Back to Journals » Medical Devices: Evidence and Research » Volume 7
Minimally invasive arthrodesis for chronic sacroiliac joint dysfunction using the SImmetry SI Joint Fusion system
Received 5 March 2014
Accepted for publication 11 April 2014
Published 7 May 2014 Volume 2014:7 Pages 125—130
DOI https://doi.org/10.2147/MDER.S63575
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Larry E Miller,1,2 Jon E Block2
1Miller Scientific Consulting, Inc., Asheville, NC, USA; 2The Jon Block Group, San Francisco, CA, USA
Abstract: Chronic sacroiliac (SI) joint-related low back pain (LBP) is a common, yet under-diagnosed and undertreated condition due to difficulties in accurate diagnosis and highly variable treatment practices. In patients with debilitating SI-related LBP for at least 6 months duration who have failed conservative management, arthrodesis is a viable option. The SImmetry® SI Joint Fusion System is a novel therapy for SI joint fusion, not just fixation, which utilizes a minimally invasive surgical approach, instrumented fixation for immediate stability, and joint preparation with bone grafting for a secure construct in the long term. The purpose of this report is to describe the minimally invasive SI Joint Fusion System, including patient selection criteria, implant characteristics, surgical technique, postoperative recovery, and biomechanical testing results. Advantages and limitations of this system will be discussed.
Keywords: arthrodesis, fusion, minimally invasive, sacroiliac, SImmetry
Introduction
Low back pain (LBP) is one of the most common physical ailments worldwide with a point prevalence of 18% and a 1-year prevalence of 38%.1 While most cases of acute LBP eventually resolve, one in five cases will persist for more than 1 month. Patients with chronic LBP often present a diagnostic dilemma to the clinician. More than 85% of LBP cases are non-specific since they cannot be readily attributed to a specific disease or spinal abnormality.2 Furthermore, there is little consensus on appropriate clinical evaluation methods for LBP,3 which is evident by studies showing large variations in the utilization of diagnostic tests.4,5
The contribution of the sacroiliac (SI) joint in chronic LBP has been recognized for decades. The reported prevalence of LBP originating from the SI joint ranges from 13% to 30%,6–10 with the largest series reporting a 23% prevalence in 1,300 well-selected patients.6 Despite the high prevalence of chronic SI joint-related LBP, accurate diagnosis of this condition remains challenging and has historically resulted in suboptimal and misdirected treatment offerings. Nonsurgical therapies for chronic LBP of SI origin, including analgesics, physical therapy, spinal manipulation, intra-articular steroid injections, and radiofrequency denervation, provide modest and temporary pain relief.11 In chronically debilitating cases refractory to at least 6 months of nonsurgical care, SI joint fusion becomes a viable treatment option in well-selected patients.12
Traditional SI joint fusion is a complex and invasive procedure involving open exposure of the joint, decortication of the articular surfaces, bone graft harvesting, and instrumented fixation using plates, screws, and/or rods. In addition to less than ideal patient outcomes reported in many series,13 open SI joint fusion is associated with significant procedural complications such as blood loss (average: 290 mL),14 neurovascular injury, disruption of musculoligamentous structures, autograft harvest-related morbidity, extended hospitalization (average: 5 days),14 and protracted time to return to work (average: 5 months).13–17 In order to lower surgical morbidity associated with SI joint fusion, minimally invasive surgical approaches for SI joint fixation and fusion have recently been developed. The purpose of this report is to describe a novel minimally invasive system (SImmetry® SI Joint Fusion System; Zyga Technology Inc., Minnetonka, MN, USA) intended for SI joint arthrodesis, including patient selection criteria, implant characteristics, surgical technique, postoperative recovery, and biomechanical testing results.
Patient selection criteria
Accurate differential diagnosis and careful patient selection are mandatory for therapeutic success with SI joint treatments. Correct identification of SI joint-generated LBP involves multiple ordered diagnostic steps, since SI joint pain often mimics discogenic or lumbar radicular symptoms.18 Typical patient symptoms indicative of chronic SI joint pathology include pain with sitting or lying that is intensified by climbing hills or stairs, dull ache below L5 unilaterally, and/or buttock pain possibly radiating to the groin or thigh. Careful review of a patient’s medical history will often reveal a history of trauma, significant leg-length disparities, or prior lumbar fusion. Physical examination includes a general back examination and magnetic resonance imaging to rule out other pathologies. If suspicion of SI joint-originating LBP remains, the patient is evaluated by conducting a series of SI joint-specific provocative tests, including pelvic compression, thigh thrust, flexion abduction external rotation (FABER), distraction, and Gaenslen’s test. Fortin’s Finger Test is also regularly employed. The SI joint is suspected to be the primary pain generator if at least three of five tests are positive.19 Finally, SI joint dysfunction is confirmed with two separate fluoroscopically guided, intra-articular diagnostic SI joint blocks that provide >75% immediate symptom relief.20
Implant characteristics
The SImmetry® SI Joint Fusion System includes titanium implants that are available in lengths ranging from 30 mm to 70 mm and are characterized by a spiral thread design and self-tapping corticocancellous threads. The threaded design allows controlled insertion and removal of the implants, and the deep threads provide a macroscale scaffold for long-term fixation. A microscale texture is produced by blasting with biocompatible calcium phosphate, which later dissolves, leaving a titanium surface free from embedded media. This resorbable blast media (RBM) surface treatment provides a 2–4 μm surface roughness, which has been demonstrated to be optimal for osteointegration in contrast to either smoother or rougher finishes.21,22 The RBM finish is immune to delamination during insertion, ongrowth, or removal, unlike hydroxyapatite-coated or titanium plasma-sprayed (TPS) surfaces.23
Surgical technique
The surgical procedure involves four main steps: minimally invasive lateral access, joint preparation, bone graft insertion, and implant delivery (Figure 1). Surgeons must be intimately familiar with the relevant lumbosacral spine and iliac anatomy, including dysplastic variations, in order to ensure accurate instrument trajectory and to avoid iatrogenic neurovascular injury. Under general anesthesia, the patient is positioned prone on a radiolucent operating table and the pelvis is prepped and draped for a lateral incision on the buttocks. A C-arm image intensifier is prepared to obtain anteroposterior, lateral, pelvic inlet, pelvic inlet-oblique, pelvic outlet and pelvic outlet-oblique projections.
  | Figure 1 Major procedural steps including: (A) access, (B) joint preparation, (C) bone grafting, and (D) fixation. |
Under anteroposterior, outlet, and lateral fluoroscopic views, the skin is marked to identify the ideal access and trajectory. The lateral view should utilize a gantry which superimposes the sacral slopes on a single image. A 1.5–2 cm longitudinal incision is made, with the skin and subcutaneous tissue divided down to the gluteal fascia. Under lateral fluoroscopic guidance, a 6 mm dilator with internal obturator is advanced to the planned entry point on the outer ilium. The obturator is then exchanged for a guide pin, which is drilled just enough to maintain its position within the outer ilium cortex. After accurate pin trajectory is confirmed with inlet, inlet-oblique, and outlet-oblique fluoroscopic views, the guide pin is advanced into the SI joint, just touching the sacral cortex. The pin should have a trajectory as perpendicular to the SI joint as possible. The lateral ilium cortex is then sequentially dilated to 9 mm to allow advancement of a working cannula to approximately 1 cm beyond the lateral cortex. Proper intraosseous trajectory and depth are confirmed with pelvic inlet and outlet projections. Next, a 9 mm cannulated drill creates an osseous tunnel though the ilium over the guide pin, and care is taken with radiographic guidance to avoid drilling across the sacral cortex of the SI joint. Ilium drillings are collected and incorporated into bone graft material for later grafting, which is advantageous since it avoids direct iliac crest bone graft harvesting, which can cause complications such as iliac crest fracture, chronic pain, and nerve damage.24
SI joint preparation begins by clearing cartilage to allow a decorticator to be fully seated on the sacral cortex of the SI joint. Joint surface preparation is accomplished by use of a unique flexible decorticator that extends out and follows the undulating surface of the joint while denuding cartilage and partially decorticating the joint. The joint is prepared well beyond the margins of the 12.5 mm threaded implant, with an affected region of approximately 5 cm2 of the ilium and 5 cm2 of the sacrum, exclusive of the implant. This represents approximately 50% of the articular joint area.25 Irrigation and suction are used intermittently throughout the process to extract joint tissue. Approximately 5 cc bone graft, including autologous bone from the ilium drillings (typically 2–3 cc), is then packed into the denuded cavity to promote bony fusion. Dilators are reinserted to allow accurate advancement of the drill pin to a safe depth and trajectory perpendicular to the joint. A 9 mm pilot hole is drilled through the sacral cortex, over which the initial 12.5 mm cannulated implant is advanced over the pin until fully seated. Fixation is obtained with at least one large diameter 12.5 mm implant and one or more additional smaller 6.5 mm implants to ensure rotational rigidity.
The 6.5 mm anti-rotation implant(s) are inserted just superior to the 12.5 mm implant or into S2 without the use of joint preparation and bone graft, in an otherwise similar fashion. Upon completion of placement of the implants, final lateral, inlet, inlet-oblique, outlet, and outlet-oblique images are obtained (Figure 2). The deep tissues and skin incision may be infiltrated with bupivacaine and epinephrine for postoperative pain control.
  | Figure 2 Schematic representation of the implanted SImmetry SI Joint Fusion System in (A) lateral, (B) inlet, and (C) outlet views. |
Several patient-related factors may complicate imaging interpretation and/or implant placement, including obesity, low bone density, sacral dysplasia, or small frame. The importance of preoperative planning cannot be overstated in such patients. In cases where dysmorphic patient anatomy will not allow for safe traverse medial to the neural foramen of the S1 or S2 bony corridors, the implants should be inserted only within the ala. A minimum of 1.5 cm implant purchase into the sacrum is recommended for adequate implant stability.
Postoperative recovery
Patients may be discharged on the same day, although a 1–2 day hospitalization may be required for pain control; pain medications are prescribed as needed. Patients may progress to full weight bearing as tolerated, typically prior to hospital discharge. Fusion status is assessed with computed tomography 6–12 months after the procedure (Figure 3).
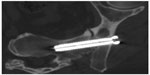  | Figure 3 Six-month postoperative orthogonal view demonstrating bridging bone with no evidence of lucency. |
Biomechanical testing
The SImmetry® SI Joint Fusion System implants were developed specifically to limit SI joint motion by achieving immediate fixation with threaded titanium implants and promoting long-term fusion by arthrodesis. The use of a two-implant construct has traditionally been utilized for fixation of stable and unstable pelvic fractures,26 with the second iliosacral screw providing significant fixation benefits.27–29 van Zwienen et al28 evaluated iliosacral screw constructs in a cadaveric fracture model. These authors reported a 28% and 44% reduction in fracture site motion and a 170% and 300% increase in rotational stiffness with the addition of a second screw for S2 and S1 placements, respectively. Yinger et al27 evaluated the effectiveness of multiple fixation constructs, including SI screws, sacral bars, and tension band plates in a hard plastic model with an Orthopaedic Trauma Association (OTA) 61-C1.2 fracture. The addition of a second iliosacral screw reduced fracture site translation by 50% and rotation by 40%. In fact, the two transverse screw construct provided rigidity that was only slightly exceeded by the strongest construct consisting of a screw and multiple anterior plates. This design is advantageous, since shear loads across the SI joint can exceed 600 N,30 and multiple implants can more effectively share loads and resist rotation. Additionally, the implant procedure requires only a posterior approach.
An additional advantage of the 12.5 mm implant relates to implant strength and durability when compared to a 6.5 mm trauma screw. Using the American Society for Testing and Materials (ASTM) standard tests, this implant had 2% greater pullout force, 22% greater failure torque, and 17% greater bending yield. Notably, this 12.5 mm implant underwent 5 million cycles in bending fatigue at 7.2 Nm with no failure, whereas the trauma screw failed at 30,000 cycles. The 12.5 mm diameter implant is six-fold stronger under shear and bending forces with double the frontal area when compared to a 6.5 mm implant. It can therefore be assumed that the use of both implants likely provides more than adequate fixation and strength to tolerate typical long-term physiological loads borne by the SI joint.
Discussion
Chronic LBP originating from the SI joint represents a diagnostic and therapeutic challenge due to complexities in differential diagnosis and the suboptimal therapeutic options available. Failure to recognize and optimally treat SI joint pathology results in undertreatment of a significant portion of chronic LBP sufferers. With the recent development of highly sensitive and specific diagnostic algorithms, awareness of the role of the SI joint in chronic LBP is increasing.
Despite recent efforts to characterize the role of the SI joint in chronic LBP, no definitive conservative, interventional, or surgical management options exist for managing chronic SI joint pain.11 Instrumented SI joint arthrodesis via large anterior or posterior surgical approaches is limited by significant risk of iatrogenic neurovascular and muscular injury, requirement for iliac crest bone harvesting, and unsatisfactory long-term patient outcomes.13 Consequently, minimally invasive techniques for fixation of the SI joint have been introduced over the last few years with encouraging early results. Several case series have reported promising mid-term outcomes with SI joint fixation using titanium plasma spray-coated implants.31–35 However, the long-term outcomes of this method are unknown, and since implants are placed without use of supplemental bone graft, the risk of late implant loosening remains a concern.36
True minimally invasive SI joint arthrodesis, accomplished with small incisions, joint decortications, and instrumented fixation supplemented with bone graft for long-term stability, is a relatively novel concept. Mason et al37 treated 73 patients with intractable SI joint pain resistant to conservative care using a percutaneous hollow modular anchorage screw supplemented with demineralized bone matrix. Throughout a mean of 3 years follow-up, back pain severity decreased by 44% on average, although fusion status was not reported. Although these results are promising, additional human studies are needed to accurately assess the therapeutic potential for this technique.
In summary, a true minimally invasive SI joint arthrodesis technique is possible in well-selected patients, combining the advantages of a minimally invasive surgical approach, proper joint preparation with decortication and bone grafting, and optimized titanium implants designed to provide a stable long-term construct.
Disclosure
LEM and JEB received financial support from Zyga Technology, Inc. (Minnetonka, MN, USA). The authors declare no other conflicts of interest in this work.
References
Hoy D, Bain C, Williams G, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64(6):2028–2037. | |
van Tulder MW, Assendelft WJ, Koes BW, Bouter LM. Spinal radiographic findings and nonspecific low back pain. A systematic review of observational studies. Spine (Phila Pa 1976). 1997;22(4):427–434. | |
Cherkin DC, Deyo RA, Wheeler K, Ciol MA. Physician variation in diagnostic testing for low back pain. Who you see is what you get. Arthritis Rheum. 1994;37(1):15–22. | |
Cherkin DC, Deyo RA, Loeser JD, Bush T, Waddell G. An international comparison of back surgery rates. Spine (Phila Pa 1976). 1994;19(11):1201–1206. | |
Volinn E, Mayer J, Diehr P, Van Koevering D, Connell FA, Loeser JD. Small area analysis of surgery for low-back pain. Spine (Phila Pa 1976). 1992;17(5):575–581. | |
Bernard TN Jr, Kirkaldy-Willis WH. Recognizing specific characteristics of nonspecific low back pain. Clin Orthop Relat Res. 1987;(217):266–280. | |
Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine (Phila Pa 1976). 1995;20(1):31–37. | |
Maigne JY, Aivaliklis A, Pfefer F. Results of sacroiliac joint double block and value of sacroiliac pain provocation tests in 54 patients with low back pain. Spine (Phila Pa 1976). 1996;21(16):1889–1892. | |
DePalma MJ, Ketchum JM, Saullo T. What is the source of chronic low back pain and does age play a role? Pain Med. 2011;12(2):224–233. | |
Katz V, Schofferman J, Reynolds J. The sacroiliac joint: a potential cause of pain after lumbar fusion to the sacrum. J Spinal Disord Tech. 2003;16(1):96–99. | |
Hansen H, Manchikanti L, Simopoulos TT, et al. A systematic evaluation of the therapeutic effectiveness of sacroiliac joint interventions. Pain Physician. 2012;15(3):E247–E278. | |
Ashman B, Norvell DC, Hermsmeyer JT. Chronic sacroiliac joint pain: fusion versus denervation as treatment options. Evid Based Spine Care J. 2010;1(3):35–44. | |
Schütz U, Grob D. Poor outcome following bilateral sacroiliac joint fusion for degenerative sacroiliac joint syndrome. Acta Orthop Belg. 2006;72(3):296–308. | |
Buchowski JM, Kebaish KM, Sinkov V, Cohen DB, Sieber AN, Kostuik JP. Functional and radiographic outcome of sacroiliac arthrodesis for the disorders of the sacroiliac joint. Spine J. 2005;5(5):520–528; discussion 529. | |
Simpson LA, Waddell JP, Leighton RK, Kellam JF, Tile M. Anterior approach and stabilization of the disrupted sacroiliac joint. J Trauma. 1987;27(12):1332–1339. | |
Dabezies EJ, Millet CW, Murphy CP, Acker JH, Robicheaux RE, D’Ambrosia RD. Stabilization of sacroiliac joint disruption with threaded compression rods. Clin Orthop Relat Res. 1989;(246):165–171. | |
Waisbrod H, Krainick JU, Gerbershagen HU. Sacroiliac joint arthrodesis for chronic lower back pain. Arch Orthop Trauma Surg. 1987;106(4):238–240. | |
Sembrano JN, Polly DW Jr. How often is low back pain not coming from the back? Spine (Phila Pa 1976). 2009;34(1):E27–E32. | |
Szadek KM, van der Wurff P, van Tulder MW, Zuurmond WW, Perez RS. Diagnostic validity of criteria for sacroiliac joint pain: a systematic review. J Pain. 2009;10(4):354–368. | |
Simopoulos TT, Manchikanti L, Singh V, et al. A systematic evaluation of prevalence and diagnostic accuracy of sacroiliac joint interventions. Pain Physician. 2012;15(3):E305–E344. | |
Wennerberg A, Albrektsson T. Effects of titanium surface topography on bone integration: a systematic review. Clin Oral Implants Res. 2009;20 Suppl 4:172–184. | |
Schwartz Z, Raz P, Zhao G, et al. Effect of micrometer-scale roughness of the surface of Ti6Al4V pedicle screws in vitro and in vivo. J Bone Joint Surg Am. 2008;90(11):2485–2498. | |
Franchi M, Orsini E, Martini D, et al. Destination of titanium particles detached from titanium plasma sprayed implants. Micron. 2007;38(6):618–625. | |
Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;(329):300–309. | |
Mahato NK. Variable positions of the sacral auricular surface: classification and importance. Neurosurg Focus. 2010;28(3):E12. | |
Tile M. Pelvic ring fractures: should they be fixed? J Bone Joint Surg Br. 1988;70(1):1–12. | |
Yinger K, Scalise J, Olson SA, Bay BK, Finkemeier CG. Biomechanical comparison of posterior pelvic ring fixation. J Orthop Trauma. 2003;17(7):481–487. | |
van Zwienen CM, van den Bosch EW, Snijders CJ, Kleinrensink GJ, van Vugt AB. Biomechanical comparison of sacroiliac screw techniques for unstable pelvic ring fractures. J Orthop Trauma. 2004;18(9):589–595. | |
Korovessis PG, Magnissalis EA, Deligianni D. Biomechanical evaluation of conventional internal contemporary spinal fixation techniques used for stabilization of complete sacroiliac joint separation: a 3-dimensional unilaterally isolated experimental stiffness study. Spine (Phila Pa 1976). 2006;31(25):E941–E951. | |
Pel JJ, Spoor CW, Pool-Goudzwaard AL, Hoek van Dijke GA, Snijders CJ. Biomechanical analysis of reducing sacroiliac joint shear load by optimization of pelvic muscle and ligament forces. Ann Biomed Eng. 2008;36(3):415–424. | |
Cummings J Jr, Capobianco RA. Minimally invasive sacroiliac joint fusion: one-year outcomes in 18 patients. Ann Surg Innov Res. 2013;7(1):12. | |
Rudolf L. MIS fusion of the SI joint: does prior lumbar spinal fusion affect patient outcomes? Open Orthop J. 2013;7:163–168. | |
Rudolf L. Sacroiliac joint arthrodesis-MIS technique with titanium implants: report of the first 50 patients and outcomes. Open Orthop J. 2012;6:495–502. | |
Sachs D, Capobianco R. One year successful outcomes for novel sacroiliac joint arthrodesis system. Ann Surg Innov Res. 2012;6(1):13. | |
Sachs D, Capobianco R. Minimally invasive sacroiliac joint fusion: one-year outcomes in 40 patients. Adv Orthop. 2013;2013:536128. | |
Shaffrey CI, Smith JS. Stabilization of the sacroiliac joint. Neurosurg Focus. 2013;35(Suppl 2):Editorial. | |
Mason LW, Chopra I, Mohanty K. The percutaneous stabilisation of the sacroiliac joint with hollow modular anchorage screws: a prospective outcome study. Eur Spine J. 2013;22(10):2325–2331. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
