Back to Journals » Journal of Pain Research » Volume 12
MicroRNA And Circular RNA Expression In Affected Skin Of Patients With Postherpetic Neuralgia
Authors Cao S, Zhang D, Yuan J, Liu C, Zhou W, Zhang L, Yu S, Qin B, Li Y, Deng W
Received 2 July 2019
Accepted for publication 30 September 2019
Published 16 October 2019 Volume 2019:12 Pages 2905—2913
DOI https://doi.org/10.2147/JPR.S221615
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Song Cao,1,2 Dexin Zhang,1 Jie Yuan,1,2 Chengxi Liu,2 Wenjing Zhou,2 Lin Zhang,2 Shouyang Yu,2 Bangyong Qin,1 Ying Li,1 Wenwen Deng3
1Department of Pain Medicine, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, People’s Republic of China; 2Guizhou Key Laboratory of Anesthesia and Organ Protection, Zunyi Medical University, Zunyi 563003, People’s Republic of China; 3Department of Cardiology, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, People’s Republic of China
Correspondence: Song Cao
Department of Pain Medicine, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, People’s Republic of China
Tel +8618212170434 Email [email protected]
Wenwen Deng
Department of Cardiology, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, People’s Republic of China
Tel +8615085064354
Email [email protected]
Abstract: Mechanisms of postherpetic neuralgia (PHN) are still not clear. Transcripts such as microRNA (miRNA) and circular RNA (circRNA) in the affected skin may take part in the initiation and development of this neuropathic pain; however, their expression profiles in skins of PHN patients have not been reported. The PHN affected skin and the mirror skin were collected and subjected to miRNA and circRNA microarray, and expression profiles were comparatively analyzed. There were 317 differently expressed miRNAs in PHN affected skin compared with mirror skin (fold change ≥2.0), and 13 of them showed fold change >10 in the PHN skin. Only one circRNA, hsa_circRNA_405463 showed fold change >2 in PHN skin, however, 31 circRNAs with fold change ≥1.5. To evaluate functions of differential miRNAs, their target mRNAs were predicted and bioinformatics analyses including gene ontology, Kyoto Encyclopedia of Genes and Genomes pathway were conducted. Target mRNAs significantly (P<0.05) enriched in 85 pathways, such as FoxO, AMPK, MAPK and pathway. These data reported for the first time that miRNA and circRNA differentially expressed in the PHN skin and these transcripts with abnormal expression could be potential targets to treat PHN.
Keywords: neuropathic pain, postherpetic neuralgia, microRNA, circular RNA
Introduction
Postherpetic neuralgia (PHN) is a typical neuropathic pain, which lasts more than 1 month,1 and profoundly affects the quality of life.2 The mechanisms of PHN are complicated, including the peripheral pathology3–5 and plastic changes in the central nervous system.4–8 Elucidating molecular changes during neuropathic pain is fundamental for the development of mechanism-oriented treatments.9 Understanding the molecular changes in PHN skin may help develop skin-targeting external medications or treatments.
MicroRNAs (miRNAs) are small non-coding molecules, which post-transcriptionally modulate pathological processes including neuropathic pain.10,11 Existing studies have shown that miRNAs are involved in the development of neuropathic pain and are expected to become clinical biomarkers and intervention targets.12,13 miRNA expression differences were detected in serum between two kinds of patients: herpes zoster and PHN.14 In patients with peripheral neuropathy, abnormal expression of miR-21-5p, miR-146a, miR-132-3p and miR-155 were reported in the skin, blood leukocytes and sural nerves.15,16 Patients with fibromyalgia syndrome showed abnormal expression of miR-let-7d in skin and leukocytes, which may be associated with lesions in peripheral skin neurofibromas.17
Circular RNAs (circRNAs) are non-coding RNAs which interact with miRNAs.18 circRNAs can act as miRNA sponges19–21 and regulate gene expression through a circRNA-miRNA-mRNA pattern.22,23 To date, there are few reports on the expression change and mechanism of circRNA in pain situation.24–26 We previously found that circRNA expression profile changed in the dorsal spinal cord of CCI rats.27 It is reported that intrathecally silence circHIPK3 with shRNA alleviated neuropathic pain in diabetic rats.24
It seems feasible and effective to interfere with the expression of miRNAs in skin. Animal studies suggested that interfering with the expression of miRNAs in the skin may affect the prognosis of diseases. Skin is an important lesion site and potential drug delivery location3 in PHN; however, the expression profiles of miRNA and circRNA in PHN skin have not been reported. In this study, the differential expression of miRNA and circRNA were detected with microarrays, and differential miRNAs’ target mRNAs were subjected to gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses to evaluate their possible functions and mechanisms in PHN situation.
Methods
Participants
This study was conducted in accordance with the Declaration of Helsinki. The present study was approved by the Medical Ethics Committee of the Affiliated Hospital of Zunyi Medical University and registered at the Chinese Clinical Trial Registry (ChiCTR1800017821) and all patients provided written informed consent. Patients were recruited from the Department of Pain Medicine of the local hospital from September 2018 to March 2019. All PHN patients in this study were diagnosed by two or more chief physicians. Pain intensity was evaluated with an 11-point numeric rating scale (NRS-11, values from 0 to 10). All of the patients recruited claimed intense pain (NRS scores ≥5) and pain durations were more than 1 month after the HZ healed. For easy sampling and to ensure that the skin locations were identical and comparable, only PHN lesion in skin of the thoracic segment was enrolled. Five PHN patients were enrolled in the microarray experiments and described in Table 1.
 |
Table 1 Demographic And Clinical Variables Of Five Patients In Microarray Experiments |
Skin Collection
For each patient, two circular skins on the back were sampled. Patients took a prone position, local anesthetics (1% lidocaine) was injected at the lesion site and the mirror site (symmetrical site of the opposite side) before the skin was taken with biopsy punchers (with 5 mm diameter and circular cross section). The bottom of the skin samples reaches the dermis, with a thickness of ~2 mm.
RNA Isolation, Purification And Hybridization
Total RNA from each sample was isolated and purified as we reported.28 miRNA labeling and array hybridization was according to Exiqon’s manual. After stopping the labeling procedure, the Hy3™-labeled samples were hybridized on the miRCURYTM LNA Array (v.19.0, Exiqon, USA) according to array manual. Then, the slides were scanned using the Axon GenePix 4000B microarray scanner (Axon Instruments, Foster City, USA).
For circRNA microarray, the RNA preparation and microarray hybridization were performed as we reported27 based on Arraystar’s standard protocols.29 Total RNA from each sample was treated with Rnase R (Epicentre Inc.) to remove linear RNAs and to enrich circRNA. Then, the enriched circRNAs were amplified and transcribed into fluorescent cRNA using a random priming method (Arraystar Super RNA Labeling Kit; Arraystar, USA). The labeled cRNAs were hybridized onto the Arraystar Human circRNA Array (v.2.0, 8×15K, Arraystar). At last, the arrays were scanned by the Agilent Scanner G2505C (Agilent, USA).
Microarray Data Analysis
For miRNAs, scanned images were imported into GenePix Pro 6.0 software (Axon) for grid alignment and data extraction. Replicated miRNAs were averaged and miRNAs that intensities ≥30 in all samples were chosen for calculating normalization factor. Expressed data were normalized using the median normalization. For circRNAs, quantile normalization of raw data and subsequent data processing were performed using the R software limma package. After quantile normalization of the raw data, low-intensity filtering was performed, and the circRNAs that at least 5 of 10 samples have flags in “P” or “M” (“All Targets Value”) were retained for further analyses. After normalization, significant differentially expressed miRNAs and circRNAs between two groups were identified through fold change and P-value. MiRNAs and circRNAs with fold changes ≥2 and P-value ≤0.05 were selected as differentially expressed genes. Hierarchical clustering was used to display the distinguishable expression pattern between PHN and control samples.
Real-Time Quantitative PCR Validation
Five differential miRNAs were randomly selected and subjected to real-time quantitative PCR (RT-qPCR). RNAiso Plus (Takara, Japan) was used to isolate total RNA from skins according to manufacturer’s instructions. Total RNA was prepared as standard template for reverse transcription with the Mir-X miRNA First-Strand Synthesis Kit (Clontech Laboratories, Japan). The miRNA-specific 5ʹ primers were as follows: hsa-miR-3664-3p, 5ʹ-CGTCTCAGGAGTAAAGACAGAGTT-3ʹ; hsa-miR-4714-3p, 5ʹ-CCAACCTAGGTGGTCAGAGTT-3ʹ; hsa-miR-16-5p, 5ʹ-CCGTAGCAGCACGTAAATATTGGCG-3ʹ; hsa-miR-20a-5p, 5ʹ-CGGCTAAAGTGCTTATAGTGCAGGTAG-3ʹ; hsa-miR-505-5p, 5ʹ-ACGGGAGCCAGGAAGTATTGATGT-3ʹ; hsa-let-7a-5p, 5ʹ-CGGCGTGAGGTAGTAGGTTGTATAGTT-3ʹ. RT-qPCR was performed on a CFX Connect Real-Time system (Bio-Rad, USA) in a 25 μL tube containing 12.5 μL of TB Green™ Premix Ex Taq™ II (Takara), and 0.5 μL of mRQ 3ʹ Primer according to manufacturer’s instructions. U6 was used as an endogenous reference. The 2−ΔΔCt method was used to calculate the relative expression of miRNAs.
MiRNA Prediction
Target mRNAs of the top 100 differential miRNAs were predicted with Arraystar’s home-made miRNA target prediction software based on the TargetScan (v.7.1, http://www.targetscan.org)30 and miRDB (v.5.0, http://mirdb.org/) prediction algorithm. Only the mRNAs predicted by two databases were included in the GO and pathway analyses.
Bioinformatics Analysis
GO and pathway analyses were performed to explore the function of differential miRNAs. The mRNAs of top 100 differential miRNAs were subjected to GO annotation in terms of Biological Processes, Cellular Components and Molecular Functions. Pathways defined by KEGG, Biocarta and Reactome (http://www.genome.jp/kegg/) were identified by DAVID (https://david.ncifcrf.gov/).
Statistical Analysis
The results were reported as mean±SD. Statistically significant differences of miRNA expression between PHN and control group were evaluated with paired t-tests with GraphPad Prism (v.7.0, GraphPad Software, USA). P<0.05 was considered to be statistically significant.
Results
miRNA Profiles
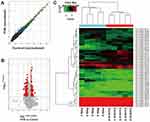
Ten samples from five PHN patients were subjected to miRNA microarray (Figure 1). The miRCURY™ LNA Array (v7.0, Exiqon) detected 2080 human miRNAs (Supplementary material 1). Overall, 317 of them were calculated as differential miRNAs (fold change ≥2.0, P<0.05), 67 were significantly up-regulated, and the other 250 were down-regulated in PHN group (Supplementary material 2). The miRNA, hsa-miR-4772-5p, down-regulated 21 times in PHN skin. Thirteen of these differentially expressed miRNAs showed expression fold change >10. The top 10 up- and down-regulated miRNAs are listed in Table 2.
 |
Table 2 Differential miRNAs Between PHN Skin And Mirror Skin (n=5) |
RT-qPCR Validation
To validate the microarray data, RT-qPCR was employed to detect miRNA expression in PHN skin and normal mirror skin in another cohort of PHN patients (n=12). Five miRNAs were randomly selected. Expression levels detected by the microarray and RT-qPCR are presented in Figure 2A. The expression trends detected by the two methods were consistent, which suggested high reliability of the microarray results.
Bioinformatics Analyses Of Target mRNA Of Differential miRNAs
A total of 4077 target mRNAs of the top 100 miRNAs were predicted by miRDB and TargetScan. These mRNAs were subjected to GO enrichment and the top 20 significantly enriched (P<0.05) GO terms are listed in Figure 2B–D. These mRNAs enriched in Biological Processes, Cellular Component and Molecular Function terms, such as Protein binding (GO: 0005515, with 542 genes) etc.
KEGG pathway analysis showed that the 4077 target mRNAs significantly (P<0.05) enriched in 85 pathways (Supplementary material 3), and the top 20 pathways are listed in Figure 3. The FoxO (45 mRNAs), ErbB (31 mRNAs), MAPK (78 mRNAs) and AMPK (39 mRNAs) pathway were among the top 10 (Figure 3).
Overview Of circRNA Profiles
Ten samples from five PHN patients were subjected to circRNA microarray. The Arraystar Human CircRNA Array v.2.0 detected 12,257 circRNAs (Supplementary material 4). Hierarchical clustering and scatter plot visualization showed the circRNAs expression levels were different (Figure 4), although only the hsa_circRNA_405463 show differential expression fold change ≥2.0 between the PHN and control group. There were 23 circRNAs were up-regulated and 8 were down-regulated in PHN skin (fold change ≥1.5), and if the threshold was set as 1.2, up to 260 circRNAs were up-regulated and 154 were down-regulated in PHN skin (fold change ≥1.2, Supplementary material 5). The top 10 up- and down-regulated circRNAs are listed in Table 3.
 |
Table 3 Differential circRNAs Between PHN Skin And Mirror Skin (n=5) |
Discussion
Skin is the lesion site and the location where hyperalgesia and allodynia appear in PHN patients. For the first time, the expression profiles of miRNAs and circRNAs in the PHN skin were detected with microarrays. Comprehensive expression changes of transcripts, especially miRNAs, were found between PHN and normal skin. This indicates that PHN skin and these differentially expressed molecules can be targets for PHN treatment.
miRNAs can regulate biological processes by inhibiting the function of their target genes. In this study, as many as 317 miRNAs were found differentially expressed in PHN skin, and 13 of them showed expression fold change >10, indicating that these differential miRNAs may influence their target genes’ expression as well as PHN pathologies. To evaluate functions of these miRNAs, their target mRNAs were predicted and KEGG pathway results indicated that they may influence the PHN pathology via AMPK, MAPK, ErbB, FoxO pathway, etc. It has been extensively reported that AMPK,31,32 MAPK13,33,34 and ErbB35,36 pathway involved in chronic and neuropathic pain in animal studies. Although FoxO’s function has not been confirmed in neuropathic pain model, bioinformatics predicted that FoxO pathway in the dorsal spinal cord27 or dorsal root ganglion37 was related to neuropathic pain.
It seems feasible and effective to interfere with the expression of miRNAs in skin. Animal studies suggested that interfering with the miRNA expression in the skin affects the prognosis of diseases. For example, interfering NR1 subunit of the NMDA receptor by intradermally injecting lentiviral vector-encoded miRNA-based shRNA reduced inflammatory pain in rats.38 Intradermally injecting miR-129 and/or miR-335 agomirs into the wound edges accelerated wound healing in rats with diabetes.39 Some miRNAs have been intradermally given and tested in clinical trials.40
Exploration of circRNAs’ roles in pain situation is still in infancy. Only a few studies focused on the expression change and mechanism of circRNA in pain situation.24–27 This study added evidence and suggested that PHN induced expression changes of circRNA in peripheral tissue. Further studies with gain- and/or loss-of-function experiments of these miRNAs and circRNAs will help understand their functions in PHN situation.
It is hard to tell whether the differential miRNAs and circRNAs are from the nerve endings of DRG neurons or cells that make up the skin. It is also not certain that these transcripts are present in cells because neurons and other cells may release miRNAs41 and circRNAs42 to the extracellular space via extracellular vesicles, such as exosomes. In situ hybridization experiments may help explain this question. Nevertheless, interventions targeting these differential miRNAs or circRNAs at the affected skin may help to alleviate PHN.
Conclusions
MiRNAs and circRNAs differentially expressed in the skin of PHN patients. Target genes of differential miRNAs enriched into pathways related to pathological pain regulation. These differential miRNAs and circRNAs as well as these predicted pathways could be potential targets to treat PHN.
Acknowledgments
The study was supported by a scientific research project from The Science and Technology Department of Guizhou Province (LH[2015]7554), the Innovative Training Program ([2015]3109) of Zunyi Medical University and the Excellent Young Talents Project of Zunyi Medical University (18zy-004). This work was also supported by a PhD Startup Fund ([2017]21) of the Affiliated Hospital of Zunyi Medical University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rowbotham MC, Davies PS, Fields HL. Topical lidocaine gel relieves postherpetic neuralgia. Ann Neurol. 1995;37(2):246–253. doi:10.1002/ana.410370216
2. Pickering G, Gavazzi G, Gaillat J, Paccalin M, Bloch K, Bouhassira D. Is herpes zoster an additional complication in old age alongside comorbidity and multiple medications? Results of the post hoc analysis of the 12-month longitudinal prospective observational ARIZONA cohort study. BMJ Open. 2016;6(2):e009689. doi:10.1136/bmjopen-2015-009689
3. Devor M. Rethinking the causes of pain in herpes zoster and postherpetic neuralgia: the ectopic pacemaker hypothesis. Pain Rep. 2018;3(6):e702. doi:10.1097/PR9.0000000000000702
4. Oaklander AL. Mechanisms of pain and itch caused by herpes zoster (shingles). J Pain. 2008;9(1 Suppl 1):S10–S18. doi:10.1016/j.jpain.2007.10.003
5. Philip A, Thakur R. Post herpetic neuralgia. J Palliat Med. 2011;14(6):765–773. doi:10.1089/jpm.2011.9685
6. Cao S, Li Y, Deng W, et al. Local brain activity differences between herpes zoster and postherpetic neuralgia patients: a resting-state functional MRI study. Pain Physician. 2017;20(5):E687–Ee699.
7. Cao S, Qin B, Zhang Y, et al. Herpes zoster chronification to postherpetic neuralgia induces brain activity and grey matter volume change. Am J Transl Res. 2018;10(1):184–199.
8. Cao S, Song G, Zhang Y, et al. Abnormal local brain activity beyond the pain matrix in postherpetic neuralgia patients: a resting-state functional MRI study. Pain Physician. 2017;20(2):E303–E314.
9. Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9(8):807–819. doi:10.1016/S1474-4422(10)70143-5
10. Andersen HH, Duroux M, Gazerani P. MicroRNAs as modulators and biomarkers of inflammatory and neuropathic pain conditions. Neurobiol Dis. 2014;71(11):159–168. doi:10.1016/j.nbd.2014.08.003
11. Jiangpan P, Qingsheng M, Zhiwen Y, Tao Z. Emerging role of microRNA in neuropathic pain. Curr Drug Metab. 2016;17(4):336–344.
12. Fernandes V, Sharma D, Vaidya S, et al. Cellular and molecular mechanisms driving neuropathic pain: recent advancements and challenges. Expert Opin Ther Targets. 2018;22(2):131–142. doi:10.1080/14728222.2018.1420781
13. Inoue K, Tsuda M. Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci. 2018;19(3):138–152. doi:10.1038/nrn.2018.2
14. Huang Y, Li X, Tao G, Zhu T, Lin J. Comparing serum microRNA levels of acute herpes zoster patients with those of postherpetic neuralgia patients. Medicine (Baltimore). 2017;96(8):e5997. doi:10.1097/MD.0000000000005997
15. Leinders M, Uceyler N, Thomann A, Sommer C. Aberrant microRNA expression in patients with painful peripheral neuropathies. J Neurol Sci. 2017;380:242–249. doi:10.1016/j.jns.2017.07.041
16. Leinders M, Uceyler N, Pritchard RA, Sommer C, Sorkin LS. Increased miR-132-3p expression is associated with chronic neuropathic pain. Exp Neurol. 2016;283(Pt A):276–286. doi:10.1016/j.expneurol.2016.06.025
17. Leinders M, Doppler K, Klein T, et al. Increased cutaneous miR-let-7d expression correlates with small nerve fiber pathology in patients with fibromyalgia syndrome. Pain. 2016;157(11):2493–2503. doi:10.1097/j.pain.0000000000000668
18. Wilusz JE, Sharp PA. A circuitous route to noncoding RNA. Science. 2013;340(6131):440–441. doi:10.1126/science.1238522
19. Hansen TB, Jensen TI, Clausen BH, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–388. doi:10.1038/nature11993
20. Zheng Q, Bao C, Guo W, et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun. 2016;7(11215). doi:10.1038/ncomms11215
21. Thomson DW, Dinger ME. Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet. 2016;17(5):272. doi:10.1038/nrg.2016.20
22. Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495(7441):333–338. doi:10.1038/nature11928
23. Wang Y, Yu X, Luo S, Han H. Comprehensive circular RNA profiling reveals that circular RNA100783 is involved in chronic CD28-associated CD8(+)T cell ageing. Immun Ageing. 2015;12(1):1–10. doi:10.1186/s12979-015-0028-x
24. Wang L, Luo T, Bao Z, Li Y, Bu W. Intrathecal circHIPK3 shRNA alleviates neuropathic pain in diabetic rats. Biochem Biophys Res Commun. 2018;505(3):644–650. doi:10.1016/j.bbrc.2018.09.158
25. Zhou J, Xiong Q, Chen H, Yang C, Fan Y. Identification of the spinal expression profile of non-coding RNAs involved in neuropathic pain following spared nerve injury by sequence analysis. Front Mol Neurosci. 2017;10:91. doi:10.3389/fnmol.2017.00091
26. Chu C, Chunshuai W, Jiajia C, et al. Transcriptional information revealed differentially expressed circular RNAs in facet joint osteoarthritis. Biochem Biophys Res Commun. 2018;497(2):790–796. doi:10.1016/j.bbrc.2018.02.157
27. Cao S, Deng W, Li Y, et al. Chronic constriction injury of sciatic nerve changes circular RNA expression in rat spinal dorsal horn. J Pain Res. 2017;10:1687–1696. doi:10.2147/JPR.S139592
28. Cao S, Liu Y, Sun W, et al. Genome-wide expression profiling of anoxia/reoxygenation in rat cardiomyocytes uncovers the role of MitoKATP in energy homeostasis. Oxid Med Cell Longev. 2015;2015:756576. doi:10.1155/2015/659750
29. Su H, Lin F, Xia D, Shen L. Profiling and bioinformatics analyses reveal differential circular RNA expression in radioresistant esophageal cancer cells. J Transl Med. 2016;14(1):225. doi:10.1186/s12967-016-0867-z
30. Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS. MicroRNA targets in Drosophila. Genome Biol. 2003;5(1):R1. doi:10.1186/gb-2003-5-1-r1
31. Xiang HC, Lin LX, Hu XF, et al. AMPK activation attenuates inflammatory pain through inhibiting NF-kappaB activation and IL-1beta expression. J Neuroinflamm. 2019;16(1):34.
32. Wang S, Kobayashi K, Kogure Y, et al. Negative regulation of TRPA1 by AMPK in primary sensory neurons as a potential mechanism of painful diabetic neuropathy. Diabetes. 2018;67(1):98–109. doi:10.2337/db17-0503
33. Lin X, Wang M, Zhang J, Xu R. p38 MAPK: a potential target of chronic pain. Curr Med Chem. 2014;21(38):4405–4418. doi:10.2174/0929867321666140915143040
34. Huang CT, Chen SH, Lin SC, Chen WT, Lue JH, Tsai YJ. Erythropoietin reduces nerve demyelination, neuropathic pain behavior and microglial MAPKs activation through erythropoietin receptors on Schwann cells in a rat model of peripheral neuropathy. Glia. 2018;66(11):2299–2315. doi:10.1002/glia.23461
35. Dai DW, Xu Z, Chen X, et al. Distinct roles of neuregulin in different models of neuropathic pain. Neurol Sci. 2014;35(4):531–536. doi:10.1007/s10072-013-1537-z
36. Calvo M, Zhu N, Grist J, Ma Z, Loeb JA, Bennett DL. Following nerve injury neuregulin-1 drives microglial proliferation and neuropathic pain via the MEK/ERK pathway. Glia. 2011;59(4):554–568. doi:10.1002/glia.21124
37. Chen CJ, Liu DZ, Yao WF, et al. Identification of key genes and pathways associated with neuropathic pain in uninjured dorsal root ganglion by using bioinformatic analysis. J Pain Res. 2017;10:2665–2674. doi:10.2147/JPR.S143431
38. Liu CC, Cheng JT, Hung KC, Chia YY, Tan PH. Lentiviral vector-encoded microRNA-based shRNA-mediated gene knockdown of N-methyl-D-aspartate receptors in skin reduces pain. Brain Behav. 2017;7(1):e00587. doi:10.1002/brb3.587
39. Wang W, Yang C, Wang XY, et al. MicroRNA-129 and −335 promote diabetic wound healing by inhibiting Sp1-mediated MMP-9 expression. Diabetes. 2018;67(8):1627–1638.
40. Singhvi G, Manchanda P, Krishna Rapalli V, Kumar Dubey S, Gupta G, Dua K. MicroRNAs as biological regulators in skin disorders. Biomed Pharmacother. 2018;108:996–1004. doi:10.1016/j.biopha.2018.09.090
41. Xu B, Zhang Y, Du XF, et al. Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell Res. 2017;27(7):882–897. doi:10.1038/cr.2017.62
42. Kim KM, Abdelmohsen K, Mustapic M, Kapogiannis D, Gorospe M. RNA in extracellular vesicles. Wiley Interdiscip Rev. 2017;8(4):e1413. doi:10.1002/wrna.2017.8.issue-4
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.