Back to Journals » Journal of Pain Research » Volume 12
Migrainous Infarction And Cerebral Vasospasm: Case Report And Literature Review
Authors Vinciguerra L, Cantone M , Lanza G , Bramanti A, Santalucia P, Puglisi V, Pennisi G , Bella R
Received 21 March 2019
Accepted for publication 19 July 2019
Published 21 October 2019 Volume 2019:12 Pages 2941—2950
DOI https://doi.org/10.2147/JPR.S209485
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Luisa Vinciguerra,1 Mariagiovanna Cantone,2 Giuseppe Lanza,3,4 Alessia Bramanti,5 Paola Santalucia,1 Valentina Puglisi,1 Giovanni Pennisi,3 Rita Bella6
1Neurology Department and Stroke Unit, IRCCS Centro Neurolesi “Bonino Pulejo”, Messina, Italy; 2Neurology Department, “Sant’Elia” Hospital, Caltanissetta, Italy; 3Department of Surgery and Medical-Surgical Specialties, University of Catania, Catania, Italy; 4Department of Neurology IC, Oasi Research Institute – IRCCS, Troina, Italy; 5Institute of Applied Sciences and Intelligent Systems (ISASI), IRCCS Centro Neurolesi “Bonino Pulejo”, Messina, Italy; 6Department of Medical and Surgical Sciences and Advanced Technology, Section of Neurosciences, University of Catania, Catania, Italy
Correspondence: Luisa Vinciguerra
Neurology Department and Stroke Unit, IRCCS Centro Neurolesi “Bonino Pulejo”, Via Provinciale Palermo, Contrada Casazza, 98124 Messina, Italy
Tel +39 090 6012 8395
Fax +39 090 6012 8850
Email [email protected]
Abstract: Migrainous infarction (MI) is a rare complication of migraines that accounts for 0.5–1.5% of all ischemic strokes. Although the pathogenesis of MI is still debated, cortical spreading depression and the consequent biochemical cascade and hemodynamic changes are presumed to play an important role. Here we describe a case of MI and systematically review the literature on the complex and possibly bidirectional relationship between migraine and stroke. A 44-year-old female with history of migraine with visual aura presented at the Emergency Department due to a sudden onset of left limb paresis and hypoesthesia. Brain magnetic resonance imaging revealed right fronto-parietal ischemic stroke. Two days after hospitalization, the patient experienced a prolonged visual aura and showed ultrasound evidence of intracranial artery vasospasm. To date, there have been 33 published articles on a total 119 patients with MI, although intracranial vasospasm has rarely been reported. Sustained hyperexcitability of cortical neurons, impairment of γ-aminobutyric acid inhibitory circuitry, altered serotonergic transmission, release of vasoconstrictive molecules, and cerebral blood flow changes have been proposed as pathogenic mechanisms of MI. The present case provides insight into the pathophysiological link between stroke and migraine, thus aiding clinicians in therapeutic decision-making although additional studies are needed to clarify the clinical, neuroradiological, and ultrasound findings that link MI and stroke-related migraine.
Keywords: biochemical change, migrainous cerebral ischemia, pathogenesis, vasospasm
Plain Language Summary
The pathophysiology of migrainous infarction is not well understood, although biochemical cascades and cerebral blood flow changes — namely, arterial vasospasm consequent to cortical spreading depression — may play a critical role. Transcranial color Doppler sonography is a non-invasive and effective tool for the early detection and monitoring of intracranial blood flow changes in migraine and its complications, providing new insight into the pathophysiological link between stroke and migraine.
Introduction
The International Classification of Headache Disorders (ICHD), Third Edition (beta version) defines migrainous infarction (MI) as a stroke developing during an attack of migraine with aura together with neuroimaging evidence of infarction in a relevant area.1 MI is a rare condition, accounting for approximately 0.5–1.5% of all ischemic strokes, although this fraction may be as high as 13% in young people with cerebral ischemia, with an estimated incidence of 0.8 per 100,000 individuals.2,3 MI is more common in women and in the posterior vascular territory (70.6%) and usually causes small strokes (64.7%), while multiple lesions are observed in 41.2% of cases.3
The pathogenesis of migraine-associated cerebral infarction remains unclear, although cortical spreading depression (CSD), biochemical alterations, hemodynamic changes, arterial vasospasm, cerebral edema, and platelet aggregation have been proposed as possible mechanisms.4,5 Vasospasm is caused by focal or diffuse reversible narrowing of vessel caliber consequent to the contraction of smooth muscle within the artery wall that is detectable by several methods, including transcranial color-coded Doppler sonography (TCCD), computed tomography (CT), magnetic resonance imaging (MRI), and cerebral angiography. TCCD is a portable and non-invasive tool that is useful not only for diagnosis but also for on-site in vivo and real-time monitoring of cerebral blood flow velocity;6,7 it is used to evaluate acute stroke as well as hemodynamic changes in chronic cerebrovascular disease.8–10
Although the temporal and causal relationship between migraine and stroke has been widely documented, the opposite situation—ie, the contribution of stroke to migraines—has rarely been investigated. Here we describe the case of a young woman who first developed ischemic stroke after a prolonged migraine followed by MI soon after hospitalization. Given the rarity and complexity of the clinical presentation, we carried out a systematic review of the literature on MI, paying particular attention to the underlying biochemical changes and relationship among migraine, vasospasm, and stroke.
Case Report
A 44-year-old woman presented at the Emergency Department with a sudden onset of difficulty in verbal expression, left facial tingling, and omolateral arm weakness following a prolonged migrainous attack that had developed two days earlier. She had a history of migraine with aura presenting with visual sensations (oscillopsia and photopsia), although she had also experienced attacks without aura, especially during her adolescence. Since starting prophylactic treatment with flunarizine within the previous 12 months, she had experienced approximately two attacks per month. The current migrainous episode was not preceded by aura, but was more severe than previous attacks and did not respond to oral triptan intake, which was repeated two hours after the first dose. The patient was not taking any oral contraceptive drugs and had no history of other illnesses, except for an episode of paroxysmal atrial fibrillation after percutaneous closure of a patent foramen ovale (PFO) three years earlier that had been successfully treated, without any further evidence of cardiac arrhythmia in repeated electrocardiogram (ECG)-Holter recordings.
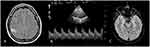
At admission, a neurological examination revealed mild paresis and hypoesthesia of the left arm, resulting in a National Institute of Health Stroke Scale (NIHSS) score of 2. Brain MRI showed an acute cortico-subcortical ischemic lesion in the right fronto-parietal region within the vascular territory of the right middle cerebral artery (MCA) (Figure 1A). TCCD revealed increased blood flow velocity in the right proximal MCA segment, with a mean value of 210.2 cm/s (Figure 1B) [cut-off flow velocity for vasospasm: >120 cm/s in the anterior circulation]6,7 and normal values in the other major brain regions. The Lindegaard ratio (LR) of 5.3 was indicative of vasospasm. Data from instrumental and laboratory tests, including thrombophilia, autoimmunity, and infectious disease screening, ECG and blood pressure monitoring, echocardiography, supra-aortic vessels sonography, and transcranial Doppler bubble test were all unremarkable.
Two days after hospitalization, the patient experienced a prolonged episode of visual aura characterized by a “fortification spectrum” with a “zigzag-type” figure and scintillating scotoma. The control TCCD showed a slight increase in blood flow velocity (mean value of 85.1 cm/s) with turbulence at the top of the basilar artery (BA) segment and a LR for posterior circulation (modified LR) of 2.9. The control MRI revealed two new bilateral lacunar strokes in the occipital lobes (Figure 1C). Treatment with antiplatelet drugs (300 mg followed by 100 mg acetylsalicylic acid)11 was initiated at admission; paracetamol was used for migraine and verapamil (120 mg three times a day) for migraine prophylaxis and vasospasm. At discharge, the patient was fully recovered from neurological deficits (NIHSS score of 0). A two-week follow-up TCCD examination showed normal blood flow velocity.
Data Source And Selection
The following Medline terms were searched in different combinations: “migrainous cerebral infarction”, “migrainous infarction”, “acute migrainous infarction”, and “migrainous stroke”. Original articles, case reports, and case series on adult patients published in English between 1990 and February 2019 were critically analyzed. Full-texts and references from relevant papers were reviewed to identify additional data sources.
Systematic Review
We identified 33 articles including 26 case reports and 7 case series describing a total of 119 patients (41 males and 78 females; mean age: 42.2 ± 18.2 years, range: 15–93 years). Clinical and demographic features and vascular territory of the stroke are summarized in Table 1.
 |
Table 1 Literature Reports Of Migrainous Infarction |
Aura was reported as visual (49.6%), sensitive (15.1%), motor (5.9%), or brainstem (2.5%) disturbances. In some patients, aura symptoms were not specified (30.3%) or were absent (4.2%). Neurological manifestations consisted of visual deficits (57%; as hemianopsia, visual distortions, and phosphenes), sensory disturbances (31.9%; hypoesthesia or paresthesia), motor deficit (28.6%), ataxia (8.4%), dysarthria (8.4%), aphasia (4.2%), diplopia (2.5%), and dizziness and hearing disorders (1.7%). In one report the MI had a fatal course.12
A review of neuroimaging correlates of MI revealed lesions in the posterior circulation in 77.3% of cases and in the middle/anterior vascular territory in 22.7%. Intracranial artery evaluation was performed in 22 studies: 13 by MR angiography (MRA; 32 patients), three by conventional angiography (5 patients), one by TCCD (9 patients), and four by more than one technique (4 patients).
Vasospasm was detected in seven studies; the diagnosis was made by MRA in two patients,12,13 by angiography in three,14–16 and by TCCD in two.17,18 TCCD revealed vasospasm in the right MCA (segment M1)17 in one patient and in both vertebral arteries in another,18 with evidence of acute ischemic lesion in the corresponding vascular territory. One subject had normal TCCD values19 and there was no narrowing of the artery lumen or enhanced resistance detected in the other six cases, although the authors did not specify whether this was determined by TCCD or by carotid duplex sonography. The same authors found reduced TCCD values for the breath-holding index in two subjects, indicating an impaired arterial response to vasodilating substances in the affected cerebral territories.20 PFO was detected in 12 patients in two studies by TCCD.3,21 One of these articles used MRA or TCCD, but the number of examined patients and obtained results were not specified.22
Some investigators have explored the biochemical mechanisms underlying MI (Table 2). One group proposed that migraine-related neuronal injury causes glial cell multiplication and accumulation of protein and fat macrophages, which restricts the motion of water molecules in MRI and yields hyperintensities in the T1-weighted image of the affected cerebral region;23 this is supported by a recent report.24 Others have demonstrated cortical hyperexcitability in the primary visual cortex, possibly due to impairment of the γ-aminobutyric acid (GABA)ergic inhibitory network and hypoactivity of thalamo-cortical circuits.25 Cortical hypermetabolism was also proposed in a case of MI with laminar necrosis.26 In another patient with MI and vasospasm, a biochemical origin was suggested for the latter based on evidence of enhanced intracellular calcium concentration and vasoactive agents such as serotonin, angiotensin II, hydroperoxides, adenosine diphosphate, and prostaglandins.16
 |
Table 2 Biochemical Implications Proposed In Migrainous Infarction |
Discussion
We described a patient who experienced a first stroke after prolonged migraine without aura. Although this episode did not meet all of the criteria for MI diagnosis, soon afterward she had a second stroke that completely satisfied the ICHD-3 beta diagnostic criteria for MI,1 with one or more otherwise typical aura symptom that persisted beyond one hour with neuroimaging confirmation of an ischemic infarction in the affected territory. The main finding of the present study is the possible bidirectional relationship between migraine and stroke, which is based on the hypothesis that vasospasm serves as a link between migrainous attack and cerebral ischemia. Indeed, the patient showed a vasospasm in the related ischemic territory.
TCCD demonstrated a high positive predictive value for detectingspasm.7,44–46 Mean flow velocity >120 cm/s in the MCA or 90 cm/s in the posterior circulation and an increase of >50 cm/s within 24 h usually indicate a vasospasm, which is generally accompanied by typical disturbances in blood flow (turbulence and musical murmurs). The LR and modified LR are often used to reliably distinguish between hyperdynamic flow and vasospasm by comparing MCA/extracranial carotid artery velocities for the anterior circulation and BA/extracranial vertebral artery velocities for the posterior circulation, respectively.6,47 Our patient had elevated blood flow velocity, suggesting vasospasm; on the other hand, she did not meet the clinical and neuroimaging diagnostic criteria for reversible cerebral vasoconstriction syndrome.48
A retrospective study of more than 130,000 migraineurs found that treatment with ergot alkaloids but not triptans was associated with increased risk of ischemic events.49 Some studies have suggested that vasospasm arises from transiently enhanced sympathetic neuronal activity through the release of noradrenaline (and consequent activation of adrenergic receptors) or vasoconstrictive molecules such as serotonin and endothelin, resulting in vasoconstriction, thrombosis, and ischemic lesions.16,50,51 A positron emission tomography study demonstrated a reversible reduction of 40% in cerebral blood flow during a migraine attack that was confirmed bysingle-photon emission computed tomography (SPECT) in a subject with basilar migraine.52,53 Similarly, extensive hypoperfusion of brain regions involved in the development of prolonged aura was reported in another SPECT study27 and in a case of cortical laminar necrosis detected by perfusion MRI.24 Hence, vasoconstrictive drugs such as ergotamines and triptans should be avoided during the acute phase of MI.
Propranolol should be prescribed with caution for migraine prophylaxis as it can limit compensatory vasodilator capacitance, and should be avoided altogether in cases of prolonged aura or migraine with aura presenting with brainstem symptoms.4 Calcium channel blockers, angiotensin receptor blockers, or angiotensin receptor antagonists are alternative choices for migraine prevention and have been shown to promote endothelial repair and decrease the level of von Willebrand factor.4,54,55 Among calcium antagonists, verapamil was shown to be particularly effective in rapidly reducing cerebral vasospasms, as detected by TCCD.56 Treatment strategies also need to take into account patients’ co-morbidities, vascular risk factors, and drug interactions. Given that pro-thrombotic alterations and platelet activation are presumed to play a role in MI, it is generally recommended that antiplatelet therapy be initiatedto prevent ischemic events.2
Except for one case with a fatal course,12 the functional outcome of MI is usually favorable. In particular, patients with posterior circulation stroke improved or recovered from neurological deficits in about 47.9% and 18.5% of cases, respectively, although mild visual field defects persisted in cases of more severe occipital infarct (approximately 32.4%). Similar results were recorded in cases of anterior circulation ischemia: approximately 74% of patients showed improvement from neurological disturbances, with 17% achieving total recovery; whereas around 26% of those with larger cerebral lesions had persistent deficits. Consistent with these data, our patient showed clinical improvement and full recovery from MI-related deficits.
The precise etiology of MI is largely unknown, although different pathomechanisms have been proposed.5 Migraine aura is generally attributed to CSD, which is a brief depolarizing wave moving at 3–5 mm/min across the cortex followed by sustained neuronal depression. During prolonged aura, long-lasting cortical neuronal hyperexcitability can result in reverberating CSD.25 Thus, inhibitory control may be lost in the primary visual cortex due to perturbation of the GABAergic system. Accordingly, a transcranial magnetic stimulation study of the human motor cortex of migraineurs found that the duration of the cortical silent period was shortened, which is a well-known index of intracortical inhibition mainly mediated by GABA-B receptor activity.57,58 Furthermore, a reduction in regional cerebral blood flow in the visual cortex during aura may lead to impairment of the GABAergic inhibitory circuitry comprising spinous stellate cells that spread horizontally and generate an inhibitory network in lamina IV of the primary visual cortex.59 Clinical studies have demonstrated that migraineurs have altered serotonergic transmission from the brainstem to the thalamus and cortex;60 this so-called “thalamo-cortical dysrhythmia” results in dysfunction of both inhibitory and excitatory cortical neurons,61 as observed by electrophysiological recordings in other neurological disorders.62–65
Activation of CSD induces cortical spreading hyperemia, followed by long-lasting oligoemia, hypoperfusion, vasoconstriction, and severely limited oxygen delivery to tissues.4,34,66 Thus, CSD leads to marked metabolic and hemodynamic changes in neurons, creating supply/demand mismatches for adenosine triphosphate, oxygen, and glucose. Consequently, the brain of migraineurs is more vulnerable to ischemia even after a mild and transient hypoxic state.27 The triggering of a chain reaction of detrimental processes including prolonged vasoconstriction due to reduced nitric oxide, increased extracellular K+, and endothelial ion channel/pump dysfunction eventually lead to vasospasm2 and brain infarction.66 CSD may also induce the release of inflammatory mediators, vasoactive peptides, and adhesion molecules, which predisposes to intravascular thrombosis.50
The neurochemical changes, pathological mechanisms, and phenotypic alterations in MI are schematically depicted in Figure 2. Interestingly, the endothelium may play a major role by inducing vasoconstriction and inhibiting vasodilatation upon neuroinflammation, suggesting that impaired cerebral endothelial function contributes to the development of ischemic stroke in migraine. This is supported by clinical evidence from studies reporting an over-representation of asymptomatic ischemic lesions in the posterior circulation of patients with migraine, especially those with aura.67 A more recent transcranial Doppler study showed that patients with migraine exhibit impaired endothelial function in the posterior cerebral circulation, implying that direct endothelial impairment contributes to posterior circulation infarcts.68 Decreased diameter and compliance of the muscular artery have also been observed in migraine patients, likely reflecting enhancedarterial tone and increasing susceptibility to vasospasm.69
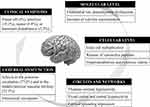 |
Figure 2 Summary of possible interactions among neurochemical and neurophysiological changes and clinical phenotype in MI patients. |
Taken together, the evidence presented here indicates that arteries of migrainous patients are more likely to develop vasospasm, thereby increasing stroke risk. Indeed, some studies have shown a transient narrowing of the left middle and posterior cerebral arteries during migraine attacks,70,71 and microvascular vasospasm in cortical regions related to the topography of aura symptoms has been reported.72 Vasospasms of varying intensity can occur during migraine attacks and may precede or accompany a headache, obstructing focal cerebral blood flow.73,74 However, further studies are needed to clarify the role of vasospasm in migraines and in the occurrence of MI.
Conclusion
Clinicians should immediately perform neuroimaging and a TCCD evaluation of migraineurs with prolonged aura for early detection of vasospasm or cerebral ischemia. If the clinical suspicion of MI is confirmed, comprehensive clinical and instrumental analyses should be carried out for accurate diagnosis and therapeutic management. Prospective multicenter studies are needed to obtain insight into the pathogenic mechanisms underlying MI and to standardize diagnostic and treatment approaches.
Ethics Approval And Informed Consent
Given that this study reports a single patient’s clinical description and instrumental exams that are routinely performed within the conventional diagnostic work-up of these subjects, it did not need ethical approval. The study subject provided written, informed consent for the publication of data and images.
Acknowledgment
The authors thank The Charlesworth Group for the professional English editing.
Disclosure
The authors report no conflicts of interest in relation to this work.
References
1. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia Int J Headache. 2013;33(9):629–808. doi:10.1177/0333102413485658
2. Plato BM. Rare complications of migraine with aura. Headache. 2016;56(8):1373–1379. doi:10.1111/head.12879
3. Wolf ME, Szabo K, Griebe M, et al. Clinical and MRI characteristics of acute migrainous infarction. Neurology. 2011;76(22):1911–1917. doi:10.1212/WNL.0b013e31821d74d5
4. Tietjen EG. Migraine and ischaemic heart disease and stroke: potential mechanisms and treatment implications. Cephalalgia Int J Headache. 2007;27(8):981–987. doi:10.1111/j.1468-2982.2007.01407.x
5. Zhang Y, Parikh A, Qian S. Migraine and stroke. Stroke Vasc Neurol. 2017;2(3):160–167. doi:10.1136/svn-2017-000077
6. Naqvi J, Yap KH, Ahmad G, et al. Transcranial doppler ultrasound: a review of the physical principles and major applications in critical care. Int J Vasc Med. 2013;2013:629378.
7. Vinciguerra L, Bösel J. Noninvasive neuromonitoring: current utility in subarachnoid hemorrhage, traumatic brain injury, and stroke. Neurocrit Care. 2017;27(1):122–140. doi:10.1007/s12028-016-0361-8
8. Malojcic B, Giannakopoulos P, Sorond FA, et al. Ultrasound and dynamic functional imaging in vascular cognitive impairment and Alzheimer’s disease. BMC Med. 2017;15(1):27. doi:10.1186/s12916-017-0799-3
9. Vinciguerra L, Lanza G, Puglisi V, et al. Transcranial Doppler ultrasound in vascular cognitive impairment-no dementia. PLoS One. 2019;14(4):e0216162. doi:10.1371/journal.pone.0216162
10. Puglisi V, Bramanti A, Lanza G, et al. Impaired cerebral haemodynamics in vascular depression: insights from transcranial doppler ultrasonography. Front Psychiatry. 2018;9:316. doi:10.3389/fpsyt.2018.00316
11. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46–110. doi:10.1161/STR.0000000000000158
12. Marshall N, Maclaurin WA, Koulouris G. MRA captures vasospasm in fatal migrainous infarction. Headache. 2007;47(2):280–283. doi:10.1111/hed.2007.47.issue-2
13. Mendizabal JE, Greiner F, Hamilton WJ, Rothrock JF. Migrainous stroke causing thalamic infarction and amnesia during treatment with propranolol. Headache. 1997;37(9):594–596. doi:10.1046/j.1526-4610.1997.3709594.x
14. Meschia JF, Malkoff MD, Biller J. Reversible segmental cerebral arterial vasospasm and cerebral infarction: possible association with excessive use of sumatriptan and Midrin. Arch Neurol. 1998;55(5):712–714. doi:10.1001/archneur.55.5.712
15. Sanin LC, Mathew NT. Severe diffuse intracranial vasospasm as a cause of extensive migrainous cerebral infarction. Cephalalgia Int J Headache. 1993;13(4):289–292. doi:10.1046/j.1468-2982.1993.1304289.x
16. Solomon S, Lipton RB, Harris PY. Arterial stenosis in migraine: spasm or arteriopathy? Headache. 1990;30(2):52–61. doi:10.1111/hed.1990.30.issue-2
17. Tsai CF, Chen CC, Wang SC, et al. Reversible vasospasm in migrainous infarction: a transcranial Doppler follow-up study. J Ultrasound Med. 2010;29(3):481–484. doi:10.7863/jum.2010.29.3.481
18. Gomez CR, Gomez SM, Puricelli MS, Malik MM. Transcranial Doppler in reversible migrainous vasospasm causing cerebellar infarction: report of a case. Angiology. 1991;42(2):152–156. doi:10.1177/000331979104200211
19. Tzoulis CH, Naess H, Thomassen L. Migrainous cerebral infarction in a previously healthy 93-year-old female patient with no risk factors for stroke. Cephalalgia Int J Headache. 2006;26(7):894–895. doi:10.1111/j.1468-2982.2006.01111.x
20. Linetsky E, Leker RR, Ben-Hur T. Headache characteristics in patients after migrainous stroke. Neurology. 2001;57(1):130–132. doi:10.1212/WNL.57.1.130
21. Decima D, Cavallo M, Leotta MR, et al. Migrainous infarction: association with vascular risk factors in a male subject. Neurol Sci. 2009;30(Suppl 1):S145–S146. doi:10.1007/s10072-009-0085-z
22. Serrano F, Arauz A, Uribe R, Becerra LC, Mantilla K, Zermeño F. Long-term follow-up of patients with migrainous infarction. Clin Neurol Neurosurg. 2018;165:7–9. doi:10.1016/j.clineuro.2017.12.008
23. Arboix A, González-Peris S, Grivé E, et al. Cortical laminar necrosis related to migrainous cerebral infarction. World J Clin Cases. 2013;1(8):256–259. doi:10.12998/wjcc.v1.i8.256
24. Morais R, Sobral F, Cunha G, et al. Advanced MRI study of migrainous infarction presenting as cortical laminar necrosis - Case report and literature review. Clin Neurol Neurosurg. 2018;167:82–85. doi:10.1016/j.clineuro.2018.02.016
25. Thissen S, Koehler PJ. Persistent aura with small occipital cortical infarction: implications for migraine pathophysiology. Case Rep Neurol. 2014;6(2):217–221. doi:10.1159/000366409
26. Liang Y, Scott TF. Migrainous infarction with appearance of laminar necrosis on MRI. Clin Neurol Neurosurg. 2007;109(7):592–596. doi:10.1016/j.clineuro.2007.04.005
27. Mancini V, Mastria G, Frantellizzi V, et al. Migrainous infarction in a patient with sporadic hemiplegic migraine and cystic fibrosis: a 99mTc-HMPAO brain SPECT study. Headache. 2019;59(2):253–258. doi:10.1111/head.13472
28. Campagna G, Vickers A, Ponce CMP, et al. Homonymous hemianopsia as the presenting sign of migrainous infarction. Can J Ophthalmol. 2018;53(6):e229–32. doi:10.1016/j.jcjo.2018.01.007
29. Khardenavis V, Karthik DK, Kulkarni S, et al. Cortical laminar necrosis in a case of migrainous cerebral infarction. BMJ Case Rep. 2018;2018.
30. Kreling GAD, de Almeida NR, Dos Santos PJ. Migrainous infarction: a rare and often overlooked diagnosis. Autopsy Case Rep. 2017;7(2):61–68. doi:10.4322/acr.2017.018
31. Renard D, Nerrant E, Freitag C. Early recurrence of migrainous infarction. Acta Neurol Belg. 2015;115(4):675–676. doi:10.1007/s13760-015-0444-x
32. Parks NE, Rigby HB, Gubitz GJ, et al. Dysmetropsia and Cotard’s syndrome due to migrainous infarction - or not? Cephalalgia Int J Headache. 2014;34(9):717–720. doi:10.1177/0333102414520765
33. Lai TH, Hong CT. Prolonged symptoms in sporadic hemiplegic migraine: aura or migrainous infarction? Acta Neurol Taiwanica. 2012;21(3):129–132.
34. Laurell K, Artto V, Bendtsen L, et al. Migrainous infarction: a Nordic multicenter study. Eur J Neurol. 2011;18(10):1220–1226. doi:10.1111/j.1468-1331.2011.03364.x
35. Caballero PEJ. Migrainous cerebral infarction after postcoital contraception. Cephalalgia Int J Headache. 2009;29(6):691–693. doi:10.1111/j.1468-2982.2008.01818.x
36. Schulz UG, Blamire AM, Davies P, et al. Normal cortical energy metabolism in migrainous stroke: a 31P-MR spectroscopy study. Stroke. 2009;40(12):3740–3744. doi:10.1161/STROKEAHA.109.558163
37. Arai S, Utsunomiya H, Arihiro S, Arakawa S. Migrainous infarction in an adult: evaluation with serial diffusion-weighted images and cerebral blood flow studies. Radiat Med. 2008;26(5):313–317. doi:10.1007/s11604-008-0226-y
38. Matsuo S, Suzuki Y, Hashimoto M, Ohtsuka K. Migrainous cerebral infarction triggered by alcohol intake under the burning sun. Jpn J Ophthalmol. 2006;50(4):395–397. doi:10.1007/s10384-006-0331-3
39. Frigerio R, Santoro P, Ferrarese C, Agostoni E. Migrainous cerebral infarction: case reports. Neurol Sci. 2004;25(Suppl 3):S300–S301. doi:10.1007/s10072-004-0318-0
40. Tang SC, Jeng JS, Liu HM, et al. Migrainous infarction involving two different arterial territories: report of two cases. Acta Neurol Taiwanica. 2004;13(1):20–23.
41. Lee H, Whitman GT, Lim JG, et al. Hearing symptoms in migrainous infarction. Arch Neurol. 2003;60(1):113–116. doi:10.1001/archneur.60.1.113
42. Arboix A, Massons J, García-Eroles L, et al. Migrainous cerebral infarction in the Sagrat Cor Hospital of Barcelona stroke registry. Cephalalgia Int J Headache. 2003;23(5):389–394. doi:10.1046/j.1468-2982.2003.00534.x
43. Demirkaya S, Odabasi Z, Gokcil Z, et al. Migrainous stroke causing bilateral anterior cerebral artery territory infarction. Headache. 1999;39(7):513–516. doi:10.1046/j.1526-4610.1999.3907513.x
44. Lysakowski C, Walder B, Costanza MC, Tramèr MR. Transcranial Doppler versus angiography in patients with vasospasm due to a ruptured cerebral aneurysm: a systematic review. Stroke. 2001;32(10):2292–2298. doi:10.1161/hs1001.097108
45. Sloan MA, Haley EC, Kassell NF, et al. Sensitivity and specificity of transcranial Doppler ultrasonography in the diagnosis of vasospasm following subarachnoid hemorrhage. Neurology. 1989;39(11):1514–1518. doi:10.1212/WNL.39.11.1514
46. Chen SP, Fuh JL, Chang FC, et al. Transcranial color doppler study for reversible cerebral vasoconstriction syndromes. Ann Neurol. 2008;63(6):751–757. doi:10.1002/ana.21384
47. Aaslid R, Huber P, Nornes H. Evaluation of cerebrovascular spasm with transcranial Doppler ultrasound. J Neurosurg. 1984;60(1):37–41. doi:10.3171/jns.1984.60.1.0037
48. Miller TR, Shivashankar R, Mossa-Basha M, et al. Reversible cerebral vasoconstriction syndrome, part 2: diagnostic work-up, imaging evaluation, and differential diagnosis. AJNR Am J Neuroradiol. 2015;36(9):1580–1588. doi:10.3174/ajnr.A4215
49. Velentgas P, Cole JA, Mo J, et al. Severe vascular events in migraine patients. Headache. 2004;44(7):642–651. doi:10.1111/j.1526-4610.2004.04122.x
50. Spalice A, Del Balzo F, Papetti L, et al. Stroke and migraine is there a possible comorbidity? Ital J Pediatr. 2016;42:41. doi:10.1186/s13052-016-0253-8
51. Agostoni E, Aliprandi A. The complications of migraine with aura. Neurol Sci. 2006;27(Suppl 2):S91–S95. doi:10.1007/s10072-006-0578-y
52. Woods RP, Iacoboni M, Mazziotta JC. Brief report: bilateral spreading cerebral hypoperfusion during spontaneous migraine headache. N Engl J Med. 1994;331(25):1689–1692. doi:10.1056/NEJM199407073310103
53. Seto H, Shimizu M, Futatsuya R, et al. Basilar artery migraine. Reversible ischemia demonstrated by Tc-99mHMPAO brain SPECT. Clin Nucl Med. 1994;19(3):215–218. doi:10.1097/00003072-199404000-00022
54. Schrader H, Stovner LJ, Helde G, et al. Prophylactic treatment of migraine with angiotensin converting enzyme inhibitor (lisinopril): randomised, placebo controlled, crossover study. BMJ. 2001;322(7277):19–22. doi:10.1136/bmj.322.7277.19
55. Tronvik E, Stovner LJ, Helde G, et al. Prophylactic treatment of migraine with an angiotensin II receptor blocker: a randomized controlled trial. JAMA. 2003;289(1):65–69. doi:10.1001/jama.289.1.65
56. Marsh EB, Ziai WC, Llinas RH. The need for a rational approach to vasoconstrictive syndromes: transcranial doppler and calcium channel blockade in reversible cerebral vasoconstriction syndrome. Case Rep Neurol. 2016;8(2):161–171. doi:10.1159/000447626
57. Aurora SK, Al‐Sayeed F, Welch KMA. The cortical silent period is shortened in migraine with aura. Cephalalgia. 1999;19(8):708–712. doi:10.1046/j.1468-2982.1999.019008708.x
58. Aurora SK. Exciting excitable brains: an update on migraine pathophysiology. J Headache Pain. 2003;4(1):7–12. doi:10.1007/s101940300021
59. Chronicle E, Mulleners W. Might migraine damage the brain? Cephalalgia. 1994;14(6):415–418. doi:10.1046/j.1468-2982.1994.1406415.x
60. Hamel E. Serotonin and migraine: biology and clinical implications. Cephalalgia Int J Headache. 2007;27(11):1293–1300. doi:10.1111/j.1468-2982.2007.01476.x
61. Coppola G, Pierelli F, Schoenen J. Is the cerebral cortex hyperexcitable or hyperresponsive in migraine? Cephalalgia Int J Headache. 2007;27(12):1427–1439. doi:10.1111/j.1468-2982.2007.01500.x
62. Bella R, Ferri R, Lanza G, et al. TMS follow-up study in patients with vascular cognitive impairment-no dementia. Neurosci Lett. 2013;534:155–159. doi:10.1016/j.neulet.2012.12.017
63. Bella R, Cantone M, Lanza G, et al. Cholinergic circuitry functioning in patients with vascular cognitive impairment–no dementia. Brain Stimulat. 2016;9(2):225–233. doi:10.1016/j.brs.2015.09.013
64. Pennisi G, Lanza G, Giuffrida S, et al. Excitability of the motor cortex in de novo patients with celiac disease. PLoS One. 2014;9(7):e102790. doi:10.1371/journal.pone.0102790
65. Bella R, Lanza G, Cantone M, et al. Effect of a gluten-free diet on cortical excitability in adults with celiac disease. PLoS One. 2015;10(6):e0129218. doi:10.1371/journal.pone.0129218
66. Mawet J, Kurth T, Ayata C. Migraine and stroke: in search of shared mechanisms. Cephalalgia Int J Headache. 2015;35(2):165–181. doi:10.1177/0333102414550106
67. Kruit MC, Launer LJ, Ferrari MD, van Buchem MA. Infarcts in the posterior circulation territory in migraine. The population-based MRI CAMERA study. Brain J Neurol. 2005;128(Pt 9):2068–2077. doi:10.1093/brain/awh542
68. Rajan R, Khurana D, Lal V. Interictal cerebral and systemic endothelial dysfunction in patients with migraine: a case-control study. J Neurol Neurosurg Psychiatry. 2015;86(11):1253–1257. doi:10.1136/jnnp-2014-309571
69. Vanmolkot FH, Van Bortel LM, de Hoon JN. Altered arterial function in migraine of recent onset. Neurology. 2007;68(19):1563–1570. doi:10.1212/01.wnl.0000260964.28393.ed
70. Momoki E, Fuchigami T, Kasuga Y, et al. A case of confusional migraine with transient increased cerebral blood flow. Brain Dev. 2019;41(6):559–562. doi:10.1016/j.braindev.2019.02.002
71. Fujita M, Fujiwara J, Maki T, et al. The efficacy of sodium valproate and a MRA finding in confusional migraine. Brain Dev. 2007;29(3):178–181. doi:10.1016/j.braindev.2006.11.009
72. Viola S, Viola P, Buongarzone MP, et al. Microvascular vasospasm of cerebral cortex in prolonged aura migraine. Neurol Sci. 2018;39(Suppl 1):95–96. doi:10.1007/s10072-018-3288-3
73. Skyhøj Olsen T. Migraine with and without aura: the same disease due to cerebral vasospasm of different intensity. A hypothesis based on CBF studies during migraine. Headache. 1990;30(5):269–272. doi:10.1111/hed.1990.30.issue-5
74. Nowak A, Kaciński M. Transcranial Doppler evaluation in migraineurs. Neurol Neurochir Pol. 2009;43(2):162–172.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.