Back to Journals » OncoTargets and Therapy » Volume 12
MicroRNA-940 restricts the expression of metastasis-associated gene MACC1 and enhances the antitumor effect of Anlotinib on colorectal cancer
Authors Wang Y, Zhao M, Zhao H, Cheng S , Bai R, Song M
Received 20 November 2018
Accepted for publication 28 February 2019
Published 12 April 2019 Volume 2019:12 Pages 2809—2822
DOI https://doi.org/10.2147/OTT.S195364
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
Yan Wang,1 Meng Zhao,2 Huishan Zhao,3 Shi Cheng,1 Rixing Bai,1 Maomin Song1
1Department of General Surgery, Beijing Tian Tan Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Breast Surgery, Beijing Tian Tan Hospital, Capital Medical University, Beijing, People’s Republic of China; 3Reproductive Medicine Centre, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, Yantai, Shandong, People’s Republic of China
Background: Metastasis-associated with colon cancer-1 (MACC1) is an important regulator that promotes colorectal cancer (CRC) cells’ proliferation and distant metastasis. Therefore, MACC1 is considered as a promising therapeutic target of CRC. This work aimed to identify the microRNA (miR) targeted to MACC1, and to study the potential of using the particular miR in enhancing the antitumor effect of chemotherapy.
Materials and methods: miR prediction was performed in the miR database. The effect of miR-940 on MACC1’s expression was examined by Western blot, and the effect of miR-940 on the expression of genes related to the epithelial–mesenchymal transition (EMT) was identified by quantitative real-time polymerase chain reaction experiments. In vivo growth of CRC cells were analyzed in the nude mice subcutaneous tumor model and CRC liver metastasis model.
Results: By using the database, miR-940 was identified to target to the 3ʹUTR of MACC1’s mRNA. Experimentally, transfection of miR-940 decreased the expression of MACC1 in CRC cells and inhibited the EMT process of the transfected cells. MiR-940 also enhanced the inhibitory effect of Anlotinib on CRC cells’ in vivo growth and invasion. Correspondingly, ectopic expression of MACC1 mutant, which does not contain miR-940 binding site, blocked the antitumor effect of miR-940 on CRC cells.
Conclusion: MiR-940 restricts the proliferation and invasion of CRC cells by targeting to MACC1’s mRNA, and enhances the antitumor effect of Anlotinib on CRC tumors.
Keywords: colorectal cancer, Anlotinib, metastasis-associated gene in colon cancer 1, proliferation, in vivo invasive growth
Introduction
Colorectal cancer (CRC) is a common malignant tumor in the digestive tract, and its mortality ranks third in all malignancies.1,2 In China, the incidence of CRC has increased year by year, with an average annual rate of 4–5%. In 2015, the number of new cases of and deaths caused by CRC has doubled compared with 10 years ago, reaching 377,000 and 191,100 cases, respectively.3 In recent years, with the continuous development of clinical diagnosis and treatment approaches, the prognosis of patients with primary CRC has improved and the median survival has prolonged.4,5 However, the prognosis of advanced CRC, especially with liver metastasis, is still very poor; the 5-year survival rate is only 1–2%.6–8 Thus, treating advanced CRC is a strong challenge. There are advances in treating CRC liver metastases, such as chemotherapeutic options include CAPOX,9,10 FOLFOX411,12 and FOLFIRI13,14 that alleviated disease progression, achieved degraded patient behavior scores which ultimately made surgical resection realizable. However, only 30–40% of patients can be benefited from antitumor chemotherapies due to the low response rate.7 Therefore, establishing more effective antitumor treatment strategies for CRC is of great clinical significance.
Metastasis-associated in colon cancer-1 (MACC1) is an important regulator that, as multiple works reported, promotes CRC cells’ proliferation and enhances the distant metastasis.15,16 Increasing evidence has revealed that a high level of MACC1 expression in clinical specimens is significantly associated with poor patient survival.17,18 Moreover, MACC1 participates in regulating drug resistance: Li et al (2015) reported that downregulation of MACC1 attenuated cisplatin resistance in tongue squamous cell carcinoma.19 Zhang et al (2016) showed that knockdown of MACC1 overcame the resistance of cisplatin in the cisplatin-resistant epithelial ovarian cancer cell line.20 Duan et al (2017) indicated that MACC1 induces the chemotherapeutic-resistance of gastric cancer cells by regulating FASN expression.21 Therefore, MACC1 is a promising therapeutic target to reduce chemotherapy resistance. This work screened microRNAs (MiRs) and found miR-940 targeted to MACC1. We then showed miR-940 restricts the proliferation and invasion of CRC cells.
Anlotinib is a newly approved orally administrative small-molecule receptor tyrosine kinases (RTK) inhibitor that targets to RTKs related to tumor proliferation and metastasis, including vascular endothelial growth factor receptor, c-proto-oncogene protein, platelet-derived growth factor receptor or fibroblast growth factor receptors.22,23 The low response rate in patients indicating the efficiency of Anlotinib need to be enhanced by certain means, although the effect of this agent revealed to be promising in rodent models.22,24 In the present work, we found miR-940 enhanced the antitumor effect of Anlotinib on CRC tumors.
Material and methods
Cell lines and agents
SW620 and Lovo cells, which were purchased from the Type Culture Collection of the Chinese Academy of Sciences, were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Invitrogen, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA, USA). The cDNA samples from five nontumor tissues, ten CRC tissues and five CRC liver metastases tissues were preserved in our lab, and the collection of samples were approved by Ethic Committee of the Beijing Tiantan Hospital with written consent from patients. Anlotinib was purchased from Selleck Corporation, Houston, Texas, USA. Cells were cultured in DMEM and maintained in a sterile incubator at 37°C with 5% CO2. To measure proliferation ability, Anlotinib was dissolved in dimethyl sulfoxide (DMSO) (SINOPHARM Corporation, Beijing, China) and diluted in DMEM. Vectors of MACC1 or vectors of MACC1 with a mutation of miR-940 target sequences in 3ʹUTR was purchased from Vigene Corporation, Jinan City, Shandong Province, China. Lentivirus particles of miR-940 or control miRNA were purchased from Vigene Corporation, Jinan City, Shandong Province, China. The examination kits and inhibitor of miR-940 were purchased from Thermo Fisher Corporation, Waltham, MA, USA.
Cell survival examination
Anlotinib was carefully dissolved in DMSO, and the concentration of Anlotinib in DMSO solution was 10, 3, 1, 0.3, 0.1, 0.03 or 0.01 mmol/L. For cell culture experiments, Anlotinib-DMSO solution was diluted by DMEM at a ratio of 1:1,000 (0, 3, 1, 0.3, 0.1, 0.03, or 0.01 μmol/L concentration of Anlotinib) and the final concentration of DMSO was 1‰. Cells were cultured and treated with 0, 3, 1, 0.3, 0.1, 0.03 or 0.01 μmol/L concentration of Anlotinib for 48 hrs. Next, cells were analyzed by 3-(4,5Dimethylthiazol-yl)-2,5Dimethylthiazol-2-yl)-2,5diphenyltetrazolium bromide (MTT) experiments, and the inhibition rate of Anlotinib was calculated following the methods described by Wu et al (2018) and Feng et al (2016).25,26
Luciferase experiments
Regions of sequences (781–960 or 2,941–3,060) of MACC1 mRNA’s 3ʹUTR which were containing targeting sequence (cctgccta) of miR-940 were amplified by PCR and cloned into pGL4.26 vectors to construct luciferase reporters. Then, control miRNA, miR-940 or luciferase reporters were transfected into cells. After 24 hrs, cells were harvested for luciferase used a kit (Promega Corporation, USA) following the protocol from manufacturer (Promega Corporation, Madison, WI, USA).
Western blot experiments
Cells which were transfected with plasmids were harvested for Western blot experiments. Total protein samples were extracted and analyzed by SDS-PAGE analysis. Protein bands in SDS-PAGE gel were trans-printed into PVDF films and films were blocked by 5% BSA at 37°C for 2 hrs. Then, films were incubated with primary antibody and secondary antibody. The expression of MACC1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), the loading control, was detected by their antibodies (anti-MACC1 antibody [Cat. No. ab106579, Abcam Corporation, Cambridge, CB4 0FL, UK]; anti-GAPDH antibody [Cat. No. ab8245], Abcam Corporation, Cambridge, CB4 0FL, UK).
In vivo antitumor effect of Anlotinib
Performance of animal experiments was reviewed and approved by the Institutional Animal Care and Use Committee of the Beijing Tiantan Hospital. All animal experiments were performed in accordance with the UK Animals (Scientific Procedures) Act, 1986, and its associated guidelines. Nude mice aged 4–6 weeks were purchased from Si-Bei-Fu Biotechnology Corporation, Beijing China. For the subcutaneous tumor model,27,28 cells were transfected with miRNA-940 or miR-940 + mutated MACC1 (MACC1Mut) and injected into nude mice (1×106 cells per animal). After 2–4 days, animals received oral administration of 1 mg/kg dose Anlotinib per two days. After 3–4 weeks’ growth, the mice were harvested, and tumor weights were measured. Tumor volumes were calculated following the methods provided by Wang et al (2018) and Chen et al (2018).27,28
For the in situ colorectal tumor model, nude mice received inhalation anesthesia. Cells were transfected with miRNA-940 or miR-940+ mutated MACC1 (MACC1Mut). The roving sandpaper was used to injure the anus, and the rectum was filled with cell suspensions. Next, the anus was clamped with a small clip for 15–20 mins. Three to four days after cells were injected, the mice received oral administration of 1 mg/kg dose Anlotinib per two days. After 3–4 weeks’ growth, the mice were harvested, and tumor weights were measured. Tumor volumes were calculated following the methods provided by Wang et al (2018) and Chen et al (2018).27,28
To produce a liver in situ tumor model, SW620 cells that had been transfected with vectors were seeded into the livers of nude mice, following a method described by Meng et al (2018).29 The liver organs with nodules formed by the MDA-MB-231 cells were collected for Masson staining using a standard method. Masson staining results were quantitatively analyzed, following the methods described by Shao et al (2018).30
qPCR experiments
The total RNA (Ribonucleic Acid) of cells or clinical specimens was extracted using a PARISTM Kit (Applied Biosystems, Thermo Scientific Corporation, Waltham, MA, USA) and were reverse-transcribed by MultiscribeTM Reverse Transcriptase (Applied Biosystems, Thermo Scientific Corporation). The qPCR (real-time polymerase chain reaction/Quantitative polymerase chain reaction) experiments were performed as described previously.31–33 The level of GAPDH mRNA was measured as an internal control. Primers used in qPCR are shown in Table 1.
 | Table 1 The primers used in this work |
Statistical analysis
All statistical significance analyses were performed using SPSS 16.0 statistical software (IBM Corporation, Armonk, NY, USA). IC50 values of Anlotinib on CRC cells were calculated with Origin software 8.1 (OriginLab, Northampton, MA, USA). Images were quantificationally analyzed by Image J Software (Version No.: 1.51j8, National Institutes of Health, Bethesda, MD, USA). Statistical significance was analyzed by Bonferroni correction with or without two ways ANOVA. Paired-samples were tested by the paired-sample t-test.
Results
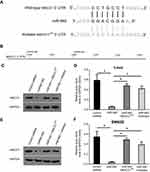
MiR-940 restricts MACC1’s expression by targeting the 3′-UTR of MACC1’s mRNA
To identify the potential miRNA targeting MACC1, the miR database was used. As shown in Figure 1, miR-940 was found to target MACC1’s mRNA with a high score (Figure 1A and B). The expression of MACC-1 and miR-940 was examined in clinical specimens. As shown in Figure S1, MACC1 is expressed higher in tumor tissues compared with non-tumor controls; MACC1 has the highest expression level In CRC samples. Interestingly, the expression of miR-940 is opposite to MACC1’s, suggest an antagonistic interaction of them. Next, Western blot experiments were carried out to confirm the effect of miR-940. Transfection of miR-940 significantly inhibited MACC1’s expression in Lovo (Figure 1C and D) or SW620 (Figure 1E and F) cells, two highly aggressive CRC cell lines. Transfection of miR-940 significantly also decreased the overexpression of MACC1 via a vector containing wild type MACC1 (Figure S2). In contrast, inhibition of miR-940 by transfection of miR-940’s inhibitor or MACC1 with a mutated miR-940 binding site (MACC1Mut) blocked the effect of miR-940 on MACC1’s expression. To further confirm the effect of miR-940 on MACC1, luciferase gene reporter assay was performed. As shown in Figure S3, miR-940 inhibited the luciferase activity of a reporter promoter contains miR-940 target sites. Therefore, miR-940 targets to MACC1.
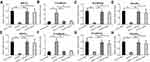
MiR-940 inhibits CRC cells’ EMT process
Next, the effect of miR-940 on migration and invasion-related pathways were examined. As shown in Figure 2, transfection of miR-940 inhibited the expression of N-cadherin and Vimentin, two mesenchymal indicators, and enhanced the expression of E-Cadherin, an epithelial indicator. These results showed that miR-940 inhibited the EMT process of CRC cells. Transfection of miR-940’s inhibitor or overexpressing MACC1Mut blocked the effect of miR-940 on EMT. Therefore, miR-940 inhibits CRC cells’ EMT process by targeting MACC1.
MiR-940 enhances the antitumor activation of Anlotinib on CRC cells
MTT were performed to examine whether targeting MACC1 could enhance the sensitivity of CRC cells to Anlotinib. As shown in Figure 3 and Table 2, Anlotinib inhibited CRC cells’ survival in a dose- and time-dependent manner. Transfection of miR-940 enhanced the activity of Anlotinib on CRC cells: the IC50 values of Anlotinib correspondingly decreased (Table 2, Figure 3). Moreover, transfection of miR-940 inhibitor or MACC1Mut blocked the effect of miR-940. Therefore, miR-940 enhances the antitumor activity of Anlotinib on CRC cells.
 | Table 2 The IC50 value of Anlotinib in CRC cells |
MiR-940 increases the in vivo antitumor activity of Anlotinib in CRC models
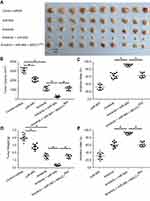
To further examine the in vivo effects of miR-940 on Anlotinib in CRC tumor, we firstly established SW620 subcutaneous tumor model on nude mice. Anlotibib was administrated orally. As shown in Figure 4, Anlotinib significantly inhibited the SW620 tumor growth in nude mice; transfection of miR-940 significantly enhanced the antitumor effect of Anlotinib. Moreover, ectopic expression of MACC1Mut in tumors could significantly block the effect of miR-940.
Next, we established SW620 in situ colorectal tumor model in nude mice, measured the volume and the weight of the in situ colorectal tumors. Similar to what we found in the subcutaneous tumor model, as shown in Figure 5, Anlotinib treatment had a strong antitumor effect, while miR-940 enhanced the effect of Anlotinib (Figure 5); and ectopic expression of MACC1Mut could significantly block the effect of miR-940.
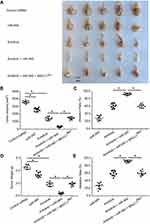
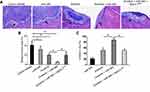
To study the effect of miR-940 in CRC liver metastasis model, SW620 tumors were established in livers. As shown in Figure 6, SW620 cells broke the capsula fibrosa to invade the liver surface. Treatment with Anlotinib significantly inhibited the invasive growth of SW620 cells into the livers of nude mice. Transfection of miR-940 enhanced the antitumor effect of Anlotinib on the invasive growth of SW620 cells into the livers of nude mice. Ectopic expression of MACC1Mut significantly decreased the effect of miR-940. Therefore, transfection of miR-940 increases the in vivo antitumor effect of Anlotinib.
Discussion
Despite the advances in treatment options in recent years, CRC is still a fatal disease with increasing incidence worldwide.34,35 In the past two decades, systemic chemotherapy b one of the foremost choices for advanced CRC treatment.36 Recently, molecule-targeted agents, such as cetuximab,37 panitumumab,38,39 bevacizumab,40 regorafenib41,42 and ramucirumab,43,44 that inhibit RTKs activation have been approved for use in CRC treatment. Downregulation of RTKs’ activity can attenuate tumor cell growth, metastasis, and angiogenesis.45–47 Anlotinib is newly approved and has shown an antitumor effect against several cancers, such as advanced medullary thyroid cancer, metastatic renal cell carcinoma, advanced non-small cell lung cancer (NSCLC), and advanced soft tissue sarcoma.48,49 In the present work, we showed that Anlotinib inhibited the proliferation and invasive growth of CRC cells. Our data extended the understanding of Anlotinib in treating human cancers.
It is well known that MACC1 participates in the regulation of cancer cell proliferation and metastasis that mediate poor clinical outcomes.50 Recently, MACC1 was also found to be a key regulator of therapeutic resistance. Yang et al (2013) showed that high levels of MACC1 predict poor outcomes of cryoablation therapy for advanced HCC.51 Liu et al (2016) revealed a new mechanism of MACC1-mediated trastuzumab resistance in gastric cancer. They found MACC1 promotes trastuzumab resistance via modulating PI3K/AKT signaling pathway and inducing the Warburg effect on gastric cancer cells.52 Moreover, Wang et al (2017) reported that MACC1 facilitates the chemoresistance of CRC cells by inducing the cancer stem cell‑like properties of CRC cells.53 In the present work, we showed downregulation of MACC1 expression via miR-940 enhanced the antitumor effect of Anlotinib on CRC cells’ proliferation and invasive growth. Mechanism studies showed that overexpression of miR-940 inhibited the EMT process of CRC cells. Inhibition of the EMT could enhance the sensitivity of human cancer cells to therapeutic strategies, such as sorafenib and radiofrequency ablation.53–66 Therefore, miR-940 is a promising approach to enhance the sensitivity of CRC cells to Anlotinib.
The liver metastasis is closely related to the poor prognosis of CRC patients.58,59 To mimic CRC liver metastasis, we established an intrahepatic invasion model. In the present work, we found CRC cells were adhered to the surface of the liver, destroyed the liver capsule and allows CRC cells to invade the liver tissue. The degree of CRC cells’ invasive growth into the liver tissue can quantitatively reflect the metastasis of the tumors. This model is more representative and efficient to mimic the in vivo invasion of human cancer cells compared to other tumor metastasis model: tumor cells were injected through tail-vein injection to form tumor nodules in nude mice’s lung organs.32,33 The massive capillary system in the lung allows cells to locate in the entire organ, but the pathological invasion of cells cannot be simply mimicked in this model. Therefore, our model is a useful tool to evaluate the therapeutic effect of antitumor therapies on cancer cells’ invasive growth.
MiRs are non-coding RNAs and very important components of the post-transcriptional regulation of the gene expression profile.59 MiRs are promising tools to attenuating the metastasis of human cancer cells.61,62 MiR-940 is a potential negative regulator of human cancer cells. Gu et al (2018) reported that transfection of MiR-940 inhibits the progression of NSCLC by targeting FAM83F.63 Wang et al (2017) showed that miR-940 suppresses cell proliferation of ovarian cancer OVCAR3 cells.64 Ding et al (2016) and Yuan et al (2015) revealed that miR-940 functions as a tumor suppressor in hepatocellular carcinoma.65,66 Interestingly, Fan et al (2018) and Lin et al (2018) reported that MiR-940 could be a positive regulator of cellular proliferation in gastric cancer cells or osteosarcoma cells.67,68 In this work, our results demonstrated that overexpression of miR-940 via preparation it as lentivirus particles suppressed MACC1’s expression and inhibited CRC cells’ EMT process. Transfection of miR-940 enhanced the antitumor effect of Anlotinib on CRC cells’ proliferation and invasive growth. Therefore, our work extended the knowledge of miR-940 in regulating cancer development.
Conclusion
MiR-940 represses the expression of MACC1 via targeting its mRNA’s 3ʹUTR. MiR-940 restricts the proliferation and invasion of CRC cells by inhibiting the EMT process, and enhances the antitumor effect of Anlotinib on CRC tumors.
Abbreviation list
MACC1, Metastasis-associated in colon cancer-1; CRC, colorectal cancer; miRs, microRNA; EMT, epithelial–mesenchymal transition; qPCR, quantitative real-time polymerase chain reaction; RTK, receptor tyrosine kinases; NSCLC, non-small cell lung cancer; STS, soft tissue sarcoma.
Acknowledgment
We would like to thank the teachers from the tumor invasion and metastasis mechanism research laboratory, capital medical university and the animal experiment center of PLA 302 hospital for their great help.
Author contributions
All authors made substantial contributions to the design and conception; acquisition, analysis or interpretation of data. All authors took part in either drafting or revising the manuscript. At the same time, all authors gave final approval of the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Xu J, Kim TW, Shen L, et al. Results of a randomized, double-blind, placebo-controlled, phase III Trial of Trifluridine/Tipiracil (TAS-102) monotherapy in Asian patients with previously treated metastatic colorectal cancer: the TERRA Study. J Clin Oncol. 2018;36(4):350–358. doi:10.1200/JCO.2017.74.3245
2. Sepulveda AR, Hamilton SR, Allegra CJ, et al. Molecular biomarkers for the evaluation of colorectal cancer: guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J Clin Oncol. 2017;35(13):1453–1486. doi:10.1200/JCO.2016.71.9807
3. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
4. Vardy JL, Dhillon HM, Pond GR, et al. Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: a prospective, longitudinal, controlled study. J Clin Oncol. 2015;33(34):4085–4092. doi:10.1200/JCO.2015.63.0905
5. Calderaro J, Azoulay D, Zafrani ES. Hepatocellular carcinoma and nodular regenerative hyperplasia after chemotherapy for metastatic colorectal carcinoma. Hepatology. 2014;60(4):1440–1441. doi:10.1002/hep.27115
6. Adam R, De Gramont A, Figueras J, et al;
7. Van Cutsem E, Nordlinger B, Cervantes A;
8. Nordlinger B, Van Cutsem E, Rougier P, et al;
9. André T, Vernerey D, Mineur L, et al;
10. Sobrero A, Lonardi S, Rosati G, et al;
11. André T, de Gramont A, Vernerey D, et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol. 2015;33(35):4176–4187. doi:10.1200/JCO.2015.63.4238
12. Lin H, Qiu X, Zhang B, Zhang J. Identification of the predictive genes for the response of colorectal cancer patients to FOLFOX therapy. Onco Targets Ther. 2018;11:5943–5955. doi:10.2147/OTT.S167656
13. Modest DP, Stintzing S, von Weikersthal LF, et al. Impact of subsequent therapies on outcome of the FIRE-3/AIO KRK0306 trial: first-line therapy with FOLFIRI plus cetuximab or bevacizumab in patients with KRAS wild-type tumors in metastatic colorectal cancer. J Clin Oncol. 2015;33(32):3718–3726. doi:10.1200/JCO.2015.61.2887
14. Casadei Gardini A, Scarpi E, Orlandi E, et al. Prognostic role of aspartate aminotransferase-lymphocyte ratio index in patients with metastatic colorectal cancer: results from the randomized ITACa trial. Onco Targets Ther. 2018;11:5261–5268. doi:10.2147/OTT.S166614
15. Kim HJ, Moon SJ, Kim SH, Heo K, Kim JH. DBC1 regulates Wnt/β-catenin-mediated expression of MACC1, a key regulator of cancer progression, in colon cancer. Cell Death Dis. 2018;9(8):831. doi:10.1038/s41419-018-1111-y
16. Raats DA, Frenkel N, van Schelven SJ, Rinkes IH, Laoukili J, Kranenburg O. CD95 ligand induces senescence in mismatch repair-deficient human colon cancer via chronic caspase-mediated induction of DNA damage. Cell Death Dis. 2017;8(3):e2669. doi:10.1038/cddis.2017.518
17. Li X, Zhao Q, An B, et al. Prognostic and predictive value of the macroscopic growth pattern in patients undergoing curative resection of colorectal cancer: a single-institution retrospective cohort study of 4,080 Chinese patients. Cancer Manag Res. 2018;10:1875–1887. doi:10.2147/CMAR.S165279
18. Li Z, Yanfang W, Li J, et al. Tumor-released exosomal circular RNA PDE8A promotes invasive growth via the miR-338/MACC1/MET pathway in pancreatic cancer. Cancer Lett. 2018;432:237–250. doi:10.1016/j.canlet.2018.04.035
19. Li HF, Liu YQ, Shen ZJ, et al. Downregulation of MACC1 inhibits invasion, migration and proliferation, attenuates cisplatin resistance and induces apoptosis in tongue squamous cell carcinoma. Oncol Rep. 2015;33(2):651–660. doi:10.3892/or.2014.3612
20. Zhang R, Shi H, Ren F, et al. Knockdown of MACC1 expression increases cisplatin sensitivity in cisplatin-resistant epithelial ovarian cancer cells. Oncol Rep. 2016;35(4):2466–2472. doi:10.3892/or.2016.4585
21. Duan J, Chen L, Zhou M, et al. MACC1 decreases the chemosensitivity of gastric cancer cells to oxaliplatin by regulating FASN expression. Oncol Rep. 2017;37(5):2583–2592. doi:10.3892/or.2017.5519
22. Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11(1):120. doi:10.1186/s13045-018-0664-7
23. Han B, Li K, Wang Q, et al. Effect of Anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol. 2018;4(11):1569–1575. doi:10.1001/jamaoncol.2018.3039
24. Zhong CC, Chen F, Yang JL, et al. Pharmacokinetics and disposition of anlotinib, an oral tyrosine kinase inhibitor, in experimental animal species. Acta Pharmacol Sin. 2018;39(6):1048–1063. doi:10.1038/aps.2017.199
25. Wu M, Zhao G, Zhuang X, et al. Triclosan treatment decreased the antitumor effect of sorafenib on hepatocellular carcinoma cells. Onco Targets Ther. 2018;11:2945–2954. doi:10.2147/OTT.S165436
26. Jia H, Yang Q, Wang T, et al. Rhamnetin induces sensitization of hepatocellular carcinoma cells to a small molecular kinase inhibitor or chemotherapeutic agents. Biochim Biophys Acta. 2016;1860(7):1417–1430. doi:10.1016/j.bbagen.2016.04.007
27. Wang L, Zhao L, Jia X, et al. Aminophenols increase proliferation of thyroid tumor cells by inducing the transcription factor activity of estrogen receptor α. Biomed Pharmacother. 2018;109:621–628. doi:10.1016/j.biopha.2018.10.168
28. Chen Z, Jiang Z, Zhang W, He B. Silencing the expression of copine-III enhances the sensitivity of hepatocellular carcinoma cells to the molecular targeted agent sorafenib. Cancer Manag Res. 2018;10:3057–3067. doi:10.2147/CMAR.S167781
29. Meng D, Lei M, Han Y, et al. MicroRNA −645 targets urokinase plasminogen activator and decreases the invasive growth of MDA-MB-231 triple-negative breast cancer cells. Onco Targets Ther. 2018;11:7733–7743. doi:10.2147/OTT.S187221
30. Shao Z1, Li Y2, Dai W3, et al. ETS-1 induces Sorafenib-resistance in hepatocellular carcinoma cells via regulating transcription factor activity of PXR. Pharmacol Res. 2018;135:188–200. doi:10.1016/j.phrs.2018.08.003
31. Li L, Liang Y, Kang L, et al. Transcriptional regulation of the Warburg effect in cancer by SIX1. Cancer Cell. 2018;33(3):368–385.e7. doi:10.1016/j.ccell.2018.01.010
32. Roskoski R
33. Liu X, Chen X, Zeng K, et al. DNA-methylation-mediated silencing of miR-486-5p promotes colorectal cancer proliferation and migration through activation of PLAGL2/IGF2/β-catenin signal pathways. Cell Death Dis. 2018;9(10):1037. doi:10.1038/s41419-018-1111-y
34. Ji Q, Xu X, Li L, et al. miR-216a inhibits osteosarcoma cell proliferation, invasion and metastasis by targeting CDK14. Cell Death Dis. 2017;8(10):e3103. doi:10.1038/cddis.2017.518
35. Liang Y, Xu X, Wang T, et al. The EGFR/miR-338-3p/EYA2 axis controls breast tumor growth and lung metastasis. Cell Death Dis. 2017;8(7):e2928. doi:10.1038/cddis.2017.518
36. Vaes N, Schonkeren SL, Brosens E, et al. A combined literature and in silico analysis enlightens the role of the NDRG family in the gut. Biochim Biophys Acta Gen Subj. 2018;1862(10):2140–2151. doi:10.1016/j.bbagen.2018.07.004
37. D’Onofrio M, Zanzoni S, Munari F, Monaco HL, Assfalg M, Capaldi S. The long variant of human ileal bile acid-binding protein associated with colorectal cancer exhibits sub-cellular localization and lipid binding behaviour distinct from those of the common isoform. Biochim Biophys Acta Gen Subj. 2017;1861(9):2315–2324. doi:10.1016/j.bbagen.2017.07.004
38. Kanat O, Ertas H, Caner B. Dual HER2 inhibition strategies in the management of treatment-refractory metastatic colorectal cancer: history and status. World J Clin Cases. 2018;6(11):418–425. doi:10.12998/wjcc.v6.i11.418
39. Dote S, Itakura S, Kamei K, et al. Oral mucositis associated with anti-EGFR therapy in colorectal cancer: single institutional retrospective cohort study. BMC Cancer. 2018;18(1):957. doi:10.1186/s12885-018-4242-8
40. Jia J, Morse MA, Nagy RJ, Lanman RB, Strickler JH. Cell-free DNA profiling to discover mechanisms of exceptional response to cabozantinib plus panitumumab in a patient with treatment refractory metastatic colorectal cancer. Front Oncol. 2018;8:305. doi:10.3389/fonc.2018.00305
41. Jayson GC, Zhou C, Backen A, et al. Plasma Tie2 is a tumor vascular response biomarker for VEGF inhibitors in metastatic colorectal cancer. Nat Commun. 2018;9(1):4672. doi:10.1038/s41467-018-07174-1
42. Chambers AE, Frick J, Tanner N, Gerkin R, Kundranda M, Dragovich T. Chemotherapy re-challenge response rate in metastatic colorectal cancer. J Gastrointest Oncol. 2018;9(4):679–686. doi:10.21037/jgo.2018.04.08
43. Schirripa M, Pasqualetti G, Giampieri R, et al. Prognostic value of thyroid hormone ratios in patients with advanced metastatic colorectal cancer treated with regorafenib: the TOREADOR study. Clin Colorectal Cancer. 2018;17(3):e601–e615. doi:10.1016/j.clcc.2018.05.013
44. Yoshino T, Portnoy DC, Obermannová R, et al. Biomarker analysis beyond angiogenesis: RAS/RAF mutation status, tumour sidedness, and second-line ramucirumab efficacy in patients with metastatic colorectal carcinoma from RAISE, a global phase 3 study. Ann Oncol. 2018. doi: 10.1093/annonc/mdy461. [Epub ahead of print]
45. Grothey A, Yoshino T, Bodoky G, et al. Association of baseline absolute neutrophil counts and survival in patients with metastatic colorectal cancer treated with second-line antiangiogenic therapies: exploratory analyses of the RAISE trial and validation in an electronic medical record data set. ESMO Open. 2018;3(3):e000347. doi:10.1136/esmoopen-2018-000347
46. Bignucolo A, De Mattia E, Cecchin E, Roncato R, Toffoli G. Pharmacogenomics of targeted agents for personalization of colorectal cancer treatment. Int J Mol Sci. 2017;18(7):
47. Martini G, Troiani T, Cardone C, et al. Present and future of metastatic colorectal cancer treatment: a review of new candidate targets. World J Gastroenterol. 2017;23(26):4675–4688. doi:10.3748/wjg.v23.i26.4675
48. Sun Y, Niu W, Du F, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol. 2016;9(1):105. doi:10.1186/s13045-016-0332-8
49. Syed YY. Anlotinib: first global approval. Drugs. 2018;78(10):1057–1062. doi:10.1007/s40265-018-0939-x
50. Guo T, Zhao S, Wang P, et al. YB-1 regulates tumor growth by promoting MACC1/c-Met pathway in human lung adenocarcinoma. Oncotarget. 2017;8(29):48110–48125. doi:10.18632/oncotarget.18262
51. Yang YP1, Qu JH, Chang XJ, et al. High intratumoral metastasis-associated in colon cancer-1 expression predicts poor outcomes of cryoablation therapy for advanced hepatocellular carcinoma. J Transl Med. 2013;11:41. doi:10.1186/1479-5876-11-41
52. Liu J, Pan C, Guo L, et al. A new mechanism of trastuzumab resistance in gastric cancer: MACC1 promotes the Warburg effect via activation of the PI3K/AKT signaling pathway. J Hematol Oncol. 2016;9(1):76. doi:10.1186/s13045-016-0302-1
53. Wang J, Wang W, Cai H, et al. MACC1 facilitates chemoresistance and cancer stem cell-like properties of colon cancer cells through the PI3K/AKT signaling pathway. Mol Med Rep. 2017;16(6):8747–8754. doi:10.3892/mmr.2017.7721
54. Livitsanou M, Vasilaki E, Stournaras C, Kardassis D. Modulation of TGFβ/Smad signaling by the small GTPase RhoB. Cell Signal. 2018;48:54–63. doi:10.1016/j.cellsig.2018.04.007
55. Liu X, Li C, Zhang R, et al. The EZH2- H3K27me3-DNMT1 complex orchestrates epigenetic silencing of the wwc1 gene, a Hippo/YAP pathway upstream effector, in breast cancer epithelial cells. Cell Signal. 2018;51:243–256. doi:10.1016/j.cellsig.2018.08.011
56. Zhang PF, Wang F, Wu J, et al. LncRNA SNHG3 induces EMT and sorafenib resistance by modulating the miR-128/CD151 pathway in hepatocellular carcinoma. J Cell Physiol. 2018. doi: 10.1002/jcp.27095. [Epub ahead of print]
57. Ikemoto T, Shimada M, Yamada S. Pathophysiology of recurrent hepatocellular carcinoma after radiofrequency ablation. Hepatol Res. 2017;47(1):23–30. doi:10.1111/hepr.12705
58. Huang S, Tan X, Huang Z, Chen Z, Lin P, Fu SW. microRNA biomarkers in colorectal cancer liver metastasis. J Cancer. 2018;9(21):3867–3873. doi:10.7150/jca.28588
59. Deng Q, Geng Y, Zhao L, et al. NLRP3 inflammasomes in macrophages drive colorectal cancer metastasis to the liver. Cancer Lett. 2018;442:21–30. doi:10.1016/j.canlet.2018.10.030
60. Bhayadia R, Krowiorz K, Haetscher N, et al. Endogenous tumor suppressor microRNA-193b: therapeutic and prognostic value in acute myeloid leukemia. J Clin Oncol. 2018;36(10):1007–1016. doi:10.1200/JCO.2017.75.2204
61. Li R, Qiao M, Zhao X, Yan J, Wang X, Sun Q. MiR-20a-3p regulates TGF-β1/Survivin pathway to affect keratinocytes proliferation and apoptosis by targeting SFMBT1 in vitro. Cell Signal. 2018;49:95–104. doi:10.1016/j.cellsig.2018.06.003
62. Xiong H, Yan T, Zhang W, et al. miR-613 inhibits cell migration and invasion by downregulating Daam1 in triple-negative breast cancer. Cell Signal. 2018;44:33–42. doi:10.1016/j.cellsig.2018.01.013
63. Gu GM, Zhan YY, Abuduwaili K, et al. MiR-940 inhibits the progression of NSCLC by targeting FAM83F. Eur Rev Med Pharmacol Sci. 2018;22(18):5964–5971. doi:10.26355/eurrev_201809_15927
64. Wang R, Wu Y, Huang W, Chen W. MicroRNA-940 targets INPP4A or GSK3β and activates the Wnt/β-catenin pathway to regulate the malignant behavior of bladder cancer cells. Oncol Res. 2018;26(1):145–155. doi:10.3727/096504017X14902261600566
65. Ding D, Zhang Y, Yang R, et al. miR-940 suppresses tumor cell invasion and migration via regulation of CXCR2 in hepatocellular carcinoma. Biomed Res Int. 2016;2016:7618342. doi:10.1155/2016/7618342
66. Yuan B, Liang Y, Wang D, Luo F. MiR-940 inhibits hepatocellular carcinoma growth and correlates with prognosis of hepatocellular carcinoma patients. Cancer Sci. 2015;106(7):819–824. doi:10.1111/cas.12688
67. Fan Y, Che X, Hou K, et al. MiR-940 promotes the proliferation and migration of gastric cancer cells through up-regulation of programmed death ligand-1 expression. Exp Cell Res. 2018;373:180–187. pii: S0014-4827(18)31118-2. doi:10.1016/j.yexcr.2018.10.011. [Epub ahead of print].
68. Lin ZW, Zhang W, Jiang SD, Wei WB, Li XF Inhibition of microRNA-940 suppresses the migration and invasion of human osteosarcoma cells through the secreted frizzled-related protein 1-mediated Wnt/β-catenin signaling pathway. J Cell Biochem. 2018. doi: 10.1002/jcb.27580. [Epub ahead of print]
Supplementary Materials
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.