotherapy in ovarian cancer. Expert Rev Anticancer Ther. 2018;18(6):555–566. doi:10.1080/14737140.2018.1458614
5. Shao Y, Li H, Du R, Meng J, Yang G. Involvement of non-coding RNAs in chemotherapy resistance of ovarian cancer. J Cancer. 2018;9(11):1966–1972. doi:10.7150/jca.24550
6. Christie EL, Bowtell DDL. Acquired chemotherapy resistance in ovarian cancer. Ann Oncol. 2017;28:viii13–5. doi:10.1093/annonc/mdx446
7. Niu L, Ni H, Hou Y, Du Q, Li H. miR-509-3p enhances platinum drug sensitivity in ovarian cancer. Gene. 2019;686:63–67. doi:10.1016/j.gene.2018.11.011
8. Xu ZH, Yao TZ, Liu W. miR-378a-3p sensitizes ovarian cancer cells to cisplatin through targeting MAPK1/GRB2. Biomed Pharmacother. 2018;107:1410–1417. doi:10.1016/j.biopha.2018.08.132
9. Li HY, Yang S, Li JC, Feng JX. Galectin 3 inhibition attenuates renal injury progression in cisplatin-induced nephrotoxicity. Biosci Rep. 2018;38(6):piiBSR20181803. doi:10.1042/BSR20181803
10. Bishr A, Sallam N, Nour El-Din M, Awad AS, Kenawy SA. Ambroxol attenuates cisplatin-induced hepatotoxicity and nephrotoxicity via inhibition of p-JNK/p-ERK. Can J Physiol Pharmacol. 2019;9(1):55–64. doi:10.1139/cjpp-2018-0528
11. Zhang Y, Huang F, Luo Q, et al. Inhibition of XIAP increases carboplatin sensitivity in ovarian cancer. Onco Targets Ther. 2018;11:8751–8759. doi:10.2147/OTT.S171053
12. Alberts DS. Carboplatin versus cisplatin in ovarian cancer. Semin Oncol. 1995;22(5 Suppl 12):88–90.
13. Zhang J, Xiong X, Hua X, et al. Knockdown of FUSE binding protein 1 enhances the sensitivity of epithelial ovarian cancer cells to carboplatin. Oncol Lett. 2017;14(5):5819–5824. doi:10.3892/ol.2017.6978
14. Barghout SH, Zepeda N, Vincent K, et al. RUNX3 contributes to carboplatin resistance in epithelial ovarian cancer cells. Gynecol Oncol. 2015;138(3):647–655. doi:10.1016/j.ygyno.2015.07.009
15. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi:10.1016/S0092-8674(04)00045-5
16. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi:10.1016/j.cell.2009.01.002
17. Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9(1):287–314. doi:10.1016/j.cell.2009.01.002
18. Li C, Hashimi SM, Good DA, et al. Apoptosis and microRNA aberrations in cancer. Clin Exp Pharmacol Physiol. 2012;39(8):739–746. doi:10.1111/j.1440-1681.2012.05700.x
19. Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer. 2006;94(6):776–780. doi:10.1038/sj.bjc.6603023
20. Xu X, Tao Y, Shan L, et al. The role of MicroRNAs in hepatocellular carcinoma. J Cancer. 2018;9(19):3557–3569. doi:10.7150/jca.26350
21. Marjaneh RM, Khazaei M, Ferns GA, Avan A, Aghaee-Bakhtiari SH. The role of microRNAs in 5-FU resistance of colorectal cancer: possible mechanisms. J Cell Physiol. 2019;234(3):2306–2316. doi:10.1002/jcp.27221
22. Shi X, Xiao L, Mao X, et al. miR-205-5p mediated downregulation of PTEN contributes to cisplatin resistance in C13K human ovarian cancer cells. Front Genet. 2018;9:555. doi:10.3389/fgene.2018.00555
23. Yuan LL, Li L, Liu JN, Mei J, Lei CJ. Down-regulation of miR-29a facilitates apoptosis of colorectal carcinoma cell SW480 and suppresses its paclitaxel resistance. Eur Rev Med Pharmacol Sci. 2018;22(17):5499–5507. doi:10.26355/eurrev_201809_15810
24. Jiang Y, Jiang J, Jia H, Qiao Z, Zhang J. Recovery of miR-139-5p in ovarian cancer reverses cisplatin resistance by targeting C-Jun. Cell Physiol Biochem. 2018;51(1):129–141. doi:10.1159/000495169
25. Qin Z, Liu X. miR-124, a potential therapeutic target in colorectal cancer. Onco Targets Ther. 2019;12:749–751. doi:10.2147/OTT.S179501
26. Wang S, Wu G, Han Y, et al. miR-124 regulates STAT3-mediated cell proliferation, migration and apoptosis in bladder cancer. Oncol Lett. 2018;16(5):5875–5881. doi:10.3892/ol.2018.9341
27. Wang P, Zhang LD, Sun MC, Gu WD, Geng HZ. Over-expression of mir-124 inhibits MMP-9 expression and decreases invasion of renal cell carcinoma cells. Eur Rev Med Pharmacol Sci. 2018;22(19):6308–6314. doi:10.26355/eurrev_201810_16041
28. Hu FY, Cao XN, Xu QZ, et al. miR-124 modulates gefitinib resistance through SNAI2 and STAT3 in non-small cell lung cancer. J Huazhong Univ Sci Technolog Med Sci. 2016;36(6):839–845. doi:10.1007/s11596-016-1672-x
29. Du J, He Y, Wu W, et al. Targeting EphA2 with miR-124 mediates Erlotinib resistance in K-RAS mutated pancreatic cancer. J Pharm Pharmacol. 2019;71(2):196–205. doi:10.1111/jphp.12941
30. Wang S, Wang N, Zheng Y, Zhang J, Zhang F, Wang Z. Caveolin-1: an oxidative stress-related target for cancer prevention. Oxid Med Cell Longev. 2017;2017:7454031. doi:10.1155/2017/7454031
31. Zou H, Volonte D, Galbiati F, Song L. Interaction of caveolin-1 with Ku70 inhibits bax-mediated apoptosis. PLoS One. 2012;7(6):e39379. doi:10.1371/journal.pone.0039379
32. Burgermeister E, Liscovitch M, Rochen C, Schmid RM, Ebert MP. Caveats of caveolin-1 in cancer progression. Cancer Lett. 2008;268(2):187–201. doi:10.1016/j.canlet.2008.03.055
33. Tang ZY, Sheng MJ, Qi YX, Wang LY, He DY. Metformin enhances inhibitive effects of carboplatin on HeLa cell proliferation and increases sensitivity to carboplatin by activating mitochondrial associated apoptosis signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22(23):8104–8112. doi:10.26355/eurrev_201812_16501
34. Zhu X, Zhang Y, Li Q, et al. β-carotene induces apoptosis in human esophageal squamous cell carcinoma cell lines via the CAV1/AKT/NF-κB signaling pathway. J Biochem Mol Toxicol. 2016;30(3):148–157. doi:10.1002/jbt.21773
35. Zou L, Zhang G, Liu L, Chen C, Cao X, Cai J. Caveolin-1 is critical in the proliferative effect of leptin on osteoblasts through the activation of Akt. Mol Med Rep. 2016;14(3):1915–1922. doi:10.3892/mmr.2016.5461
36. Westphal D, Kluck RM, Dewson G. Building blocks of the apoptotic pore: how Bax and Bak are activated and oligomerize during apoptosis. Cell Death Differ. 2014;21(2):196–205. doi:10.1038/cdd.2013.139
37. Zhang R, Xu J, Zhao J, Bai J. Knockdown of miR-27a sensitizes colorectal cancer stem cells to TRAIL by promoting the formation of Apaf-1-caspase-9 complex. Oncotarget. 2017;8(28):45213–45223. doi:10.18632/oncotarget.16779
38. Ying HC, Xu HY, Lv J, Ying TS, Yang Q. MicroRNA signatures of platinum-resistance in ovarian cancer. Eur J Gynaecol Oncol. 2015;36(1):16–20.
39. Ma Y, Qin H, Cui Y. MiR-34a targets GAS1 to promote cell proliferation and inhibit apoptosis in papillary thyroid carcinoma via PI3K/Akt/Bad pathway. Biochem Biophys Res Commun. 2013;441(4):958–963. doi:10.1016/j.bbrc.2013.11.010
40. Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13(22):2905–2927. doi:10.1101/gad.13.22.2905
41. Datta SR, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91(2):231–241. doi:10.1016/S0092-8674(00)80405-5
42. Todt F, Cakir Z, Reichenbach F, et al. Differential retrotranslocation of mitochondrial Bax and Bak. EMBO J. 2015;34(1):67–80. doi:10.15252/embj.201488806
43. Todt F, Cakir Z, Reichenbach F, Youle RJ, Edlich F. The C-terminal helix of Bcl-x(L) mediates Bax retrotranslocation from the mitochondria. Cell Death Differ. 2013;20(2):333–342. doi:10.1038/cdd.2012.131
44. Edlich F. The great migration of Bax and Bak. Mol Cell Oncol. 2015;2(3):e995029. doi:10.4161/23723556.2014.995029
45. Schellenberg B, Wang P, Keeble JA, et al. Bax exists in a dynamic equilibrium between the cytosol and mitochondria to control apoptotic priming. Mol Cell. 2013;49(5):959–971. doi:10.1016/j.molcel.2012.12.022
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.