Back to Journals » Medical Devices: Evidence and Research » Volume 9
Minimal adverse effects profile following implantation of periauricular percutaneous electrical nerve field stimulators: a retrospective cohort study
Authors Roberts A, Sithole A, Sedghi M, Walker CA, Quinn TM
Received 29 February 2016
Accepted for publication 24 May 2016
Published 3 November 2016 Volume 2016:9 Pages 389—393
DOI https://doi.org/10.2147/MDER.S107426
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Arthur Roberts,1–3 Alec Sithole,4 Marcos Sedghi,5 Charles A Walker,6,7 Theresa M Quinn,8
1Indiana Craniofacial Center, 2Department of Oral Pathology, Medicine and Radiology, Indiana University School of Dentistry, Indianapolis, IN, USA; 3Department of Anesthesia, Critical Care, and Pain Management, College of Medicine and Veterinary Medicine, University of Edinburgh, Edinburgh, UK; 4Department of Computer Science, Mathematics and Physics, Missouri Western State University, St Joseph, MO, 5Conservative Care Specialists Medical Group, Los Angeles, CA, 6Walker Pain Management Center, Jackson, TN, 7Bethel University, Paris, TN, 8Wisconsin Pain Solutions, Menomonee Falls, WI, USA
Abstract: The periauricular percutaneous implantation of the Neuro-Stim System™ family of devices EAD, MFS, and BRIDGE is a procedure involving the use of a non-opiate, neuromodulation analgesic for relieving acute and chronic pain. It has been approved as a minimal-risk procedure by multiple governmental and institutional facilities. This retrospective report of findings will help quantify the incidence of clinically observed bleeding, localized dermatitis, and infections at the implantation sites of the electrode/needle arrays, dermatitis at the site of the generator, and patient syncope. A total of 1,207 devices, each producing up to 16 percutaneous punctures, for a total of 19,312 punctures were monitored for adverse effects, based on retrospective chart audits conducted at six clinical facilities over a 1-year period.
Keywords: clinical risks and discomfort, percutaneous auricular neuro-stimulation, Neuro-Stim System™ devices, EAD, MFS, Bridge, neuromodulation, adverse effects
Introduction
The objective of this study is to determine the incidence of specified adverse effects during Neuro-Stim System™ (NSS; Innovative Health Solutions, Versailles, IN, USA) therapy.
Stimulation of the auricular branch of the vagal nerve, utilizing both a transcutaneous and a percutaneous approach, to relieve chronic and acute pain, depression, dysautonomia, and inflammation has been reported in recent literature.1–7 Reports on the NSS have been published with similar findings.8
The NSS (EAD) was approved by the US Food and Drug Administration (FDA) in October 2014, and has been categorized as a minimal-risk device by the FDA, the Independent Review Board of the Defense and Veterans Center for Integrative Pain Management, and the Children’s Hospital of Wisconsin.9
The NSS devices (EAD, MFS, and BRIDGE) were percutaneously implanted targeting cranial nerves V, VII, IX, and X, and branches of the greater and lesser occipital nerves and their associated neurovascular bundles (Figure 1).
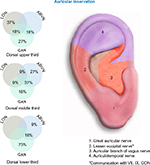  | Figure 1 Concentration of cranial nerves. Abbreviations: LON, lesser occipital nerve; ABVN, auricular branch of vagus nerve; GAN, great auricular nerve; GON, greater occipital nerve. |
Evidence supports the mechanism of action of auricular stimulation via activation of the nucleus tractus solitarius, the hypothalamus, the amygdala, and the rostral ventromedial medulla, affecting both sympathetic and parasympathetic feedback loops. The results are disruption of ascending nociceptive stimuli and mitigation of descending nociceptive signals into the gray matter of the dorsal horn of the spinal column.
Persistent irritation of the microglia is thought to distort the feedback patterns of the neuromatrix with subsequent endocrine, immune, and autonomic nervous system changes, and impairment of descending medullary inhibition. One element of our hypothesis is that the neuromodulating signal of the devices reduces this irritation and the associated microglial inflammation.5,6,8,10–14
Materials and methods
The NSS is a group of neuro-stimulation medical devices consisting of a battery-operated solid-state generator externally affixed by double-sided tape to the skin behind the ear, four wire connecting leads, and four attached electrode/needle arrays each consisting of four 1.5 mm needles designed to be percutaneously implanted into the dermis of the external ear (Figure 2).
  | Figure 2 Assembled Neuro-Stim System™ device. |
For this report of findings, the EAD, MFS, and BRIDGE, although packaged separately, are collectively referred to as the NSS.
The NSS devices are provided in disposable convenience kits consisting of a generator, sterile wire leads with arrays attached, transilluminator, tweezers, surgical marking pen, several adhesives, bandages, disinfectant, and instructions for use. The devices and convenience kits are assembled at Key Electronics in Jeffersonville, IN, USA (Figure 3).
  | Figure 3 Device convenience kit. |
Licensed clinicians at multiple centers across the US who have placed >60 NSS devices in the past year (including multiple placements on the same patient) were asked to retrospectively review their charting for the outlined adverse patient observations.
Inclusion criteria
All patients from the six participating centers, who qualified within the accepted clinical guidelines for placement of the NSS, were included in this study. The sample included both male and female patients, aged 16–70 years. All patients provided written informed consent to take part in this study. The Indiana University Institutional Review Board (IRB) IRB00000220/IRB-01 approved the protocol of this study and was in compliance with (as applicable) 45 CFR 46.109 (d) and IU Standard Operating Procedures (SOPs) for Research Involving Human Subjects.
Transillumination
The use of transillumination to visualize, isolate, and target the auricular neurovascular bundles allows the clinician to avoid direct implantation into the larger vessels within the designated physiological zone (Figures 1 and 4).
  | Figure 4 Neurovascular bundles isolated by transillumination. |
Upon percutaneous implantation of the arrays (Figure 5), and activation of the generator, a fractal geometric electronic field is produced which is designed to stimulate the targeted neurovascular bundles.15
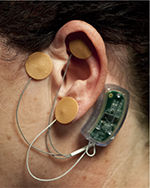  | Figure 5 Activated device properly implanted. |
Retrospective design
Six centers, from a wide geographic and interdisciplinary background, performed a retrospective chart audit to ascertain the incidence of specified adverse reactions for the preceding 12 months to placement of a total of 1,207 NSS devices. Each device had four electrode arrays, with four needles per array. These arrays were percutaneously implanted into the dorsal or ventral aspect of the external ear with a total of 16 percutaneous punctures per application. The result was a total cohort of 19,312 percutaneous punctures. Data from four different categories were collected:
- Bleeding at any puncture site
- Dermatitis at a puncture site or at the generator attachment site
- Perception of significant device pain, resulting in patient request to discontinue treatment
- Syncope at time of implantation
Results and analysis
The data on discomfort upon insertion of the electrodes obtained from six different treatment centers are presented in Table 1. Each of the 1,207 NSS devices had four arrays, and each array had four needles. Out of a total of 19,312 punctures, eleven episodes of bleeding and eleven episodes of localized dermatitis were observed at the electrode insertion sites. No incidence of syncope (fainting) or infection was observed.
Overall, bleeding and dermatitis had the highest rate of occurrences, which were 0.057% and 0.062%, respectively.
Discussion
The dorsal and ventral aspects of the auricle were heavily vascularized from branches of the superficial temporal artery, and the posterior auricular artery.16 Cranial nerves V, VII, IX, and X as well as branches of the greater and lesser occipital nerves were also present in a predicable anatomical fashion. This allowed the clinician to target specific cranial neurovascular bundles.16
Random percutaneous implantation of the arrays into a heavily vascularized area per standard NSS protocol could result in a high incidence of bleeding. The technique of transillumination avoids random placement, and thus helps reduce the incidence of insertion directly into the peripheral arterial branches, greatly reducing the potential of bleeding.
The use of the FDA-approved biocompatible materials, sterilization of the arrays, and proper skin-disinfecting technique, as outlined in the instructions for use, reduce the incidence of dermatitis, and practically eliminate the chance of infection.
There were no reported incidences of syncope in this cohort. However, due to the potential stimulation of the peripheral branches of the vagus nerve, possible vaso-vagal response should be taken into consideration. Patient’s preexisting health conditions such as seizures, bleeding disorders, on-demand electrical implants, medications, and autonomic nervous system status must also be taken into consideration.
While this report of findings supports that the use of NSS devices involves minimal risk, it does not imply that there is no risk. The clinician must evaluate the risk assessment of patients on an individual basis. Placement technique including precise array implantation, proper disinfection of implantation site, careful monitoring of the patient’s vital signs, and the availability of immediate supportive care are recommended.
Conclusion
The FDA, the Independent Review Board of the Defense and Veterans Center for Integrative Pain Management, and the Children’s Hospital of Wisconsin have all categorized the use of the NSS devices as having minimal risk.9,17,18 This is supported by the low reported incidence of bleeding, dermatitis, infection, and syncope. However, monitoring of the patient’s vital signs and immediate availability of supportive care are recommended.
Disclosure
The authors report no conflicts of interest in this work.
References
Frangos E, Ellrich J, Komisaruk BR. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul. 2015;8(3):624–636. | ||
George MS, Nahas Z, Borckardt JJ, et al. Vagus nerve stimulation for the treatment of depression and other neuropsychiatric disorders. Exp Rev Neurother. 2007;7(1):1–12. | ||
Ghoname EA, Craig WF, White PF, et al. Percutaneous electrical nerve stimulation for low back pain. JAMA. 1999;281(9):818–823. | ||
Goadsby PJ, Cohen AS, Matharu MS. Trigeminal autonomic cephalgias: diagnosis and treatment. Curr Neurol Neurosci Rep. 2007;7(2):117–125. | ||
Zhang Y, Popovic ZB, Bibevesky S, et al. Chronic vagus stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail. 2009;2(6):692–699. | ||
La Marc R, Nedeljkkvic M, Yuan L, Maecker A, Ehlert U. Effects of auricular electrical stimulation on vagal activity in healthy men: evidence from a three-armed randomized trial. Clin Sci. 2010;118(8):537–546. | ||
Li S, Zhai X, Rong P, et al. Transcutaneous auricular vagus nerve stimulation triggers melatonin secretion and is antidepressive in Zucker diabetic fatty rats. PLoS One. 2014;9(10):1–6. | ||
Roberts A, Brown C. Decrease in VAS score following placement of percutaneous peri-auricular peripheral nerve field stimulators. Clin Med Diagn. 2015;5(2):17–21. | ||
FDA. What does it mean for FDA to “classify” a medical device? 2014. Available from: http://www.fda.gov/AboutFDA/Transparency/Basics/ucm194438.htm. | ||
Zhuo M, Sengupta JN, Gebhart GF. Biphasic modulation of spinal visceral nociceptive transmission from the rostroventral medial medulla in the rat. J Neurophysiol. 2002;87(5):2225–2236. | ||
Basbaum A. Pain and the control of pain: pain transmission, regulation and management. In: Woolf CJ, editor. Central Sensitization. London: Henry Stewart Talks Ltd; 2009. | ||
Wallace DJ, Clauw DJ, editors. Fibromyalgia and Other Central Pain Syndromes. Philidelphia, PA: Lippincott Williams & Wilkins; 2005. | ||
Gary Kaplan D. Microglia and central sensitization syndrome: a new paradigm for understanding and treating chronic pain and depression. Presented at: Annual meeting of the American Academy of Pain Management; September 27, 2013; Orlando, FL. | ||
Watkins LR, Hutchinson MR, Ledeboer A, Wiesler-Frank J, Milligan ED, Maier SF. Glia as the “bad guys”: implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun. 2007;21(2):131–146. | ||
Serentz M, Gerlleri B, Hoffman J. The organism as a bioreactor. Interpretation of the reduction law of metabolism in terms of heterogeneous catalysis and fractal structure. J Theor Biol. 1985;117(2):209–230. | ||
Peuker ET, Filler TJ. The nerve supply of the human auricle. Clin Anat. 2002;15(1):35–37. | ||
Kent M. Double blind, randomized, sham-controlled study on the effects of Neuro-Stim system on pain, sleep and opioid use in pain patients. Available from: https://clinicaltrials.gov/ct2/show/NCT02673684. NLM identifier: NCT02673684. | ||
Kovacic K. Efficacy of Auricular Neurostimulation for Adolescents with Pain-Associated Functional Gastrointestinal Disorders. Milwaukee, WI: Children’s Hospital and Health System; 2015. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

